Introduction
Meningiomas of the spinal canal are relatively rare
compared with the incidence in the intracranial compartment,
accounting for ∼1.2% of all meningiomas of the central nervous
system (1,2). Spinal meningiomas are, in general,
intradural and extramedullary. Chronic compression of the spinal
cord is the main pathological mechanism and total removal of the
meningioma, for cord decompression, is the primary treatment choice
(1,3).
With the development of modern neuroradiological techniques and
standard microneurosurgical procedures, intraspinal meningiomas can
be removed with low morbidity and a good outcome (1). However, the development of a delayed
neurological deficit in the post-operative period occurs often in
patients with chronic compressive spinal cord lesions, including
cervical spondylotic myelopathy, ossification of the spinal
ligament and spinal stenosis (4–8), and can
be identified in a small subset of patients with intraspinal
meningiomas. Neurological deterioration or even paralysis
subsequent to surgery is extremely rare, but these are the most
serious complications (5–7).
Post-operative neurological deficit is most often
due to mechanical damage resulting from the surgical procedure and
intraspinal hematoma (6). However,
careful surgical techniques and intraoperative neuromonitoring can
indicate any potential trauma to neural tissue during the tumor
removal and decompression procedures (1,4,5). In the absence of clear etiology,
vascular insult and toxic mechanisms may be responsible for the
neurological deterioration (4,5). The
current study presents 284 patients that underwent surgical
intervention for a spinal meningioma at Beijing Tiantan Hospital
(Capital Medical University, Beijing, China). Special consideration
was focused on patients that experienced post-operative
neurological deterioration, in order to identify the causes and
factors.
Materials and methods
Between the 2004 and 2010, 284 patients were
pathologically diagnosed with intraspinal meningiomas at the
Department of Neurosurgery of Beijing Tiantan Hospital. The data
associated with the clinical presentation, radiological imaging,
treatment and follow-up outcomes were collected, with approval from
the Institutional Review Board of Beijing Tiantan Hospital and
written informed consent was obtained from all patients. The lesion
was clearly predominant in females, with the cohort comprising 226
female patients and 58 male patients, yielding a female to male
ratio of 3.9:1. The age of patients ranged between 12 and 86 years,
with a mean age of 52 years. The inclusion criteria consisted of:
Post-operative deterioration of motor function, due to an unknown
cause, by at least one level in a standard manual muscle test with
aggravation of extremity function; the appearance of a novel
sensory deficit subsequent to surgery; or combined deterioration of
motor and sensory function. Exclusion criteria consisted of:
Extradural, atypical, World Health Organization grade II or
recurred tumors; meningiomas associated with neurofibromatosis; or
post-operative deterioration caused by intraspinal hematoma,
incomplete cord decompression or potential iatrogenic injury, which
can be indicated by post-operative magnetic resonance imaging (MRI)
or intraoperative monitoring.
MRI with gadolinium-contrast enhancement was
performed as a standard radiological investigation prior to and
following surgical treatment. T1 and T2 weighted imaging was
performed in the sagittal and axial planes to determine the spinal
level, size and the dural attachment of the meningioma and its
association with the spinal cord.
During the initial years of the present study, mono-
or multisegmental laminectomy or hemilaminectomy was performed to
access the intraspinal meningioma. However, this strategy was later
altered and replaced as the standard surgical approach by
osteoplastic laminotomy with subsequent reconstruction of the
posterior spinal column using microplates. All patients underwent
surgical resection with intraoperative neurophysiological
monitoring of somatosensory and motor evoked potentials.
A modified McCormick classification (Table I) (9,10) was
applied to assess pre- and post-operative neurological function.
Structured telephone interviews were performed to determine the
post-operative follow-up status of the patients.
 | Table I.Modified McCormick classification. |
Table I.
