Introduction
Non-small cell lung cancer (NSCLC) is the most
common type of lung cancer, accounting for more than 80% of cases.
As a class, NSCLC is relatively insensitive to chemotherapy
compared with small cell carcinoma. A number of drugs are useful
for NSCLC as systemic therapy, including platinum agents (cisplatin
and carboplatin), taxanes (paclitaxel and docetaxel), vinorelbine,
vinblastine, etoposide, pemetrexed and gemcitabine. However,
despite advances in chemotherapeutic drugs, the prognosis of lung
cancer remains poor, with an overall 5-year survival rate of less
than 14% in the USA and even lower (5–10%) in Europe and other
countries (1).
A number of investigators have attempted to develop
specific targeting molecules, including antibodies and antagonists,
for the treatment of NSCLC. Bevacizumab is a recombinant monoclonal
antibody that blocks vascular endothelial growth factor. Erlotinib
is a small molecule inhibitor of epidermal growth factor receptor
(EGFR), and crizotinib is a small molecule inhibitor that targets
anaplastic lymphoma kinase and hepatocyte growth factor receptor
c-MET. Cetuximab is a monoclonal antibody that targets EGFR.
The pathway of interleukin 6/interleukin 6 receptor
(IL-6/IL-6R) signaling regulates diverse biological activities,
including cell growth and differentiation, in immune and
hematopoietic systems (2). However,
IL-6/IL-6R signaling is also known to promote tumor growth and
survival in several organ systems (3). The plasma IL-6 levels of cancer patients
were observed to correlate with the clinical and pathological
variety and survival in prostate cancer (4), ovarian cancer (5), breast cancer (6) and colorectal cancer (7). Tocilizumab is a humanized recombinant
IgG1k monoclonal antibody against IL-6R (8). It is approved by the FDA and used in
clinics to treat rheumatoid arthritis and juvenile idiopathic
arthritis patients (9). It has also
been demonstrated that tocilizumab has an anti-proliferative effect
on glioma cells via inhibition of the JAK-STAT3 pathway (10). Furthermore, there is evidence that
IL-6/IL-6 signaling affects NSCLC tumorigenesis (11).
In a previous study (12), we demonstrated the possibility of
using IL-6R as a selective anticancer drug target using an H460
lung cancer stem cell model. Here, we investigate the effect of
IL-6R blockage on the proliferation of NSCLC cells using the
IL-6R-targeted antibody tocilizumab.
Materials and methods
Cell culture
NSCLC cell lines H460, A549, H1299 and H358 were
obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). The
cells were grown in RPMI-1640 medium (Gibco Life Technologies,
Carlsbad, CA, USA) with 10% heat-inactivated fetal bovine serum
(Gibco) and 1% penicillin/streptomycin (Gibco) at 37°C in a
humidified incubator (5% CO2), as recommended by
KLCB.
Cell proliferation assay
The anticancer drugs methotrexate (MTX) and
5-fluorouracil (5-FU) were purchased from Sigma-Aldrich (St. Louis,
MO, USA), and tocilizumab was kindly donated by Dr Misahiko Mihara
at Chugai Pharmaceutical Co. Ltd. (Shizuoka, Japan). The
anti-proliferation activity of tocilizumab, MTX and 5-FU in NSCLC
cells in vitro was measured using the EZ-Cytox kit
(Daeillab, Seoul, Korea). Ten microliters of tocilizumab, MTX or
5-FU were added to 96-well plates containing 104 cells
per well in 100 µl medium. The final concentrations of tocilizumab
were 10, 100 and 1000 ng/ml. The final concentrations of MTX and
5-FU were 50 and 25 µg/ml, respectively. Following a 24-h
incubation, WST-1 solution (Daeillab) was added, and the optical
density was analyzed using the ELISA plate reader Magellan™ (Tecan,
Männedorf, Switzerland) at reference wavelengths of 450 and 620
nm.
Cell cycle analysis
The NSCLC cells were seeded at 2.0×105
cells/well in 6-well plates. The cells were allowed to recover for
24 h and then treated with tocilizumab. To analyze the cell cycle
distribution, the cells were collected after a 24-h incubation and
washed with phosphate-buffered saline (PBS). The cells were fixed
in 70% ethanol and stored overnight at 4°C. For the analysis, the
cells were transferred to PBS and incubated with ribonuclease A (50
µg/ml) at room temperature for 5 min. The cells were then stained
with 10 µg/ml propidium iodide (PI) and incubated at 37°C for 10
min. Finally, the cells were analyzed using fluorescence-activated
cell sorting.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
qPCR was performed to identify the gene expression
level of IL-6R in the NSCLC cells based on the expression of a
housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), as an internal control. RNA was quantified by its
absorption at 260 nm and stored at −80°C before use. Briefly,
first-strand cDNA was synthesized from 2 µg total RNA with
Superscript III transcriptase (Invitrogen Life Technologies,
Carlsbad, CA, USA). PCR amplification was performed with specific
primer pairs designed from published human gene sequences (13). qPCR was performed using SYBR-Green
(Takara Bio Inc., Shiga, Japan) and a Bio-Rad machine (Bio-Rad
Laboratories Inc., Hercules, CA, USA). DNA was amplified using 60
cycles of denaturation for 5 sec at 95°C and annealing for 40 sec
at 60°C.
