Introduction
The worldwide incidence of inflammatory bowel
disease (IBD), namely ulcerative colitis (UC) and Crohn's disease,
has been increasing for a number of decades. One possible
explanation for this increase is that advances in treatment
strategies have resulted in the prolonged survival of affected
patients, however, the number of IBD patients diagnosed with
colorectal cancer [CRC; colitis-associated cancer (CAC)] has also
increased, particularly in those suffering from UC (1). CAC is considered to develop as a result
of chronic inflammation, with a recently conducted meta-analysis
estimating the incidence rate of CAC at 7 and 12 per 1,000
individuals per year in UC patients at 20 and 30 years
post-diagnosis, respectively. This high incidence rate highlights
the importance of preventing the development of CAC in high-risk UC
patients (2). Furthermore, the
expression of numerous inflammatory cytokines is associated with
the development of acute or chronic intestinal inflammation
(3–5);
for example, the upregulation of the tumor necrosis factor-α
(TNF-α) and interleukin (IL)-1β pro-inflammatory cytokines requires
the activation of transcription factor nuclear factor κB (NF-κB)
(6–8).
Feverfew (Tanacetum parthenium), a
traditional herb that has been applied medicinally in Europe for
the treatment of migraine, fever and arthritis, contains a number
of sesquiterpene lactones, including parthenolide (PT) (9). Previously conducted studies have
established that the anti-inflammatory mechanisms of feverfew
involve the inhibition of IL-1- and TNF-α-mediated NF-κB activation
(10–12). PT was recently demonstrated to be the
specific agent in feverfew that was responsible for this action,
inhibiting NF-κB activation and thus inducing apoptotic cell death
in various types of human cancer cells (13,14).
Additionally, previous studies have also demonstrated that PT is a
potent inhibitor of NF-κB activation and can inhibit the expression
of pro-inflammatory cytokines in experimental murine models
(11,15–19). Our
recent study used xenograft models to reveal that PT is a potential
chemopreventive and therapeutic agent for CRC (20); however, to date, no evidence of the
therapeutic effect of PT on CAC exists. Therefore, we hypothesized
that PT exerts its anticarcinogenic effect on CAC by inhibiting the
activation of the NF-κB signaling pathway. The present study aimed
to provide experimental evidence of this hypothesis by evaluating
the effect of PT administration on a murine model of azoxymethane
(AOM)/dextran sulfate sodium (DSS)-induced CAC.
Materials and methods
Chemicals and reagents
Parthenolide and z-VAD-fluoromethylketone were
obtained from Calbiochem (San Diego, CA, USA), AOM and DSS were
purchased from Sigma-Aldrich (St. Louis, MO, USA) and a terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL) assay kit was obtained from Promega Corporation (Madison,
WI, USA). Anti-inhibitor of κBα (IκBα, mouse monoclonal; cat. no.
sc-8404), anti-p65 (mouse monoclonal; cat. no. sc-8008),
anti-B-cell lymphoma (Bcl)-2 (rabbit polyclonal; cat. no. sc-783),
anti-Bcl-extra large (xL, mouse monoclonal; cat. no. sc-8392) and
anti-caspase 3 (rabbit polyclonal; cat. no. sc-7148) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Beverly, MA,
USA), while anti-actin (rabbit polyclonal; cat. no. A2066)
antibodies were purchased from Sigma-Aldrich.
Animal models
A total of 15 six-week-old pathogen-free female
Balb/C mice were purchased from Orient Bio Inc., (Seongnam, Korea).
Mice were given ad libitum access to water and standard
rodent food until they reached the desired weight (18–20 g). Mice
were maintained on a 12-h:12-h light/dark cycle under specific
pathogen free conditions. All procedures using the mice were
reviewed and approved by Chonbuk National University Animal Care
and Use Committee (Approval no: CBNU 2015-0013). In each group,
five mice were randomly assigned after they were weighed. The mice
were injected intraperitoneally with 7.4 mg/kg body weight of AOM
dissolved in physiological saline. After five days, 3% DSS was
administered in the drinking water for five days, followed by 16
days of regular water. This cycle was repeated three times.
Following sacrifice by cervical dislocation, the entire colon was
removed from the cecum to the anus, and the colon was then opened
longitudinally, and the number of macroscopic tumors were counted
and measured using calipers. Subsequently, the distal colons were
fixed in 10% neutral-buffered formalin for 24 h, and transferred to
70% ethanol for subsequent paraffin embedding and histological
analysis.
Histological analysis
The sections (5 µm) were stained with hematoxylin
and eosin, and histological analysis was performed by a pathologist
in a double-blind manner. The inflammation scores of mucosal
inflammation were determined as follows (21): 0, normal morphology; 1, focal
inflammatory cell infiltrate around the crypt base; 2, diffuse
infiltration of inflammatory cells around the crypts or
erosion/destruction of the lower one-third of the glands; and 3,
erosion/destruction of the lower two-thirds of the glands or loss
of all the glands. Furthermore, invasion depth was scored as
follows (22): 0, no invasion; 1,
invasion through the mucosa; 2, invasion through the submucosa; and
3, full invasion through the muscularis and into the serosa.