Modified McCormick classification.
| Grade | Definition |
|---|
| I | Neurologically
normal |
| Gait normal |
| Normal professional
activity |
| Ib | Tired after walking
several kilometers |
| Running is
impossible, or moderate sensorimotor deficit does not significantly
affect the involved limb |
| Moderate discomfort
in professional activity |
| II | Presence of
sensorimotor deficit affecting the function of the involved
limb |
| Mild to moderate gait
difficulty |
| Severe pain or
dysesthetic syndrome impairs quality of life |
| Independent function
and ambulation maintained |
| III | More severe
neurological deficit |
| Requires cane and/or
brace for ambulation or maintains significant bilateral
upper-extremity impairment |
| May or may not
function independently |
| IV | Severe neurological
deficit |
| Requires wheelchair
or cane and/or brace with bilateral upper-extremity impairment |
| Usually not
independent |
Results
Post-operative neurological deterioration with
unknown causes occurred in 10 out of 284 patients (3.5%) (Table II). This consisted of five men and
five women, aged between 32 and 62 years at presentation (mean,
46.8 years). The duration of illness from the onset of symptoms to
diagnosis ranged between 10 and 120 months (mean, 42.8 months). The
thoracic region of the spinal cord was affected in seven patients,
and the cervical region in three patients. All patients
pre-operatively presented with various degrees of sensory, motor or
sphincter dysfunctions. The pre-operative assessment revealed that
three patients were at Grade I of the modified McCormick
classification, followed by seven at Grade Ib.
 | Table II.Summary of 10 patients with
postoperative neurological deterioration. |
Table II.
Summary of 10 patients with
postoperative neurological deterioration.
|
|
|
|
|
|
|
|
| McCormick grade |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Age, years | Gender | Duration of illness,