Protein extraction and western blot
analysis
Whole-cell lysates were extracted using the Pro-Prep
protein extraction solution plus protease inhibitor cocktail
(Intron Biotechnology, Seongnam, Korea) according to the method
described in the manufacturer's guidelines. Cell lysates were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE), transferred to a nitrocellulose
membrane (Bio-Rad), and immunoblotted with antibodies against the
following: signal transducer and activator of transcription 3
(STAT3), phospho-STAT3, extracellular-signal-regulated kinases
(ERK), phospho-ERK, nuclear factor κB (NFκB) and phospho-NFκB (Cell
Signaling Technology, Inc., Beverly, MA, USA). After incubating
with the secondary antibody, the membranes were developed using
enhanced chemiluminescence. ImageJ software (NIH, USA) was used to
analyze the results.
Statistical analysis
The results are expressed as the means ± standard
deviation. Analysis of variance was used to compare differences
among the groups. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed with Statistical Analysis Systems software (SPSS version
20; IBM SPSS, Armonk, NY, USA).
Results
Cell proliferation
H460, A549, H1299 and H358 cells were treated in
triplicate with tocilizumab at concentrations of 10, 100 and 1000
ng/ml. The inhibition of cell growth was examined by a commercial
kit and an ELISA reading system after 24 h of treatment and was
calculated as the percentage of viable cells relative to untreated
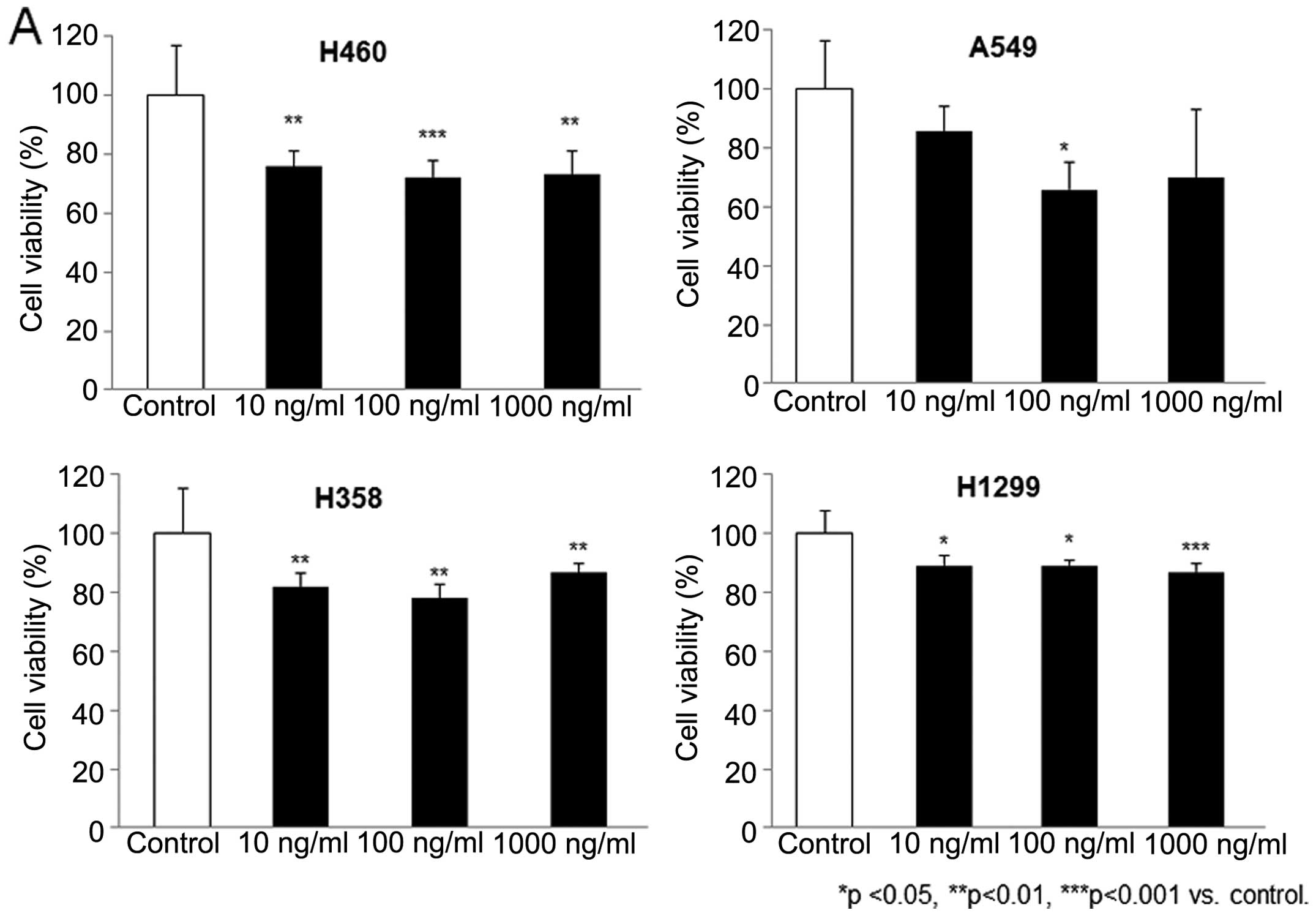
cell cultures. As shown in Fig. 1A,
tocilizumab demonstrated substantial growth inhibition in the NSCLC
cells. Following exposure to tocilizumab at a 100 ng/ml
