Introduction
Osteosarcoma (OS) is the most commonly diagnosed
primary malignant cancer affecting the bone, and one of the most
heterogeneous types of human tumor (1,2). The
incidence rates of OS are 1.7 per million and 4.2 per million, in
individuals aged 25–59 years old and ≥60 years old, respectively
(3). OS predominantly occurs in
children and young adolescents, and is characterized by a high
level of malignancy, relapse and metastasis, and poor prognoses. In
recent years, the combination of systemic chemotherapy and modern
surgery has improved the treatment of OS. However, no substantial
improvement in patient survival has been observed (4) and the five-year survival rate for
patients with metastatic OS is 20–30% (3). Therefore, it is important to understand
the molecular mechanisms underlying OS in order to improve
treatment strategies. In addition, the identification and
characterization of molecules involved in OS tumorigenesis is
required to aid advances in therapeutic strategies.
Ubiquitin-conjugating enzyme E2 C (UbcH10) belongs
to the E2 family and is involved in ubiquitin-dependent proteolysis
(5,6).
UbcH10 is highly conserved and consists of a core domain with a
catalytic Cys residue, and an N-terminal extension (7). The core domain interacts with a
ubiquitin-fold domain in the E1 enzyme to form a ubiquitin adduct,
and the N-terminal extension regulates E3 enzyme activity. Previous
studies have demonstrated that UbcH10 is crucial in mitotic
regulation, and is required for the degradation of mitotic
checkpoint proteins, cyclins (5,8) and other
mitosis-related substrates (7,9), which are
essential for spindle assembly checkpoints and mitotic exits.
Increasing evidence has indicated that UbcH10 is abnormally
overexpressed in a number of malignant tumors, including cancers of
the adrenal gland, bladder, brain, breast, cervix, colon, rectum,
esophagus, liver, lung, nasopharynx, ovary, prostate (late-stage),
pancreas, stomach, thyroid and uterus (10,11).
UbcH10 is recognized as a potential cancer biomarker (11). An overexpression of UbcH10 is
significantly associated with the pathological grading of tumors,
high cellular proliferation and poor prognoses of cancers affecting
the adrenal gland, breast, colon, liver, lung and ovary (10,11).
Furthermore, UbcH10 transgenic mice are prone to developing a range
of spontaneous and carcinogen-induced tumors (12). By contrast, silencing of UbcH10
inhibits glioma and colorectal cancer proliferation (13,14).
However, limited evidence exists regarding the biological function
of UbcH10 in OS.
In the present study, UbcH10 was knocked down in the
OS U2OS and SaOS2 cell lines through lentivirus-mediated RNA
interference (RNAi). The role of UbcH10 in OS progression was then
analyzed in vitro. The cellular proliferation, invasion,
colony formation and migration abilities were determined in UbcH10
knockdown cells. In addition, the expression of Ki-67 and matrix
metalloproteinases (MMPs) were analyzed.
Materials and methods
Cell culture
The human OS U2OS and SaOS2 cell lines were obtained
from the Institute of Biochemistry and Cell Biology (Shanghai,
China). The cells were incubated in RPMI-1640 medium supplemented
with 10% fetal calf serum (Thermo Fisher Scientific Inc., Waltham,
MA, USA) and 1% antibiotics (penicillin and streptomycin;
Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified 5%
CO2 atmosphere.
Lentivirus-mediated short hairpin RNA
(shRNA) transfection
The shRNA oligos of UbcH10 were designed according
to its sequence in the NCBI database as follows:
5′-AACCUGCAAGAAACCUACUCA-dTdT-3′. The sequence of the control shRNA
was as follows: 5′-AAAUGCACACACACAUACUCG-dTdT-3′. The fragments of
shRNA were inserted into the lentivirus vector and transfected into
HEK293 cells with packaging vectors using Lipofectamine 2000 (Life
Technologies, Grand Island, NY, USA). After 48 h, the recombinant
lentivirus was collected from the media for further infection.
The U2OS and SaOS2 cells were cultured in a 6-well
plate at a density of 12×104 cells per well. Subsequent
to a 24-h culture, the cells were transfected with the recombinant
lentivirus at a multiplicity of infection of 20. At 48 h
post-infection, the cells were observed using a fluorescence
microscope (DM IL LED; Leica Microsystems, Wetzlar, Germany). The
infection efficiencies were determined by the ratio of green
fluorescent protein (GFP)-positive cells to total cells.