Immunohistochemistry (IHC)
IHC was performed in paraffin-embedded, 5-µm tissue
sections. For the analysis of NF-κB p65, phospho-IκBα, Bcl-2,
Bcl-xL and caspase 3 protein expression levels, the slides were
hydrated and endogenous peroxidase activity quenched with 0.03%
hydrogen peroxide in MeOH. Antigen retrieval was performed using
boiling sodium citrate in a microwave (20 mM sodium citrate pH 6.5)
for 16 min at 30% power. Subsequent to blocking, primary antibody
was added (dilution ratio, 1:500) overnight at 4°C. The slides were
washed and incubated with secondary antibody for 30 min. After
this, the slides were reacted with streptavidin for 20 min, the
reaction was visualized with 3,3-diaminobenzidine
tetrahydrochloride for 5 min, and the slides were counterstained
with Meyer's hematoxylin. Sections were examined under a Olympus
BX53 microscope (Olympus America, Hauppauge, NY, USA); images of
the representative areas were captured at a magnification of x20
and the number of positively stained cells were counted.
Apoptosis was quantitatively determined by
performing a TUNEL assay using an ApopTag® in situ Apoptosis
Detection kit (EMD Millipore, Temecula, CA, USA), according to the
manufacturer's instructions. Four fields at x20 magnification were
selected at the proliferation front of each tumor and the number of
TUNEL-positive cells were counted.
Western blotting
Colon tissue samples were homogenized in lysis
buffer [20 mM Tris HCl (pH 7.5), 1% Triton X100, 0.2 M NaCl, 2 mM
EDTA, 2 mM ethylene glycol tetraacetic acid, 1 M dithiothreitol and
2 M aprotinin). Protein samples (50 µg per lane) were