concentration, cell growth was significantly decreased by
27.75±5.81, 34.23±9.49, 22.14±4.87 and 10.81±1.94% in the H460,
A549, H1299 and H358 cells, respectively. In addition, the
anti-proliferative effect of tocilizumab (100 ng/ml) was compared
with that of the conventionally used anticancer drugs MTX and 5-FU
in the NSCLC cells. The concentrations of MTX (50 mg/ml) and 5-FU
(25 mg/ml) were based on those used in our previous study (12). MTX is a novel drug that acts as an
inhibitor of folate metabolism, and 5-FU is an irreversible
inhibitor of thymidylate synthase. These drugs have been used in
the treatment of NSCLC patients for some time. As shown in Fig. 1B, the cell growth inhibition rate of
tocilizumab in the NSCLC cells was similar or only slightly lower
than that of MTX and 5-FU.
Cell cycle distribution
A flow cytometric cell cycle analysis was performed
to determine whether the results of the cell assay reflected
cytostatic or cytotoxic effects due to cell cycle arrest or
apoptosis. NSCLC cells were treated with 100 ng/ml tocilizumab.
After 24 h of drug treatment, the cells were fixed and suspended in
PI and measured in comparison with untreated cells. Morphological
changes in the apoptotic cells, including shrinkage, rounding and
membrane blebbing, were also observed following the tocilizumab
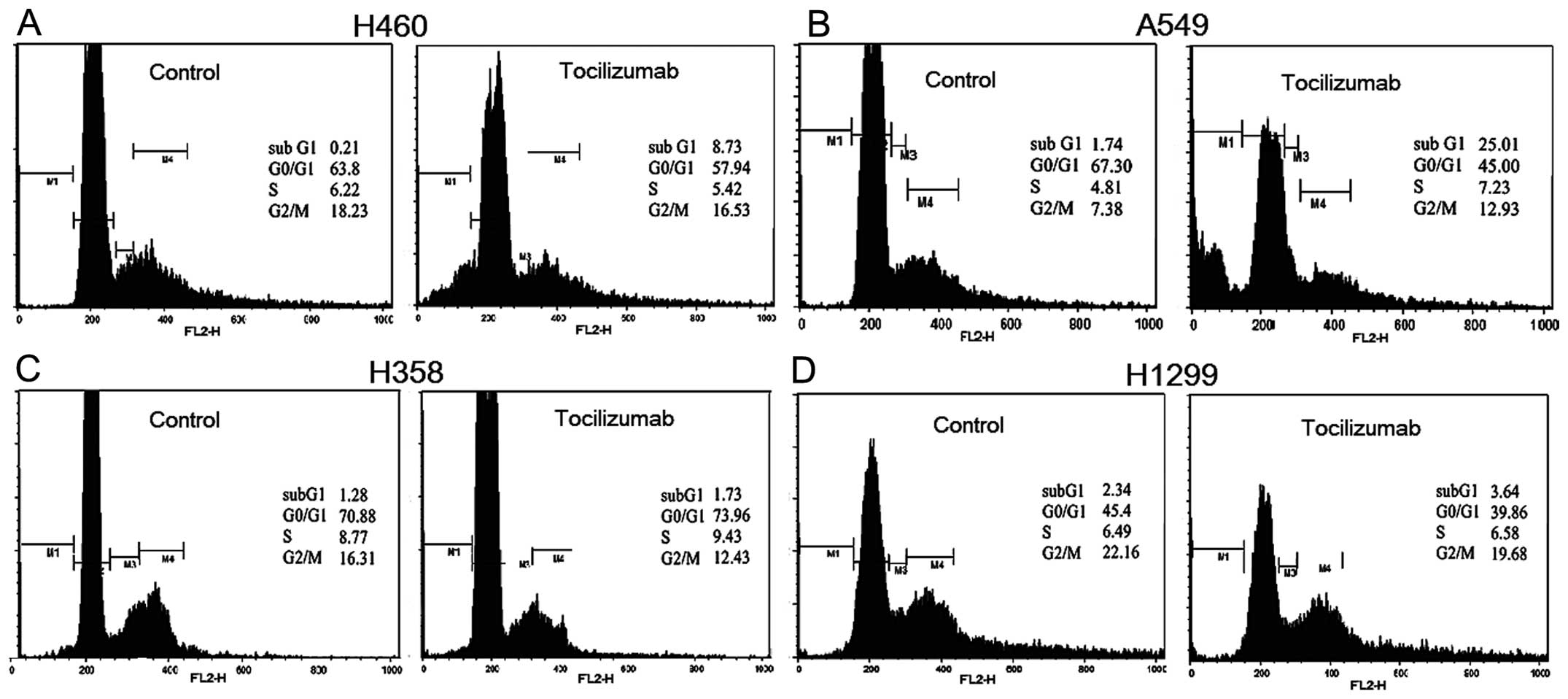
treatment. As shown in Fig. 2, a
significant accumulation of the cell population in the sub-G1 phase
was observed in the H460 and A549 cells following drug treatment.
Similar effects on the cell cycle were observed with tocilizumab
concentrations of 10 and 1000 ng/ml. Following tocilizumab