months | Tumor location | Dural attachment | Post-operative onset
time of deterioration, h | High signal changes
on T2WI | Pre-operative | Post-operative | Last FU | FU, months |
|---|
| 1 | 44 | F | 48 | C4 | Ventrolateral | 4 | + | I | III | I | 48 |
| 2 | 32 | M | 10 | C6-7 | Lateral | 6 | – | Ib | IV | Ib | 50 |
| 3 | 41 | M | 96 | C6-7 | Dorsolateral | 3 | – | Ib | IV | I | 64 |
| 4 | 62 | M | 60 | T3-4 | Lateral | 6 | – | Ib | III | Ib | 32 |
| 5 | 51 | F | 36 | T9-10 | Lateral | 4 | + | I | IV | I | 28 |
| 6 | 38 | F | 12 | T11-12 | Ventrolateral | 3 | – | Ib | IV | Ib | 24 |
| 7 | 62 | M | 120 | T11-12 | Lateral | 6 | + | Ib | III | I | 54 |
| 8 | 56 | F | 18 | T1-2 | Dorsal | 8 | – | Ib | III | I | 48 |
| 9 | 48 | M | 18 | T10-11 | Ventrolateral | 6 | – | Ib | IV | III | 52 |
| 10 | 34 | F | 10 | T11 | Ventral | 4 | + | I | IV | II | 96 |
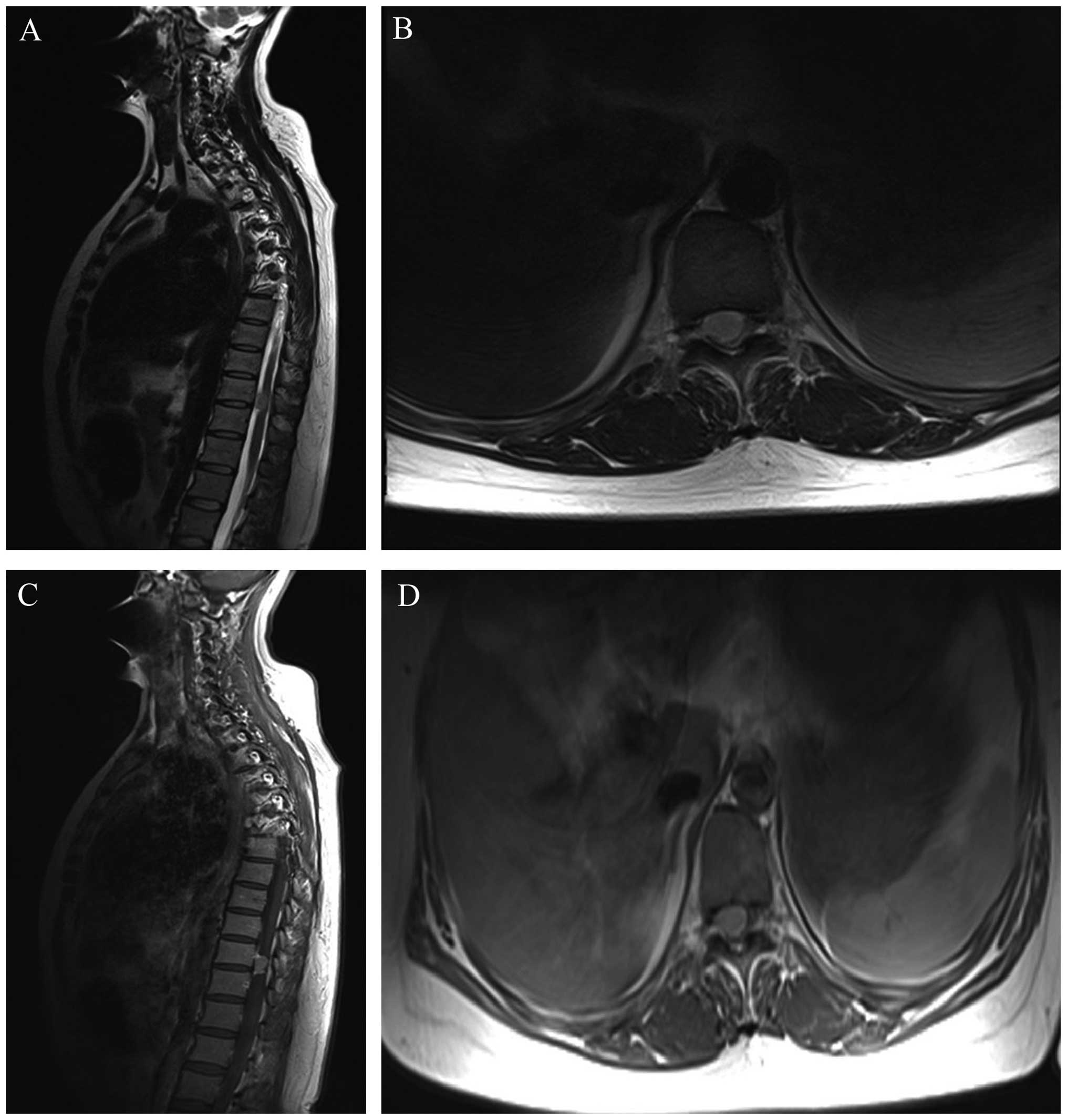
On MRI, the tumors in all cases were found to
compress the cord severely and the average invasion ratio of the
spinal canal was >70%, which was measured on axial T2 weighted
images. All lesions were well demarcated from the spinal cord
parenchyma, which facilitated their exposure and dissection. The
dural attachment of the intraspinal meningioma was predominantly
localized laterally or ventrolaterally in four and three patients
respectively, as determined by pre-operative MRI and intraoperative
exploration (Fig. 1). Laminectomy or
osteoplastic laminotomy with reconstruction of the posterior spinal
column was performed to access the tumor. Gross total removal was
achieved in all 10 cases. The dural attachment was completely
resected if the tumor was located dorsally or dorsolaterally, and
duraplasty was performed with artificial dura. In ventrally located
tumors, the dural attachment was not excised but extensively
bipolar cauterized. No somatosensory and motor-evoked potentials
were affected during the surgery.