electrophoresed on 10% SDS PAGE gels and the separated proteins
were electrophoretically transferred onto polyvinylidene difluoride
membranes. Subsequently, the membranes were incubated with
anti-phospho IκB (1:1,000), anti-p65 (1:1,000), anti-Bcl2
(1:1,000), anti-Bcl-xL (1:1,000) and anti-caspase3 (1:1,000) and
anti-β-actin (1:1,000) antibodies. The signal was detected using
enhanced WEST-one (iNtRON Biotechnology, Daejeon, Korea) and
analyzed with a luminescent image analyzer (LAS-3000; Fuji Film
Corporation, Tokyo, Japan).
Statistical analysis
The data are presented as the mean ± standard error
of the mean of a minimum of three independent experiments performed
in duplicate. Imaging analysis for IHC were performed by cellsense
standard software (standard version, Olympus America).
Representative blots are included. All data were entered into a
Microsoft Excel version 5.0 (Microsoft Corporation, Redmond, WA,
USA) spreadsheet, and SPSS software (SPSS, Inc., Chicago, IL, USA)
was used to perform the two-tailed t tests or the analysis of
variance, where appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
PT inhibits colon carcinogenesis
induced by AOM/DSS administration in a murine model
AOM is a procarcinogen that upon metabolic
activation, causes the formation of O6-methylguanine
(23). AOM induces the development of
tumors in the distal colon of rodents and is commonly used to
elicit CRC in experimental animals (24–26). Thus,
the present study used a CAC model in which six-week-old mice were
injected with a single dose of AOM followed by DSS administered in
the drinking water to analyze the antitumor activities of PT.
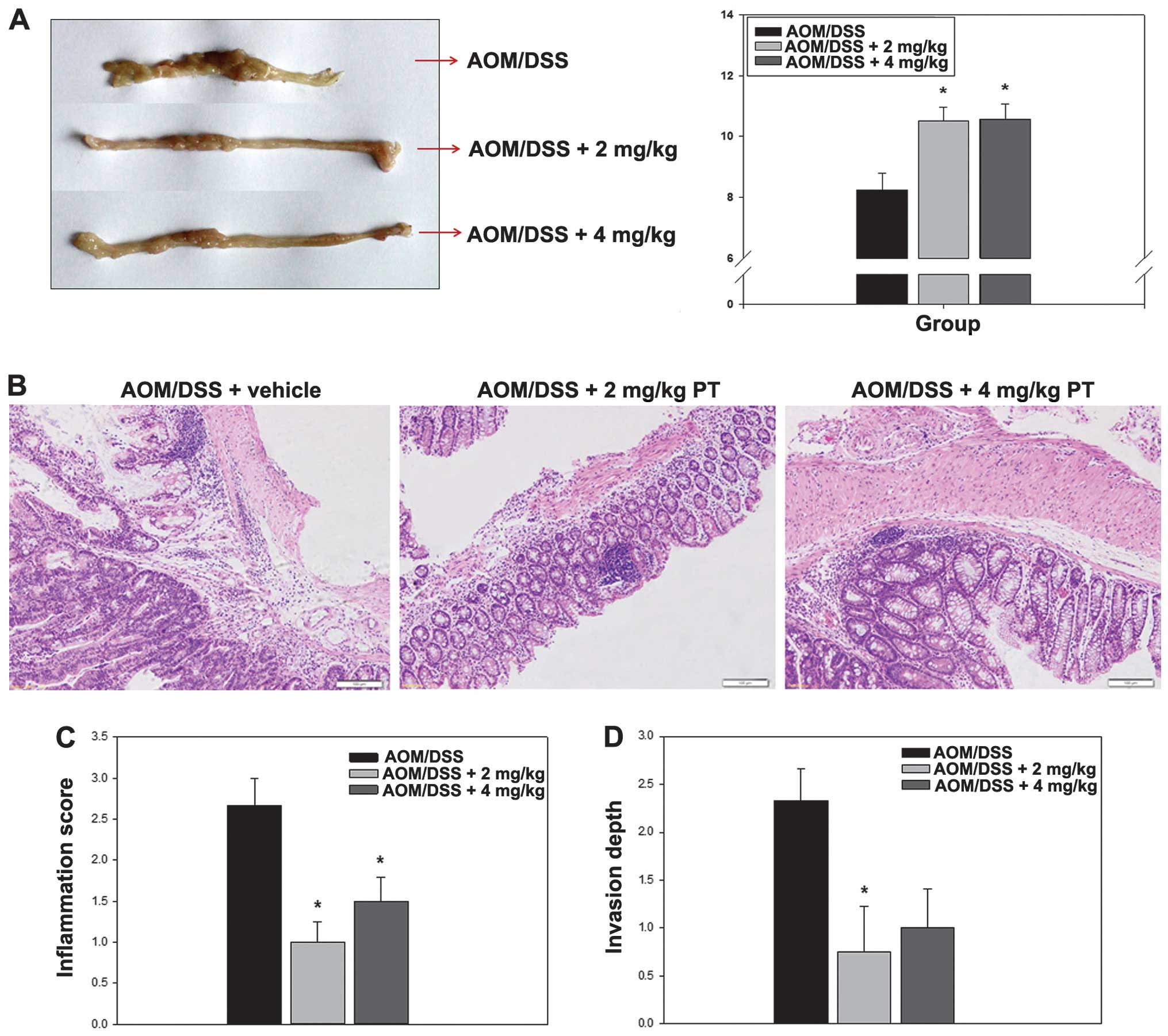