treatment, 8.73% of the H460 cell population was arrested in the
sub-G1 phase in contrast to 0.21% of the untreated control cells
(Fig. 2A). In addition, 25.01% of the
A549 cell population was in the sub-G1 phase following drug
treatment, compared with only 1.74% of the untreated control cells
(Fig. 2B). Although there was no
significant difference in the H358 and H1299 cells between the
treated and untreated groups, minor increases were observed
following tocilizumab treatment: 1.73% of H358 cells were in the
sub-G1 phase compared with 1.28% of the untreated control cells
(Fig. 2C), and 3.64% of H1299 cells
were in the sub-G1 phase compared with 2.34% of the untreated
control cells (Fig. 2D).
Gene expression and
immunoblotting
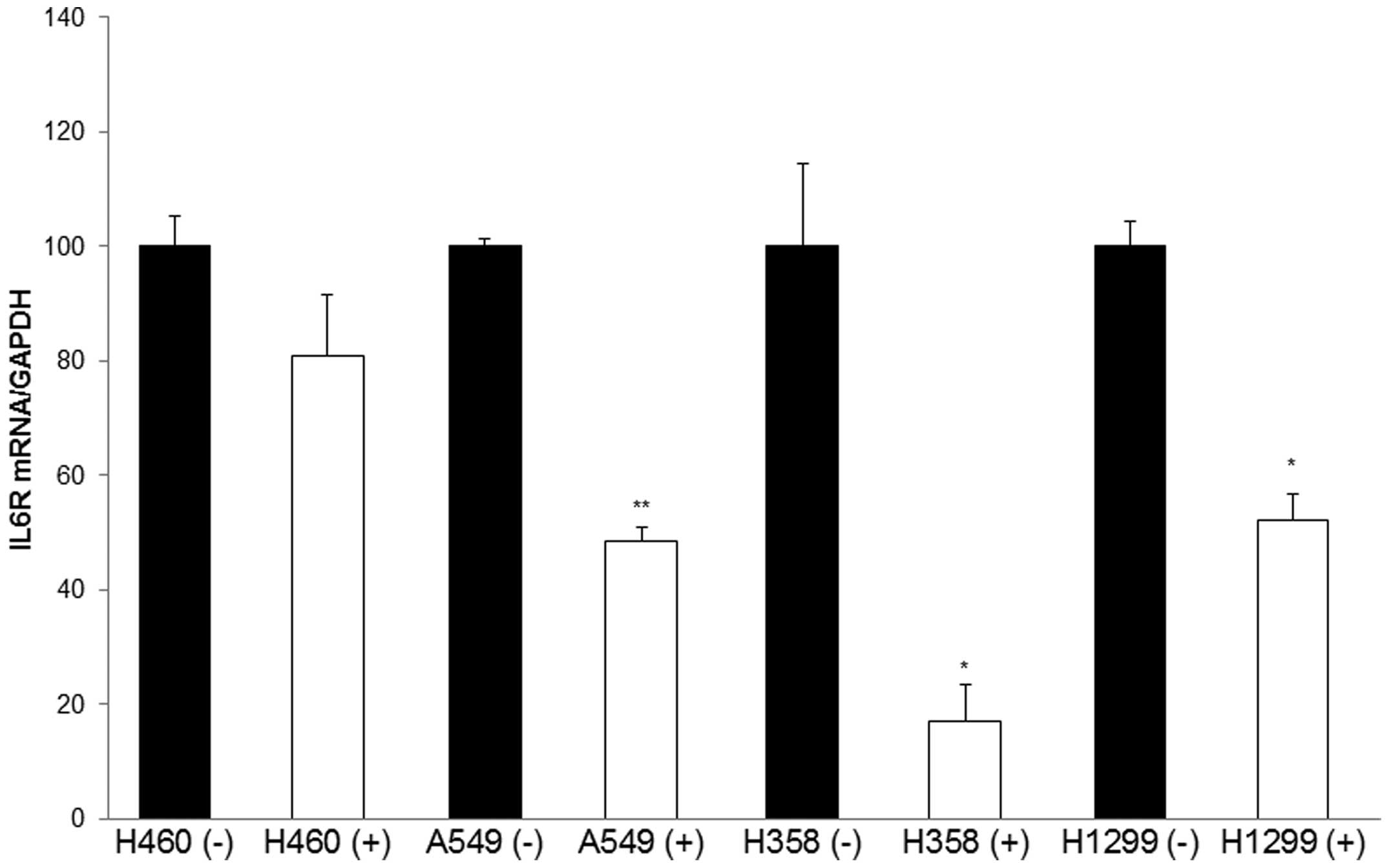
The mRNA expression of IL-6R was analyzed in H460,
A549, H1299 and H358 cells using qPCR. The transcript levels were
normalized to the expression of GAPDH. The data in Fig. 3 reveal marked decreases in IL-6R
expression with tocilizumab at a concentration of 100 ng/ml.
Tocilizumab significantly reduced the mRNA levels of IL-6R by 50%
in A549, 77% in H358, 48% in H1299, and 20% in H460 cells. The
principal transcriptional factors in the IL-6R/IL-6 signaling
pathway regulating cell differentiation and growth are ERK1/2,
STAT3 and NFκB. Thus, we estimated the influence of tocilizumab on
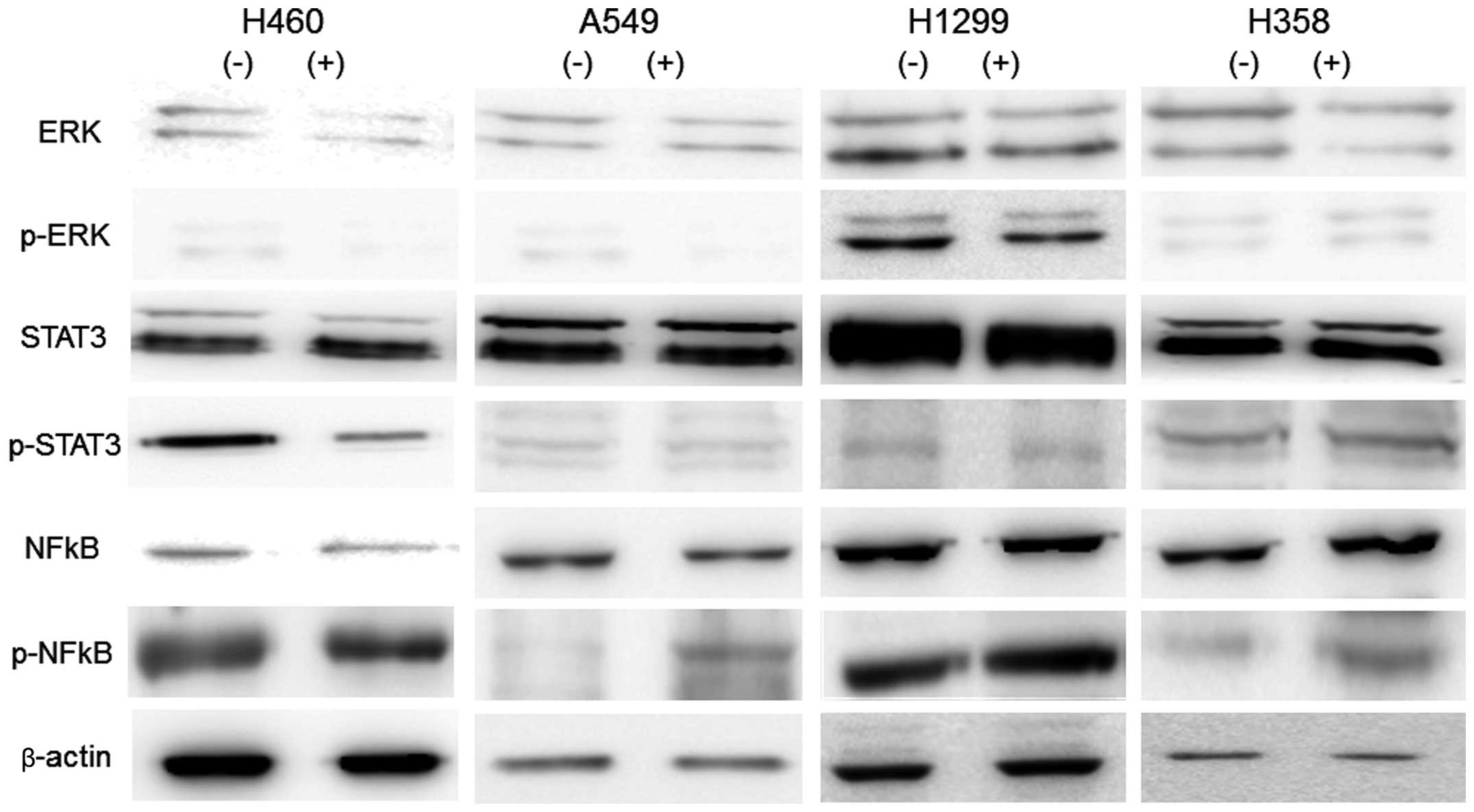
the levels of activation of these factors in NSCLC cells. The cells
were treated with 100 ng/ml tocilizumab, and protein lysates were
obtained after 24 h of drug exposure. The levels of the
transcription factors and their phosphorylated forms in whole-cell