Western blot analysis
At 3 days post lentiviral infection, the U2OS and
SaOS2 cells were collected and lysed in RIPA buffer (150 mM NaCl,
100 mM Tris-HCl, 1% Tween-20, 1% sodium deoxycholate and 0.1% SDS)
supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and a
protease inhibitor cocktail. Following centrifugation at 13,000 × g
for 15 min, the supernatant was collected and boiled with 2X SDS
protein sample buffer. The proteins were separated using SDS-PAGE
and transferred to polyvinylidene fluoride membranes. The membranes
were blocked with Tris-buffered saline and Tween 20 (TBST; Beijing
SolarBio Science & Technology Co., Ltd., Beijing, China) plus
1% bovine serum albumin (Westang Bio-Tech Co., Ltd., Shanghai,
China) for 1 h and probed with a variety of antibodies overnight at
4°C. Next, the membranes were washed with TBST for 15 min and
probed with horseradish peroxidase-conjugated secondary antibodies
for 1 h. The membranes were then washed with TBST for 15 min and
signals were detected by enhanced chemiluminescence using
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific Inc.) and the Amersham Imager 600 (GE Healthcare,
Pittsburgh, PA, USA). The primary antibodies used in the present
study were: Anti-UbcH10 (1:500; cat. no. 14234S; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-GAPDH (1:10,000; cat. no.
sc-365062; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-Ki-67 (1:1,000; cat. no. sc-7846; Santa Cruz Biotechnology,
Inc.), anti-MMP-3 (1:1,000; cat. no. 14351S; Cell Signaling
Technology, Inc.) and anti-MMP-9 (1:1,000; cat. no. sc-21733; Santa
Cruz Biotechnology, Inc.). The secondary antibodies were
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(1:2,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.).
MTT assay
In brief, the U2OS and SaOS2 cells were cultured in
a 96-well plate at a density of 104 cells per well.
Subsequent to a 24-h incubation, the cells were transfected with a
recombinant lentivirus carrying shRNA. At various time-points of 1,
2, 3, 4 and 5 days, MTT (Sigma-Aldrich) was added at a final
concentration of 5 mg/ml and incubated with the cells at 37°C for 4
h. After removing the medium, dimethyl sulfoxide was added in order
to terminate the reaction. All wells were analyzed using an ELISA
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a
wavelength of 490 nm.
Colony formation assay
After 5 days of lentivirus treatment with control
shRNA-expressing lentivirus (Lv-shCon) or UbcH10-targeted
shRNA-expressing lentivirus (Lv-shUbcH10), the U2OS and SaOS2 cells
were trypsinized, counted and then replated in a 6-well plate at a
concentration of 200 cells per well. The cell samples were then
allowed to grow for 14 days in order to form natural colonies.
Following this, the plate was washed twice with phosphate-buffered
saline solution and stained for 10 min with Giemsa (Sigma-Aldrich).
Images of the stained colonies were then captured under a
fluorescence microscope. Finally, the total number of colonies (N50
cells/colony) and the total number of cells in each colony were
counted and analyzed.
Transwell invasion assay
The invasion of U2OS and SaOS2 cells was analyzed
using BioCoat Transwell chambers (Corning Incorporated, Corning,
New York, NY, USA). The cells were serum starved for 24 h and then
harvested. In total, 2×104 cells were added to the upper
chamber, which was coated with Matrigel™ matrix (BD Biosciences,
Franklin Lakes, NJ, USA). Subsequent to a 24-h incubation at 37°C
in a humidified atmosphere of 5% CO2, the cells on the
upper surface of the chamber were removed with a cotton swab. The
cells that had invaded to the bottom surface of the insert were
fixed with 70% ethanol and stained with crystal violet. The
invasiveness was quantitated by counting the number of cells on
five different random views using a light microscope (DMi1; Leica
Microsystems) (magnification, x10). All experiments were repeated
at least three times, with more than three wells for each
treatment.
Migration assay
Cellular migration was analyzed using a wound
healing assay. The U2OS and SaOS2 cells were cultured to 90–95%
confluence and then serum starved for 24 h. Following this, the
monolayers of cells were carefully wounded using sterilized pipette
tips. The wound closure was determined using a light microscope,
and images were captured at the indicated time points.
Statistical analysis
The cell culture experiments were performed in
triplicate. Student's t-test was used to analyze the
significance of differences. Two-tailed P<0.05 was considered to
indicate a statistically significant difference, and the data are
presented as the mean ± standard deviation.