First, colon length was compared between the control (AOM/DSS plus
DMSO as vehicle) and PT-treated (AOM/DSS plus 2 mg/kg PT and
AOM/DSS plus 4 mg/kg PT) groups. Numerous nodular, polypoid and
caterpillar-like tumors were observed in the middle and distal
colon of mice in the control group. By contrast, shortening of the
colon, which is a characteristic of colon carcinogenesis, was
significantly improved in the PT-treated group (Fig. 1A). Histological analysis revealed that
the severity of inflammation and the invasion depth of the
ulcerated areas in the colons of the PT-treated mice were
significantly lower compared with that in the non-PT-treated mice
(P<0.05; Fig. 1B–D).
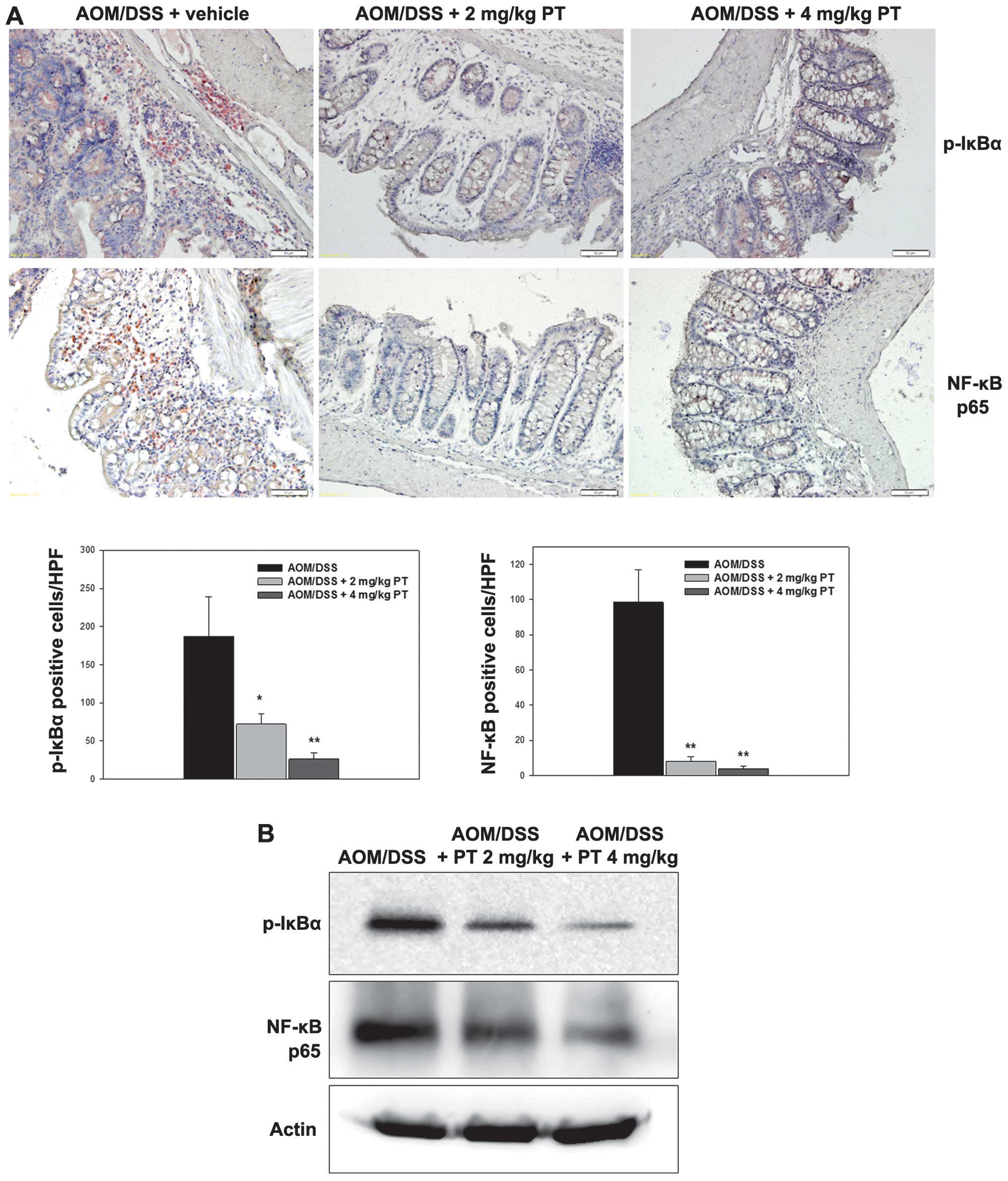
Administration of PT suppresses NF-κB
signaling by blocking IκBα phosphorylation
Degradation of IκB proteins via a phosphorylation
and ubiquitination-dependent pathway is an essential step for NF-κB
activation (27). To evaluate the
molecular basis of NF-κB inactivation by PT, the present study
examined the effects of PT on the phosphorylation and degradation
of IκBα protein. As indicated in Fig.
2A, AOM/DSS mice treated with PT displayed significantly
reduced phospho-IκBα positively-stained cells compared with the
AOM/DSS control mice treated with vehicle. Furthermore, the protein
expression level of phospho-IκBα was increased in the
AOM/DSS-treated mice, but was markedly inhibited in the PT-treated
mice (Fig. 2B).
Anti-NF-κB p65 (RelA) is one of the subunits of
NF-κB (27). Numerous anti-NF-κB p65
positively-stained cells were detected in the mice with
AOM/DSS-induced CAC, whereas treatment with PT resulted in a