lysates were analyzed by western blotting using commercially
available antibodies. As shown in Fig.
4, tocilizumab did not alter the levels of the proteins ERK1/2,
STAT3 and NFκB and phosphorylated ERK1/2 and STAT3. However,
phosphorylated NFκB was considerably increased by tocilizumab
treatment in the NSCLC cells.
Discussion
IL-6 plays a significant role in the neoplastic
process through its action on cancer cell adhesion, motility,
proliferation, tumor-specific antigen expression and thrombopoiesis
(14). IL-6, with its known functions
in immune response, acute phase reaction and hematopoiesis, was
considered a therapeutic target approximately two decades ago. The
aim of this study was to assess whether an anti-IL-6R antibody
could be utilized as a new targeting molecule for NSCLC therapy.
Another anti-IL-6 antibody, siltuximab, has been demonstrated to
have potential benefits in the treatment of various human cancers,
including multiple myeloma, breast cancer and prostate cancer,
either as a single agent or in combination with other chemotherapy
drugs (14–17). It has also been indicated that
anti-IL-6R antibodies may be a potential agent for the suppression
of colon cancer progression (18,19). These
earlier investigations have revealed IL-6R as a potent target for
antibody treatment in anticancer therapy. To our knowledge, there
has been no study describing the utilization of an anti-IL-6/IL-6R
antibody in NSCLC cancer therapy.
Tocilizumab is a fully humanized monoclonal antibody
against IL-6R that was approved for the treatment of patients with
rheumatoid arthritis (20). In Japan,
tocilizumab has also been approved for the treatment of