Results
Lentivirus-mediated RNAi efficiently
suppresses the expression of UbcH10 in OS cells
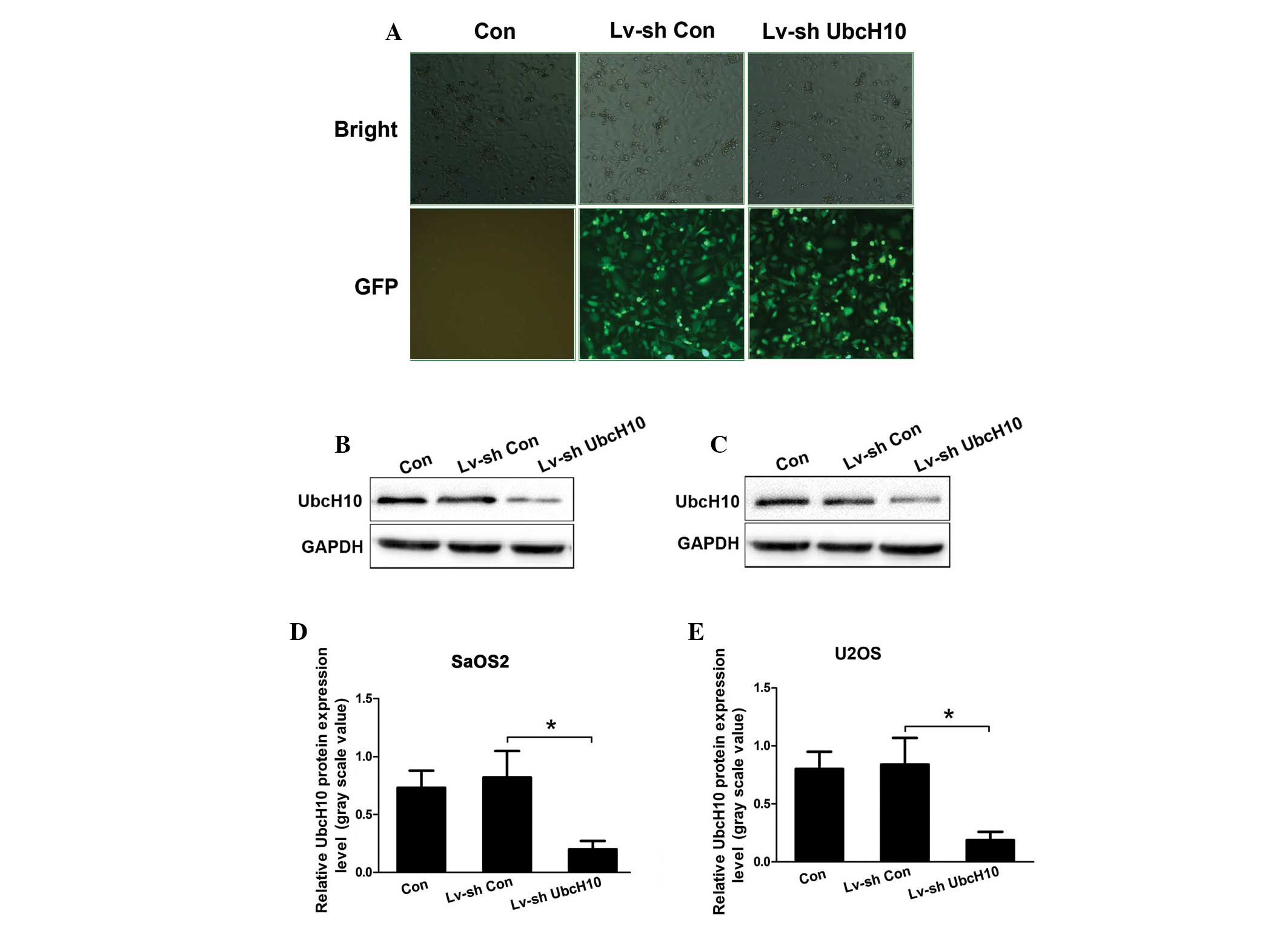
At 24 h post lentivirus-transfection, >90%
Lv-shCon and Lv-ShUbcH10 transfected U2OS and SaOS2 cells exhibited
GFP-positive signals (Fig. 1A), which
indicated that the recombinant lentivirus was able to infect the OS
cells with high efficiency. The proportion of positive cells that
were transfected with Lv-shUbcH10 was >90%, as evidenced by GFP
expression 3 days after transfection (Fig. 1A). Further western blot analysis
revealed that the protein levels of UbcH10 were significantly
reduced in Lv-shUbcH10-transfected U2OS and SaOS2 cells (P=0.018
and P=0.021, respectively; Fig.