significant reduction in the number of NF-κB p65 positively-stained
cells (Fig. 2A; P<0.05).
Furthermore, the protein expression level of NF-κB p65 was markedly
inhibited in the PT-treated mice and was highly correlated with the
IHC results (Fig. 2B).
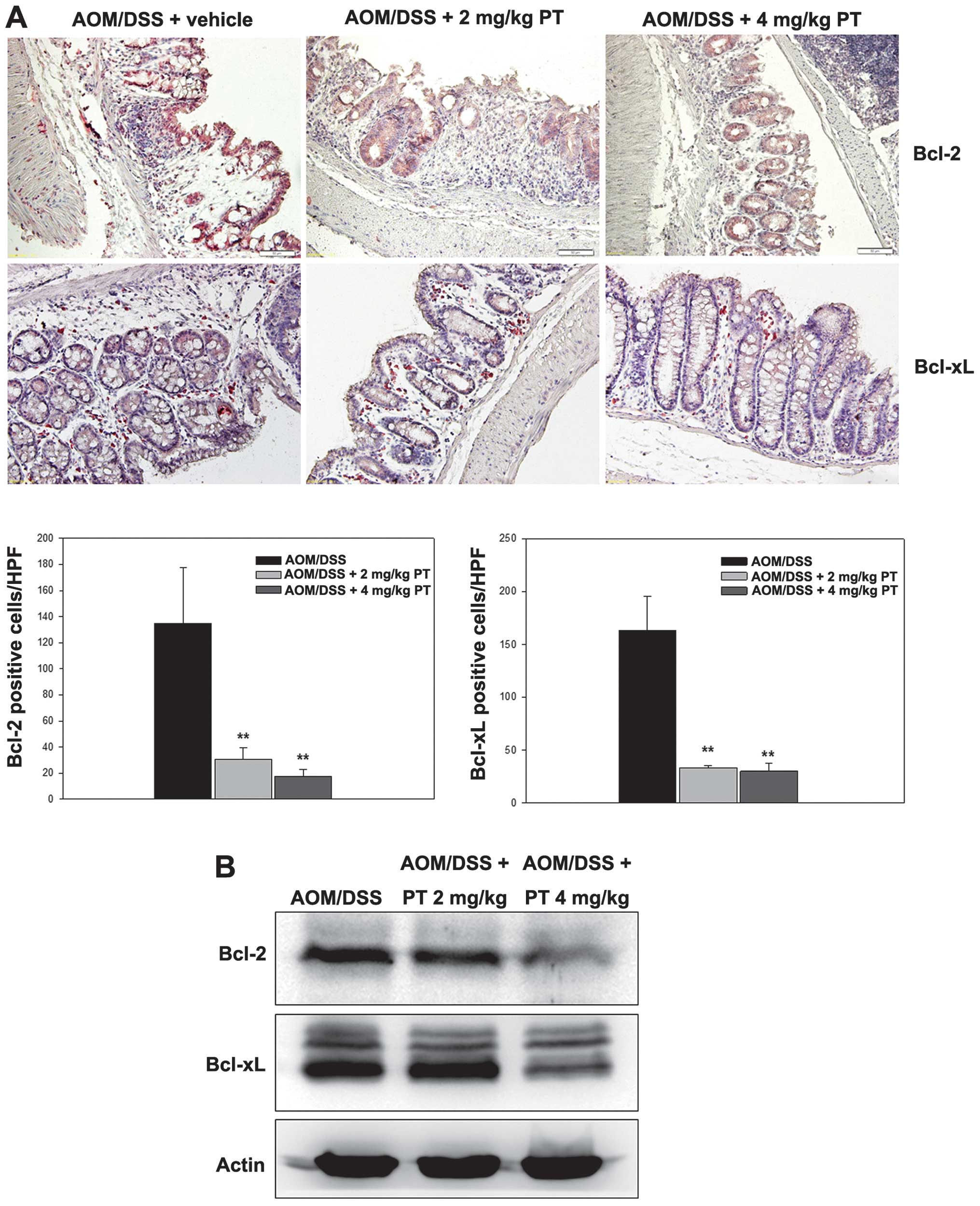
Administration of PT downregulates
anti-apoptotic proteins Bcl-2 and Bcl-xL
The regulation of Bcl-2 and Bcl-xL expression by PT
administration was examined in the CAC mice. As indicated in
Fig. 3A, cells positively
immunostained for Bcl-2 were detected in the AOM/DSS-induced CAC
group; however, the number of Bcl-2 positively-immunostained cells
was significantly reduced by PT treatment (Fig. 3A; P<0.05). Furthermore, the data
correlated well with the western blotting results (Fig. 3B). Similarly, IHC with the Bcl-xL
antibody resulted in markedly fewer positively-stained cells in the
PT-treated group compared with the vehicle-treated group (Fig. 3A), and Bcl-xL protein expression
levels were significantly downregulated following PT treatment
(Fig. 3A; P<0.05).
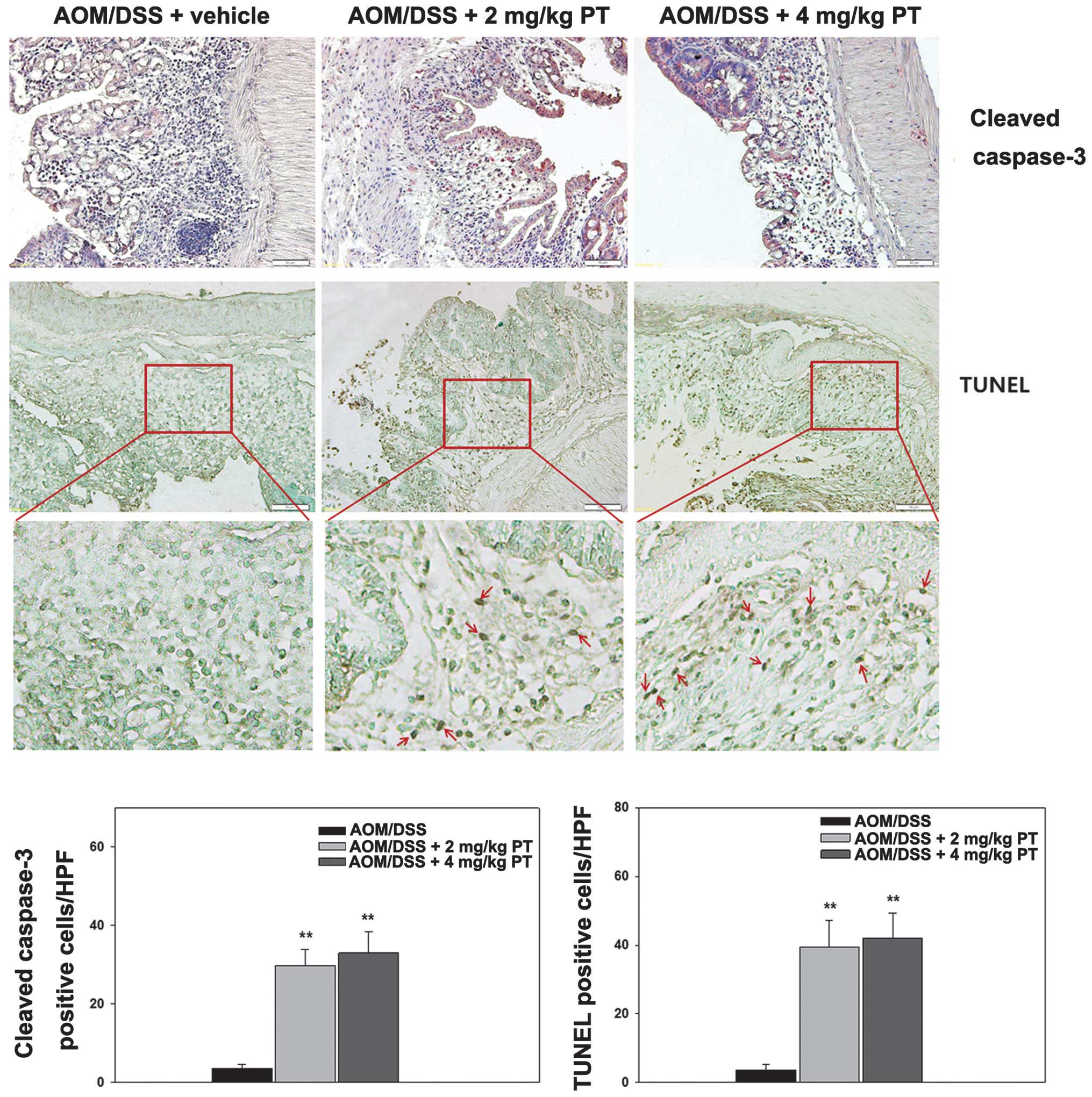
Administration of PT increases
apoptosis in dysplasia lesions in a murine CAC model
To perform additional evaluation of the effects of
PT on apoptosis, caspase 3 expression was examined by IHC, and
apoptotic cells were detected in the colonic epithelium using a
TUNEL assay. The results revealed that the number of cells
positively stained with caspase 3 and TUNEL (Fig. 4) was significantly increased in the
two PT-treated groups compared with the vehicle-treated group,
indicating that PT promotes apoptosis in the colons of CAC
mice.