polyarticular-course juvenile idiopathic arthritis, systemic-onset
juvenile idiopathic arthritis and Castleman's disease (21). However, this drug was initially
investigated in the field of oncology. Mouse monoclonal antibodies
against human IL-6 were noted to be effective in a patient with
plasma cell leukemia (22). The mouse
monoclonal antibody bound to the human IL-6R, inhibiting IL-6
function, and demonstrated strong antitumor cell activity against
multiple myeloma cells (23). In our
study, tocilizumab exhibited a significant growth inhibition in
NSCLC cells (H460, A549, H1299 and H358), with proliferation
significantly decreased by approximately 40% in A549 cells. The
growth inhibition rates of tocilizumab in NSCLC cells were
comparable with those of MTX and 5-FU, classically used anticancer
drugs. This finding indicates that tocilizumab has a potent
antitumor activity against NSCLC. Kudo et al (10) described the antitumor effect of
tocilizumab in U87MG glioma cells and the critical role of the IL-6
signaling pathway in glioma cell proliferation. We further examined
whether the result of our cell assay was a reflection of cytostatic
or cytotoxic effects due to cell cycle arrest. Compared with the
untreated control, tocilizumab treatment resulted in an approximate
40-fold increase in sub-G1 phase arrest in H460 cells and an
approximately 14-fold increase in A549 cells. H358 and H1299 cells
also exhibited approximately 1.3- and 1.5-fold increases,
respectively, in the cell population in the sub-G1 phase. The
statistical accumulation of the cell population in the sub-G1 phase
demonstrates that significant apoptosis occurred in NSCLC cells
following tocilizumab treatment.