1B–E). The control shRNA did not affect the expression of
UbcH10. These data demonstrate the high gene transfer efficiency of
lentiviruses in OS cells, and suggest that the expression of UbcH10
is efficiently knocked down by shUbcH10.
UbcH10-targeted RNAi reduces OS cell
proliferation and colony formation
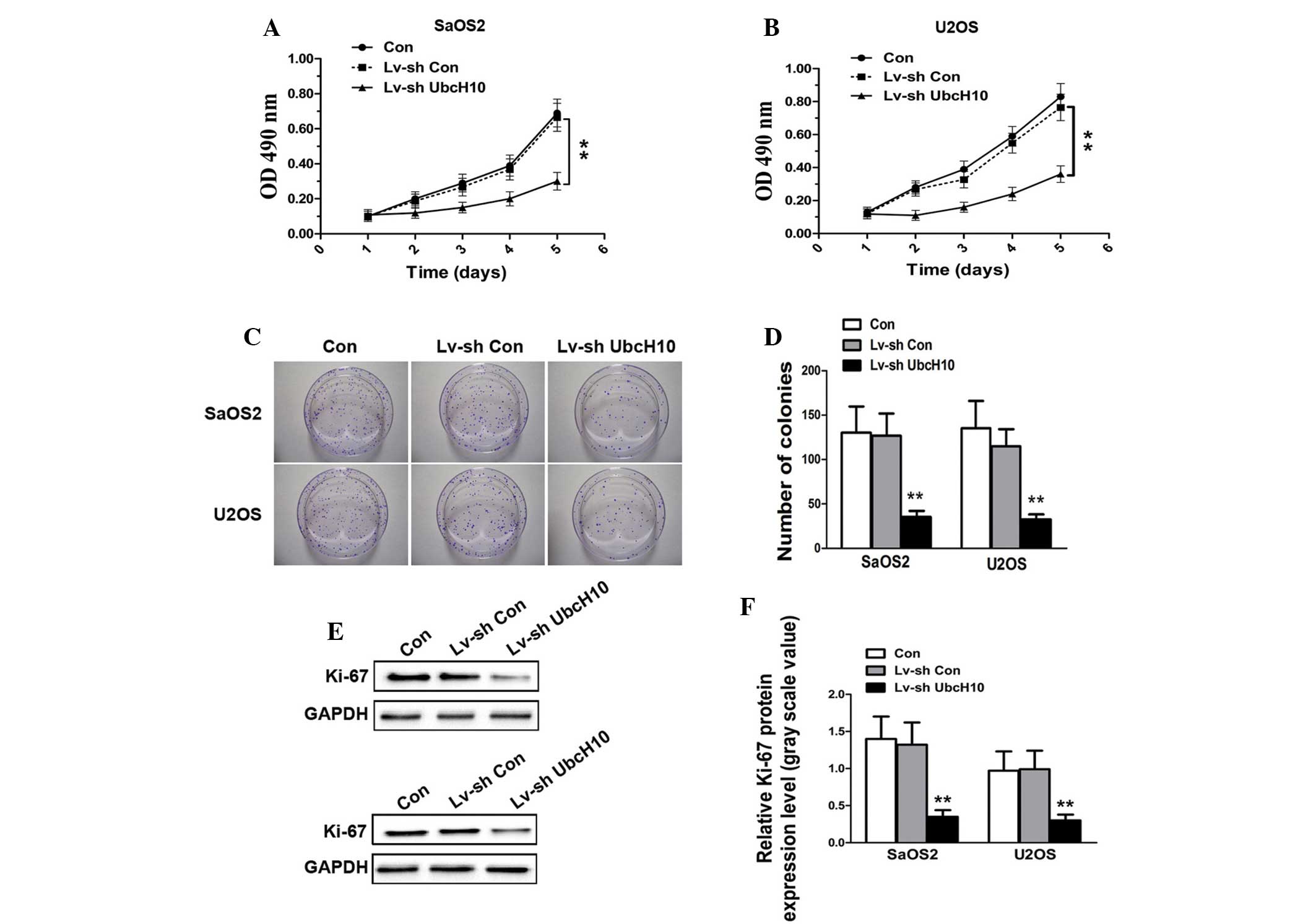
Proliferation is a key process involved in the
progression of tumors. In order to determine whether shUbcH10 has
an inhibitory effect upon OS cell growth, an MTT assay was
performed. As shown in Fig. 2A and B,
the growth curves for UbcH10 knockdown U2OS and SaOS2 cells were
significantly lower during the 5-day incubation than those for
control cells and Lv-shCon-transfected cells (P=0.0017 and
P=0.0028, respectively). The colony formation assay demonstrated
that the colony numbers of Lv-shUbcH10-transfected U2OS and SaOS2
cells were significantly reduced compared with those of the control
cells and Lv-shCon-transfected cells (P=0.0015 and P=0.0022,
respectively) (Fig. 2C and D). This
indicates that the colony formation ability is impaired in UbcH10
knock-down OS cells.