Immediately following the surgery, all patients were
able to move all extremities. The onset of the neurological deficit
occurred at post-operative hours 3–8 in all cases (mean, 5 h
post-surgery). Weakness of extremities was noted in all 10 cases,
and sensory disturbances were identified in four cases. The
neurological assessment revealed that six patients were classified
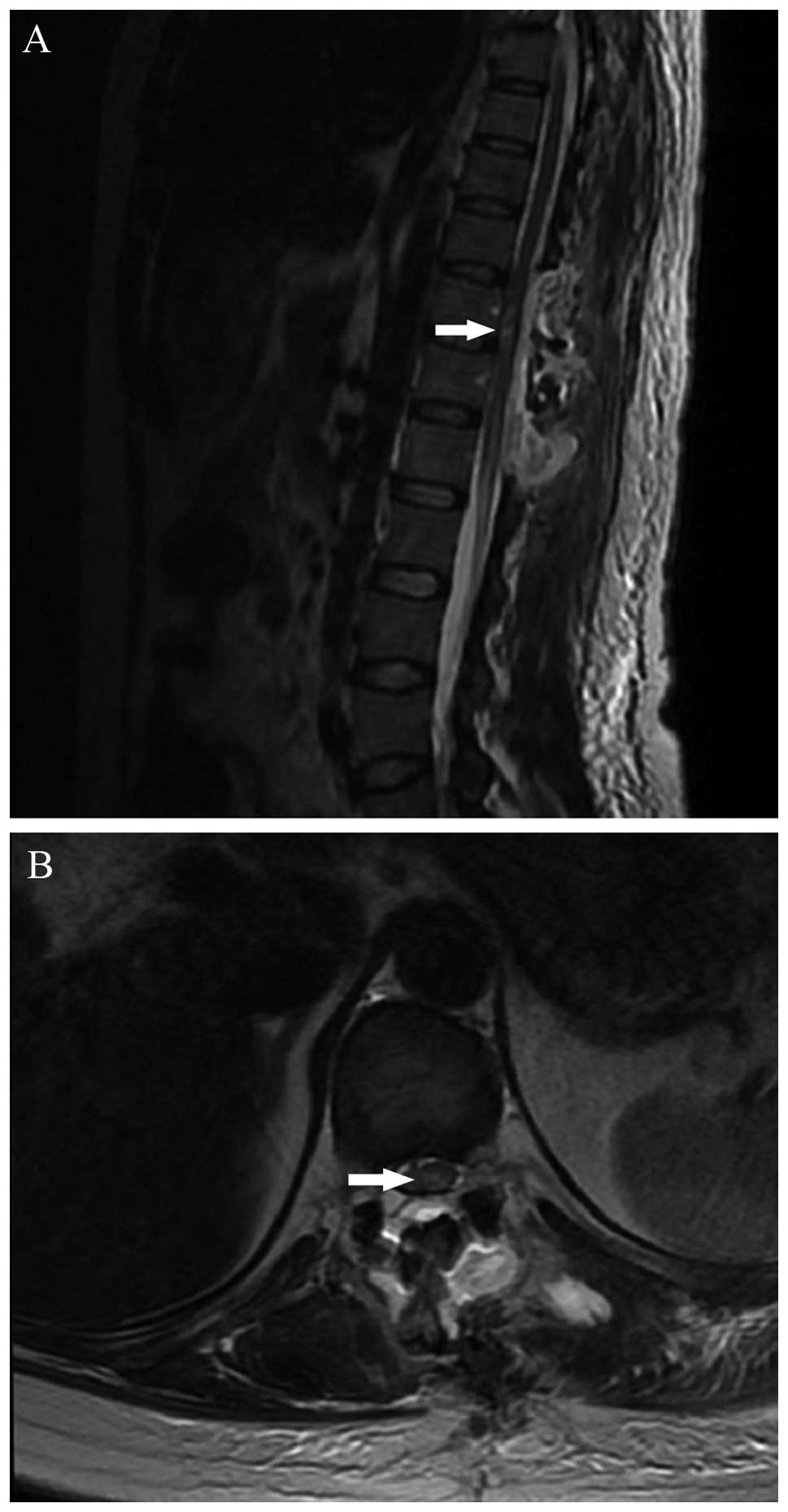
as Grade IV, followed by four as Grade III. An MRI performed
immediately subsequent to surgery demonstrated that there was no
pathological enhancement of the spinal cord lesion. However, in
four cases, T2-weighted imaging revealed an area of high signal
changes intrinsic to the cord, without residual compression, which
was considered to be consistent with spinal cord edema (Fig. 2). The steroid protocol for acute
spinal cord injury was immediately performed and tapering
intravenous dexamethasone was added. The mean follow-up period was
49.6 months (range, 24–96 months). During the telephone interviews,
nine patients reported a marked recovery in their status compared
to the pre-operative presentation, which had occurred during the
several weeks to months subsequent to surgery. The status of one
patient (Case 9) exhibited little improvement at the final
follow-up. At the most recent follow-up assessment, five patients
had returned to Grade I, three were classified as Grade Ib, one was
classified as Grade II and one was classified as Grade III on the
modified McCormick scale.
Discussion
Meningiomas are common tumors in the spinal canal.