The mRNA expression of IL-6R was analyzed in NSCLC
cells. Tocilizumab significantly reduced the mRNA levels of IL-6R
by 20–80%. Although we have no direct data for the downregulation
of IL-6R at the protein level, the above results may indicate that
tocilizumab has a regulatory function. It is known that ERK1/2,
STAT3 and NFκB are involved in the signaling pathway of IL-6R/IL-6
as vital transcriptional factors in tumor proliferation (24). In our study, tocilizumab did not alter
the levels of the ERK1/2, STAT3, NFκB and phosphorylated ERK1/2 and
STAT3 proteins, but this antibody did considerably increase the
expression of phosphorylated NFκB in NSCLC cells. This result
indicates that the phosphorylation of NFκB may be an significant
factor in the anti-proliferative activity of tocilizumab. NFκB is a
key transcriptional regulator of genes involved in inflammatory
responses as well as genes regulating cell proliferation and
metastasis in carcinogenesis (25).
NFκB is considered to function either as an inhibitor or an
activator of apoptotic cell death. The nuclear accumulation and
transcriptional activity of NFκB are increased in T-cell lymphoma
cells and are responsible for their increased proliferation
(26). However, evidence has also
revealed a pro-apoptotic role for NFκB. Martin (27) emphasized the balance of NFκB
activation with regard to pro-apoptotic and anti-apoptotic effects
at the level of target gene activation. Although a number of
phosphorylation sites on NFκB proteins have been characterized, it
remains unclear how phosphorylation regulates the activities of
related proteins and controls target gene expression (28).
Our study revealed the anti-proliferation potency of
tocilizumab on NSCLC cells via apoptosis induction and IL-6R
signaling alteration. Therefore, we suggest that the IL-6R antibody
may be utilized as a new targeting molecule in NSCLC cancer
therapies.
Acknowledgements
This paper was supported by Konkuk University in
2013.
References
|
1
|
Lam WK and Watkins DN: Lung cancer: future
directions. Respirology. 12:471–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995.PubMed/NCBI
|
|
3
|
Hodge DR, Hurt EM and Farrar WL: The role
of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer.
41:2502–2512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michalaki V, Syrigos K, Charles P and
Waxman J: Serum levels of IL-6 and TNF-alpha correlate with
clinicopathological features and patient survival in patients with
prostate cancer. Br J Cancer. 90:2312–2316. 2004.PubMed/NCBI
|
|
5
|
Plante M, Rubin SC, Wong GY, Federici MG,
Finstad CL and Gastl GA: Interleukin-6 level in serum and ascites
as a prognostic factor in patients with epithelial ovarian cancer.
Cancer. 73:1882–1888. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang GJ and Adachi I: Serum interleukin-6
levels correlate to tumor progression and prognosis in metastatic
breast carcinoma. Anticancer Res. 19:1427–1432. 1999.PubMed/NCBI
|
|
7
|
Chung YC and Chang YF: Serum interleukin-6
levels reflect the disease status of colorectal cancer. J Surg
Oncol. 83:222–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohsugi Y and Kishimoto T: The recombinant
humanized anti-IL-6 receptor antibody tocilizumab, an innovative
drug for the treatment of rheumatoid arthritis. Expert Opin Biol
Ther. 8:669–681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bongartz T: Tocilizumab for rheumatoid and
juvenile idiopathic arthritis. Lancet. 371:961–963. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kudo M, Jono H, Shinriki S, Yano S,
Nakamura H, Makino K, Hide T, Muta D, Ueda M, Ota K, et al:
Antitumor effect of humanized anti-interleukin-6 receptor antibody
(tocilizumab) on glioma cell proliferation. Laboratory
investigation. J Neurosurg. 111:219–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haura EB, Livingston S and Coppola D:
Autocrine interleukin-6/interleukin-6 receptor stimulation in
non-small-cell lung cancer. Clin Lung Cancer. 7:273–275. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi H, Cho HJ, Cho SM, Jo K, Park JA, Kim
NH, Amidon GL, Kim JS and Shin HC: Blockade of interleukin-6
receptor suppresses the proliferation of H460 lung cancer stem
cells. Int J Oncol. 41:310–316. 2012.PubMed/NCBI
|
|
13
|
Koyama Y, Mitsui N, Suzuki N, Yanagisawa
M, Sanuki R, Isokawa K, Shimizu N and Maeno M: Effect of
compressive force on the expression of inflammatory cytokines and
their receptors in osteoblastic Saos-2 cells. Arch Oral Biol.