To confirm the results, western blotting was
performed in order to analyze the expression level of the cellular
proliferation marker, Ki-67. As shown in Fig. 2E and F, there was no significant
difference in the protein level of Ki-67 in Lv-shCon-transfected
U2OS and SaOS2 cells (P=0.657). By contrast, the expression of
Ki-67 was markedly reduced in UbcH10 knock-down U2OS and SaOS2
cells (P=0.0035 and P=0.0017, respectively). These results indicate
that fewer OS cells entered the process of proliferation following
downregulation of UbcH10, a result is which is consistent with
those of the MTT and colony formation assays.
UbcH10-targeted RNAi suppresses OS
cell invasion and migration
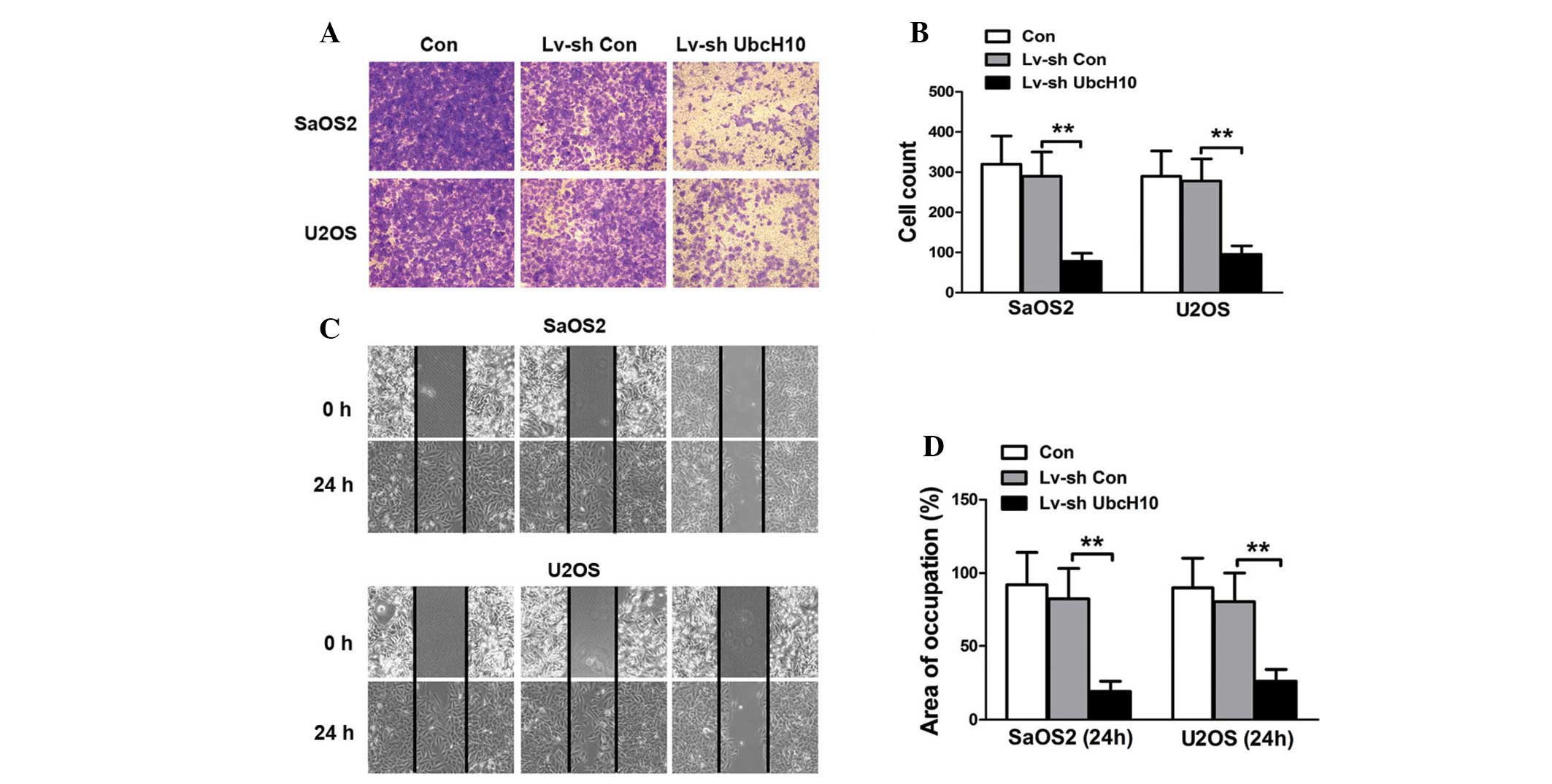
The matrix invasion and migration abilities of
cancer cells are closely associated with metastasis. Therefore, the
effect of UbcH10 suppression on the invasion of OS cells was
investigated. The results of the Transwell invasion assay revealed
fewer UbcH10 knock-down U2OS and SaOS2 cells than control and
Lv-shCon-transfected U2OS and SaOS2 cells in the lower chamber
(Fig. 3A and B). The cell migration
was investigated using a wound healing assay. The quantification of
cellular movement at 24 h revealed that cellular migration was
significantly repressed in UbcH10 knock-down U2OS and SaOS2 cells
compared with control and Lv-shCon-transfected cells (P=0.0029 and
P=0.0016, respectively) (Fig. 3C and
D). These results suggest that the invasion and migration
abilities of OS cells are impaired following knockdown of
UbcH10.
UbcH10 knockdown of OS cells