Discussion
Our previous study demonstrated that PT induces
apoptosis in a number of human CRC cell lines, including the HT-29,
LS174T and SW480 cell lines, and that it reduces tumor growth and
angiogenesis in a CRC xenograft model (28). PT is considered to be a promising
candidate as a novel type of chemotherapeutic agent for cancer
treatment; however, to the best of our knowledge, no study has yet
explained the mechanism of the anticancer effect of PT on CAC.
Therefore, the present study selected an AOM/DSS mouse model to
assess the role of PT in CAC and in NF-κB inactivation. In
agreement with our hypothesis, the results indicated that PT
administration clinically and histologically improves
AOM/DSS-induced CAC in mice, as assessed by histological injury
scores. Furthermore, the beneficial effect of PT treatment appears
to inhibit NF-κB activation via blocking IκBα phosphorylation and
is associated with the downregulation of the apoptosis-associated
molecules, Bcl-2, Bcl-xL and caspase 3. These findings indicate
that PT may be a useful therapeutic approach to treat CAC and that
PT may block IκBα phosphorylation, thus silencing NF-κB
activation.
The oncogenic potential of AOM is markedly augmented
in the setting of chronic inflammation, such as that induced by
repeated cycles of DSS treatment (29). A recent study demonstrated the power
of this model by deciphering the epithelial versus myeloid cell
contribution of IκB kinase β to polyp formation in the setting of
inflammation (30), while a different
study investigated the contributions of IL-6 and its downstream
mediator, signal transducer and activator of transcription-3
(STAT-3) (31). PT is an effective
inhibitor of IL-6-type cytokines, which activate the
phosphorylation of STAT3 on Tyr 705. PT has been demonstrated to
prevent the activity of IL-6-type cytokines by inhibiting the
phosphorylation of STAT3 on Tyr 705 (32,33),
indicating that PT, as an inhibitor of IL-6, is able to provide
protection from carcinogenesis in experimental CAC models. Although
the present study did not demonstrate the role of PT in regulating
and suppressing carcinogenesis via the IL-6/STAT3 pathway,
additional important insights into the mechanism were obtained.
Previous studies have demonstrated that NF-κB, a
central molecule involved in inflammation, regulates the expression
of a diverse array of target genes that are involved in promoting
cell proliferation, regulating immune and inflammatory responses
and contributing towards the pathogenesis of various diseases,
including cancer (34–36). In chronic inflammation, NF-κB has a
specific role in coupling inflammation to cancer. In total, >15%
of all malignancies are initiated by chronic inflammatory disease;
for example, skin inflammation initiates squamous cell carcinoma,
viral hepatitis initiates liver cancer and inflammatory bowel
disease (IBD) initiates CRC (31,37–41).
Previous studies have indicated that constitutive NF-κB activation
in IBDs may be the cause of the increased risk of developing CRC
(42–44). It has been shown that PT is a potent
inhibitor of NF-κB activation, which is able to effectively inhibit
pro-inflammatory cytokine expression in cultured cells and
experimental models (11,15–19,45).
Furthermore, a previous study using experimental murine colitis
demonstrated that the administration of PT significantly reduces
the severity of DSS-induced colitis, as assessed by NF-κB p65 and
the blockage of IκBα protein phosphorylation, and resulting in a
reduction in the expression of inflammatory mediators, including
myeloperoxidase activity, TNF-α and IL-1β (46). The present study has demonstrated that
PT suppresses IκBα phosphorylation and NF-κB p65 in a CAC mouse
model, providing experimental evidence of the potential application
of PT in CAC patients.
The association between NF-κB and apoptosis was
recently elucidated in studies demonstrating that the inhibition of
NF-κB activation, by the IκB super repressor (47,48) or in
Rel A (p65) knock-out cells (49),
results in increased apoptosis. In particular, NF-κB appears to be
responsible for the activation of genes involved in proliferation
and tumor survival, such as those of the apoptotic proteins Bcl-2
and Bcl-xL (50,51). The results of the present study
indicated that PT is essential for AOM/DSS-induced colon cancer
carcinogenesis via the apoptotic route-associated Bcl-2 family
members, which cause inhibition of NF-κB activation. Additionally,
the PT-treated cells appeared to undergo greater levels of
apoptosis compared with the vehicle-treated group, as evaluated by
caspase 3 antibody staining and TUNEL assay. Thus, the present
study indicates that NF-κB is the key regulator of PT-mediated
apoptosis via the apoptosis signaling cascade.