53:488–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang XP, Yang DC, Elliott RL and Head JF:
Down-regulation of expression of interleukin-6 and its receptor
results in growth inhibition of MCF-7 breast cancer cells.
Anticancer Res. 31:2899–2906. 2011.PubMed/NCBI
|
|
15
|
Yao X, Huang J, Zhong H, Shen N, Faggioni
R, Fung M and Yao Y: Targeting interleukin-6 in inflammatory
autoimmune diseases and cancers. Pharmacol Ther. 141:125–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fizazi K, De Bono JS, Flechon A,
Heidenreich A, Voog E, Davis NB, Qi M, Bandekar R, Vermeulen JT,
Cornfeld M and Hudes GR: Randomised phase II study of siltuximab
(CNTO 328), an anti-IL-6 monoclonal antibody, in combination with
mitoxantrone/prednisone versus mitoxantrone/prednisone alone in
metastatic castration-resistant prostate cancer. Eur J Cancer.
48:85–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hunsucker SA, Magarotto V, Kuhn DJ,
Kornblau SM, Wang M, Weber DM, Thomas SK, Shah JJ, Voorhees PM, Xie
H, et al: Blockade of interleukin-6 signalling with siltuximab
enhances melphalan cytotoxicity in preclinical models of multiple
myeloma. Br J Haematol. 152:579–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu CP, Chen YL, Huang CC, Chou CC, Liu
CL, Hung CH, Kao TY and Chung YC: Anti-interleukin-6 receptor
antibody inhibits the progression in human colon carcinoma cells.
Eur J Clin Invest. 41:277–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider MR, Hoeflich A, Fischer JR, Wolf
E, Sordat B and Lahm H: Interleukin-6 stimulates clonogenic growth
of primary and metastatic human colon carcinoma cells. Cancer Lett.
151:31–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yilmaz S and Simsek I: Early intervention
in the treatment of rheumatoid arthritis: focus on tocilizumab.
Ther Clin Risk Manag. 9:403–408. 2013.PubMed/NCBI
|
|
21
|
Oldfield V, Dhillon S and Plosker GL:
Tocilizumab: a review of its use in the management of rheumatoid
arthritis. Drugs. 69:609–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klein B, Wijdenes J, Zhang XG, Jourdan M,
Boiron JM, Brochier J, Liautard J, Merlin M, Clement C,
Morel-Fournier B, et al: Murine anti-interleukin-6 monoclonal
antibody therapy for a patient with plasma cell leukemia. Blood.
78:1198–1204. 1991.PubMed/NCBI
|
|
23
|
Sato K, Tsuchiya M, Saldanha J, Koishihara
Y, Ohsugi Y, Kishimoto T and Bendig MM: Reshaping a human antibody
to inhibit the interleukin 6-dependent tumor cell growth. Cancer
Res. 53:851–856. 1993.PubMed/NCBI
|
|
24
|
Stärkel P, Charette N, Borbath I,
Schneider-Merck T, De Saeger C, Abarca J, Leclercq I and Horsmans
Y: Ras inhibition in hepatocarcinoma by
S-trans-trans-farnesylthiosalicyclic acid: association of its tumor
preventive effect with cell proliferation, cell cycle events and
angiogenesis. Mol Carcinog. 51:816–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sorriento D, Illario M, Finelli R and
Iaccarino G: To NFκB or not to NFκB: The Dilemma on How to Inhibit
a Cancer Cell Fate Regulator. Transl Med UniSa. 4:73–85.
2012.PubMed/NCBI
|
|
26
|
Chang TP and Vancurova I: NFκB function
and regulation in cutaneous T-cell lymphoma. Am J Cancer Res.
3:433–445. 2013.PubMed/NCBI
|
|
27
|
Martin AG: NFκB anti-apoptotic or
pro-apoptotic, maybe both. Cell Cycle. 9:3131–3132. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Viatour P, Merville MP, Bours V and
Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins:
implications in cancer and inflammation. Trends Biochem Sci.
30:43–52. 2005. View Article : Google Scholar : PubMed/NCBI
|