downregulates the expression of MMPs
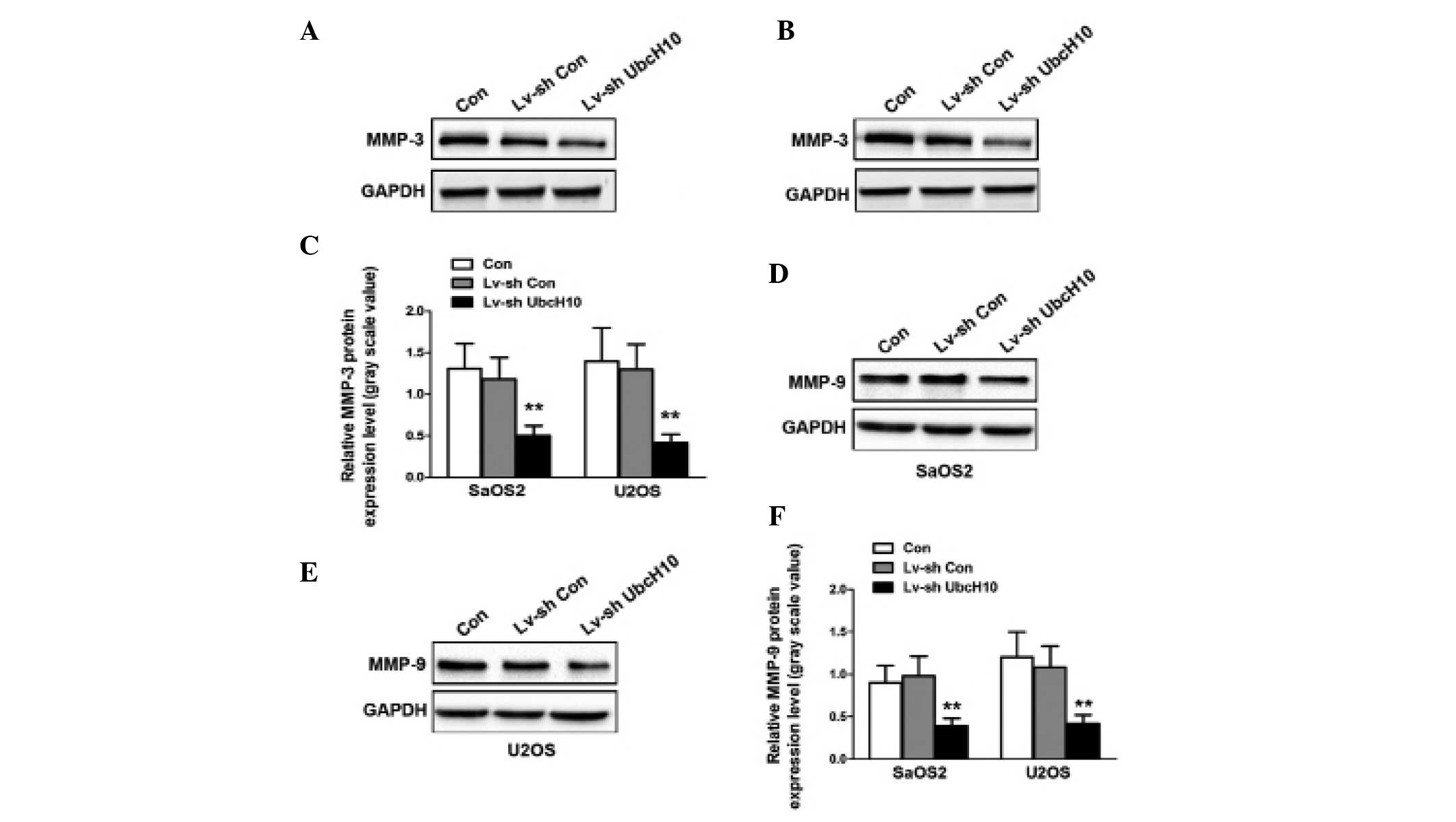
The expression of MMPs in U2OS and SaOS2 cells was
investigated. The levels of MMP-3 protein were significantly
reduced in Lv-shUbcH10-transfected U2OS and SaOS2 cells compared
with control and Lv-shCon-transfected cells (P=0.0043 and P=0.0061,
respectively) (Fig. 4A–C). Similar
results were observed for MMP-9 (Fig.
4D–F). These results indicate that the MMP signaling pathway is
disrupted in UbcH10 knockdown OS cells.
Discussion
UbcH10 is a potential cancer biomarker that is
overexpressed in a variety of cancers. In order to investigate its
functions in OS, UbcH10 was knocked down in the OS U2OS and SaOS2
cell lines. Western blot analysis revealed that the protein levels
of UbcH10 in Lv-shUbcH10 decreased to approximately one quarter of
that in the control cells. This indicated that the recombinant
lentivirus containing UbcH10-targeted shRNA could successfully
knockdown UbcH10 in U2OS and SaOS2 cells. Lentiviruses are
therefore useful for gene-targeted RNAi in OS cells in
vitro.
The present study also identified that, as a result
of decreased Ki-67 levels, a downregulation in the expression of
UbcH10 inhibited cellular proliferation and colony formation in
vitro. This confirmed that the expression of UbcH10 is
correlated with the proliferation activity of cancer cells. In
accordance with the results of the present study, a previous study
demonstrated that a knockdown of UbcH10 inhibited the cellular
proliferation of other cancer cells, including those of lung,
glioma and colorectal cancers (13–15). It is