In conclusion, the present study demonstrated that
the administration of PT appears to significantly inhibit the
inflammation-carcinoma sequence and may be a crucial regulator of
experimental CAC. Possibly via the negative regulation of NF-κB, PT
reduces IκBα phosphorylation, and Bcl-2 and Bcl-xL expression,
increases the expression of caspase 3, and promotes cell apoptosis,
resulting in the suppression of tumorigenesis. Therefore, PT may be
a novel chemopreventive agent for the treatment of CAC.
Acknowledgements
The present study was supported by the 18th grant
from the Kye-Nam, Kim Jae Jung Memorial Fund. The authors thank
Professor Mie-Jae Im for proofreading the original manuscript and
for all contributions.
References
|
1
|
Podolsky DK: Inflammatory bowel disease. N
Engl J Med. 347:417–429. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eaden JA, Abrams KR and Mayberry JF: The
risk of colorectal cancer in ulcerative colitis: a meta-analysis.
Gut. 48:526–535. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papadakis KA and Targan SR: Role of
cytokines in the pathogenesis of inflammatory bowel disease. Annu
Rev Med. 51:289–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rogler G and Andus T: Cytokines in
inflammatory bowel disease. World J Surg. 22:382–389. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neurath MF, Fuss I, Schürmann G, et al:
Cytokine gene transcription by NF-kappa B family members in
patients with inflammatory bowel disease. Ann NY Acad Sci.
859:149–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blackwell TS and Christman JW: The role of
nuclear factor-kappa B in cytokine gene regulation. Am J Respir
Cell Mol Biol. 17:3–9. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knight DW: Feverfew: chemistry and
biological activity. Nat Prod Rep. 12:271–276. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphy JJ, Heptinstall S and Mitchell JR:
Randomised double-blind placebo-controlled trial of feverfew in
migraine prevention. Lancet. 2:189–192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hehner SP, Heinrich M, Bork PM, et al:
Sesquiterpene lactones specifically inhibit activation of NF-kappa
B by preventing the degradation of I kappa B-alpha and I kappa
B-beta. J Biol Chem. 273:1288–1297. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lyss G, Knorre A, Schmidt TJ, Pahl HL and
Merfort I: The anti-inflammatory sesquiterpene lactone helenalin
inhibits the transcription factor NF-kappaB by directly targeting
p65. J Biol Chem. 273:33508–33516. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Ong CN and Shen HM: Critical
roles of intracellular thiols and calcium in parthenolide-induced
apoptosis in human colorectal cancer cells. Cancer Lett.
208:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen J, You KR, Lee SY, Song CH and Kim DG:
Oxidative stress-mediated apoptosis = The anticancer effect of the
sesquiterpene lactone parthenolide. J Biol Chem. 277:38954–38964.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sweeney CJ, Mehrotra S, Sadaria MR, et al:
The sesquiterpene lactone parthenolide in combination with
docetaxel reduces metastasis and improves survival in a xenograft
model of breast cancer. Mol Cancer Ther. 4:1004–1012. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saadane A, Masters S, Di Donato J, Li J
and Berger M: Parthenolide inhibits IkappaB kinase, NF-kappaB
activation, and inflammatory response in cystic fibrosis cells and
mice. Am J Respir Cell Mol Biol. 36:728–736. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyata N, Gon Y, Nunomura S, et al:
Inhibitory effects of parthenolide on antigen-induced microtubule
formation and degranulation in mast cells. Int Immunopharmacol.
8:874–880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hehner SP, Hofmann TG, Dröge W and Schmitz
ML: The antiinflammatory sesquiterpene lactone parthenolide
inhibits NF-kappa B by targeting the I kappaB kinase complex. J
Immunol. 163:5617–5623. 1999.PubMed/NCBI
|
|
19
|
Kwok BH, Koh B, Ndubuisi MI, et al: The
anti-inflammatory natural product parthenolide from the medicinal
herb Feverfew directly binds to and inhibits IkappaB kinase. Chem
Biol. 8:759–766. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SL, Trang KT, Kim SH, et al:
Parthenolide suppresses tumor growth in a xenograft model of
colorectal cancer cells by inducing mitochondrial dysfunction and
apoptosis. Int J Oncol. 41:1547–1553. 2012.PubMed/NCBI
|
|
21
|
Wang Y, Zhang HX, Sun YP, et al: Rig-I-/-
mice develop colitis associated with downregulation of G alpha i2.
Cell Res. 17:858–868. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arthur JC, Perez-Chanona E, Mühlbauer M,
et al: Intestinal inflammation targets cancer-inducing activity of
the microbiota. Science. 338:120–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pegg AE: Methylation of the O6 position of
guanine in DNA is the most likely initiating event in
carcinogenesis by methylating agents. Cancer Invest. 2:223–231.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Popivanova BK, Kitamura K, Wu Y, et al:
Blocking TNF-alpha in mice reduces colorectal carcinogenesis
associated with chronic colitis. J Clin Invest. 118:560–570.