therefore hypothesized that OS cell growth inhibition is caused by
UbcH10-mediated cell cycle regulation. In order to address this,
the present study analyzed the expression of the cellular
proliferation marker, Ki-67 (16).
Following UbcH10 knockdown, the decreased expression of Ki-67
indicated the presence of fewer dividing OS cells. This is
consistent with its role in the regulation of mitotic exit and cell
cycle progression through the destruction of mitosis-related
substrates (5,7–9).
A downregulation in the expression of UbcH10 was
also observed to impair the invasion and migration ability of OS
cells. In lung cancer cells, UbcH10-targeted RNAi also inhibits
cellular migration (15). In order to
determine the underlying mechanisms involved in the impaired
invasion and migration of UbcH10 knockdown OS cells, the expression
of MMP-3 and MMP-9 was analyzed. The levels of MMP-3 and −9
proteins decreased significantly. MMPs are a family of
zinc-dependent endopeptidases, which degrade proteins in the
extracellular matrix (17), and are
crucial in cancer cell invasion and migration (17,18).
Previous studies have demonstrated that an overexpression of MMP-3
in normal breast epithelium results in invasive tumor formation
(19). Additionally, in a recent
study, MMP-9 was identified as a potential biomarker for OS
(20). The downregulation of MMP-3
and −9 confirms that tumorigenesis is inhibited in UbcH10 knockdown
OS cells. However, whether MMP-3 and −9 are direct substrates of
UbcH10-mediated ubiquitin-dependent proteolysis remains to be
elucidated.
In conclusion, the results of the present study
demonstrate that, via the deregulation of Ki-67 and MMPs,
lentivirus-mediated UbcH10-targeted RNAi can lead to cell growth
inhibition, decreased colony formation, and impaired cellular
invasion and migration in the human OS U2OS and SaOS2 cell lines.
These results indicate an important role for UbcH10 in OS
progression, which suggests that UbcH10 may be a potential
therapeutic target for the treatment of OS. Therapeutic strategies
which target the UbcH10 gene or compounds that inhibit UbcH10
activity may be of use clinically for the treatment of OS and thus,
further studies are required to investigate these methods.
References
|
1
|
Bacci G, Longhi A, Versari M, Mercuri M,
Briccoli A and Picci P: Prognostic factors for osteosarcoma of the
extremity treated with neoadjuvant chemotherapy: 15-year experience
in 789 patients treated at a single institution. Cancer.
106:1154–1161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The etiology of
osteosarcoma. Cancer Treat Res. 152:15–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akiyama T, Dass CR and Choong PF: Novel
therapeutic strategy for osteosarcoma targeting osteoclast
differentiation, bone-resorbing activity, and apoptosis pathway.
Mol Cancer Ther. 7:3461–3469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Townsley FM, Aristarkhov A, Beck S,
Hershko A and Ruderman JV: Dominant-negative cyclin-selective
ubiquitin carrier protein E2-C/UbcH10 blocks cells in metaphase.
Proc Natl Acad Sci USA. 94:2362–2367. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okamoto Y, Ozaki T, Miyazaki K, Aoyama M,
Miyazaki M and Nakagawara A: UbcH10 is the cancer-related E2
ubiquitin-conjugating enzyme. Cancer Res. 63:4167–4173.
2003.PubMed/NCBI
|
|
7
|
Lin Y, Hwang WC and Basavappa R:
Structural and functional analysis of the human mitotic-specific
ubiquitin-conjugating enzyme, UbcH10. J Biol Chem. 277:21913–21921.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aristarkhov A, Eytan E, Moghe A, Admon A,
Hershko A and Ruderman JV: E2-C, a cyclin-selective ubiquitin
carrier protein required for the destruction of mitotic cyclins.
Proc Natl Acad Sci USA. 93:4294–4299. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rape M, Reddy SK and Kirschner MW: The
processivity of multiubiquitination by the APC determines the order
of substrate degradation. Cell. 124:89–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao Z, Zhang H and Cowell J:
Ubiquitin-conjugating enzyme UBE2C: molecular biology, role in
tumorigenesis, and potential as a biomarker. Tumour Biol.
33:723–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie C, Powell C, Yao M, Wu J and Dong Q:
Ubiquitin-conjugating enzyme E2C: a potential cancer biomarker. Int
J Biochem Cell Biol. 47:113–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Ree JH, Jeganathan KB, Malureanu L and
van Deursen JM: Overexpression of the E2 ubiquitin-conjugating
enzyme UbcH10 causes chromosome missegregation and tumor formation.
J Cell Biol. 188:83–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Bao Y, Luo C, et al: Knockdown of
ubiquitin-conjugating enzyme E2C/UbcH10 expression by RNA
interference inhibits glioma cell proliferation and enhances cell
apoptosis in vitro. J Cancer Res Clin Oncol. 136:211–217. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen SM, Jiang CY, Wu JY, et al: RNA
interference-mediated silencing of UBCH10 gene inhibits colorectal
cancer cell growth in vitro and in vivo. Clin Exp Pharmacol
Physiol. 37:525–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pallante P, Malapelle U, Berlingieri MT,
et al: UbcH10 overexpression in human lung carcinomas and its
correlation with EGFR and p53 mutational status. Eur J Cancer.
49:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43:Suppl. S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies KJ: The complex interaction of
matrix metalloproteinases in the migration of cancer cells through
breast tissue stroma. Int J Breast Cancer. 2014:8390942014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Shi Q, Yuan TX, et al: Matrix
metalloproteinase 9 (MMP-9) in osteosarcoma: review and
meta-analysis. Clin Chim Acta. 433:225–231. 2014. View Article : Google Scholar : PubMed/NCBI
|