2008.PubMed/NCBI
|
|
25
|
Tanaka T, Suzuki R, Kohno H, et al:
Colonic adenocarcinomas rapidly induced by the combined treatment
with 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine and dextran
sodium sulfate in male ICR mice possess beta-catenin gene mutations
and increases immunoreactivity for beta-catenin, cyclooxygenase-2
and inducible nitric oxide synthase. Carcinogenesis. 26:229–238.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kohno H, Suzuki R, Sugie S and Tanaka T:
Beta-Catenin mutations in a mouse model of inflammation-related
colon carcinogenesis induced by 1,2-dimethylhydrazine and dextran
sodium sulfate. Cancer Sci. 96:69–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SL, Trang KT, Kim SH, et al:
Parthenolide suppresses tumor growth in a xenograft model of
colorectal cancer cells by inducing mitochondrial dysfunction and
apoptosis. Int J Oncol. 41:1547–1553. 2012.PubMed/NCBI
|
|
29
|
Okayasu I, Ohkusa T, Kajiura K, et al:
Promotion of colorectal neoplasia in experimental murine ulcerative
colitis. Gut. 39:87–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Greten FR, Eckmann L, Greten TF, et al:
IKKbeta links inflammation and tumorigenesis in a mouse model of
colitis-associated cancer. Cell. 118:285–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grivennikov S, Karin E, Terzic J, et al:
IL-6 and Stat3 are required for survival of intestinal epithelial
cells and development of colitis-associated cancer. Cancer Cell.
15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sobota R, Szwed M, Kasza A, et al:
Parthenolide inhibits activation of signal transducers and
activators of transcription (STATs) induced by cytokines of the
IL-6 family. Biochem Biophys Res Commun. 267:329–333. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song JM, Qian X, Upadhyayya P, et al:
Dimethylaminoparthenolide, a water soluble parthenolide, suppresses
lung tumorigenesis through down-regulating the STAT3 signaling
pathway. Curr Cancer Drug Targets. 14:59–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo JL, Kamata H and Karin M:
IKK/NF-kappaB signaling: balancing life and death - a new approach
to cancer therapy. J Clin Invest. 115:2625–2632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lawrence T, Willoughby DA and Gilroy DW:
Anti-inflammatory lipid mediators and insights into the resolution
of inflammation. Nat Rev Immunol. 2:787–795. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vakkila J and Lotze MT: Inflammation and
necrosis promote tumour growth. Nat Rev Immunol. 4:641–648. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schottenfeld D and Beebe-Dimmer J: Chronic
inflammation: a common and important factor in the pathogenesis of
neoplasia. CA Cancer J Clin. 56:69–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schottenfeld D and Beebe-Dimmer JL:
Advances in cancer epidemiology: understanding causal mechanisms
and the evidence for implementing interventions. Annu Rev Public
Health. 26:37–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Neurath MF, Pettersson S, Meyer zum
Büschenfelde KH and Strober W: Local administration of antisense
phosphorothioate oligonucleotides to the p65 subunit of NF-kappaB
abrogates established experimental colitis in mice. Nat Med.
2:998–1004. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rogler G, Brand K, Vogl D, Page S,
Hofmeister R, et al: Nuclear factor kappaB is activated in
macrophages and epithelial cells of inflamed intestinal mucosa.
Gastroenterology. 115:357–369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ardite E, Panés J, Miranda M, et al:
Effects of steroid treatment on activation of nuclear factor kappaB
in patients with inflammatory bowel disease. Br J Pharmacol.
124:431–433. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang BY, Chung SW and Kim TS: Inhibition
of interleukin-12 production in lipopolysaccharide-activated mouse
macrophages by parthenolide, a predominant sesquiterpene lactone in
Tanacetum parthenium:involvement of nuclear factor-kappaB. Immunol
Lett. 77:159–163. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao ZJ, Xiang JY, Liu L, Huang XL and Gan
HT: Parthenolide, an inhibitor of the nuclear factor-κB pathway,
ameliorates dextran sulfate sodium-induced colitis in mice. Int
Immunopharmacol. 12:169–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang CY, Mayo MW and Baldwin AS Jr: TNF-
and cancer therapy-induced apoptosis: potentiation by inhibition of
NF-kappaB. Science. 274:784–787. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Van Antwerp DJ, Martin SJ, Kafri T, Green
DR and Verma IM: Suppression of TNF-alpha-induced apoptosis by
NF-kappaB. Science. 274:787–789. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Beg AA and Baltimore D: An essential role
for NF-kappaB in preventing TNF-alpha-induced cell death. Science.
274:782–784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang YW, Wang SJ, Zhou YN, Pan SH and Sun
B: Escin augments the efficacy of gemcitabine through
down-regulation of nuclear factor-κB and nuclear
factor-κB-regulated gene products in pancreatic cancer both in
vitro and in vivo. J Cancer Res Clin Oncol. 138:785–797. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun JG, Chen CY, Luo KW, et al:
3,5-Dimethyl-H-furo[3,2-g]chromen-7-one as a potential anticancer
drug by inducing p53-dependent apoptosis in human hepatoma HepG2
cells. Chemotherapy. 57:162–172. 2011. View Article : Google Scholar : PubMed/NCBI
|