Since cord compression is the main pathogenic mechanism, surgical
treatment is usually successful and the outcomes are generally
promising (6). Total removal of the
chronic compressive spinal cord lesion in the absence of any direct
trauma to the cord resulted in delayed, but severe, neurological
deterioration in all 10 of the presented cases. To date, the
underlying pathophysiology of such a finding remains unclear
(4–8).
Post-operative neurological deteriorations are usually detected on
physical and neurological examination and may be a substantial
disability to the patients (4,6,8). There are several theories for
neurological deterioration subsequent to cord decompression, and
iatrogenic cord insult is a well-known possible etiology (11,12).
However, the timing, nature and underlying circumstances of the
neurological deficits observed in the present patients suggests
that alternative underlying mechanisms more likely than direct cord
trauma (4–6,8).
Post-operative hematomas may be a cause of neurological
deterioration (13), but the patients
are able to move all extremities following the surgical procedure
(5,14–18). In
addition, no post-operative hematomas or any compressive lesions
were identified in the present study, which was consistent with
previous studies (4,5). Another possible etiology of
post-operative paralysis subsequent to thoracic decompression is
the presence of microthrombi compromising the watershed regions of
arterial supply (14,15). Neurapraxia during recoil in
cross-sectional dimensions of the cord and a sudden drop in blood
pressure also require consideration as possible etiologies for
neurological deterioration (16–18).
In the present study, all tumors chronically and
severely compressed the spinal cord, but the patients appeared to
have compensated for the compression. It was therefore hypothesized
that the acute removal and decompression of the tumors resulted in
immediate cord expansion within the open canal space, and the
long-term ischemic compressed segment of the cord was exposed to a
rush in blood supply. This sudden cord expansion and reperfusion
may have led to disruption in the blood-spinal cord barrier, and
triggered a cascade of reperfusion injury, resulting in
post-operative neurological deterioration. In addition, since all
10 patients experienced severe cord compression with a long
duration of illness, it is proposed that the long duration of
symptoms and the degree of compression is a risk factor for
reperfusion injury. In the literature, ischemia-reperfusion injury
has previously been indicated to be a cause for post-operative
paralysis by Hasegawa et al, who reported that 49 out of 857
patients (5.7%) experienced upper extremity palsy subsequent to
decompression surgery for chronic compressive spinal cord disorders
(19). In 2006, a Japanese study
group reported the case of a patient that experienced delayed
transient paraplegia subsequent to laminectomy for thoracic
ossification of the posterior longitudinal ligament and ossification
of the ligamentum flavum, in the absence of deleterious changes on
intraoperative neuromonitoring (20).
In this study, a neurological examination performed immediately
subsequent to surgery produced a baseline result. However, over the
following 18 h, the patient neurologically deteriorated to complete
paraplegia (20).
Microcirculatory disturbance due to reperfusion can
occur at any level and in any region where surgical decompression
was performed to treat a chronic compressive lesion (19,21,22).
Reperfusion of neural tissue is well-known to result in deleterious
clinical sequelae that are likely to be associated with the role of
reactive oxygen radical-mediated neuronal cell death (23,24).
Animal models have demonstrated that superoxide-mediated injury
occurs following reperfusion during acute neuronal ischemic events
(24,25). Furthermore, spinal cord
ischemic-reperfusion injury may also appear contingent on
mitochondria-dependent apoptosis, inflammatory reactions and
specific phospholipid signaling cascades, resulting in neuronal
injury. It has been suggested that acute and chronic spinal cord
ischemic injury may induce the passage of blood-borne or
neurotrophic substances, in particular TNF-α, through the blood
brain barrier (BBB) past the saturation point (4,5,23,25).
Decoupling of astrocyte foot processes from endothelial cell
surfaces appears to inhibit tight junction function in the BBB.
Transport systems and ionic buffering then become disrupted,
allowing worsened reperfusion injury upon decompression of a
previously ischemic spinal cord (4,23).
However, a potential mechanism for decompression-associated
reperfusion injury of chronic ischemia has not been established at
present (16–18). Substantial efforts have focused on the
mitigation of spinal cord ischemic injury (4). These efforts have included surgical
techniques, pharmacological interventions, and mechanical methods
(4,23,24). More
recently, it has been suggested that potent antioxidants may play
an important role in the management of spinal cord ischemic
reperfusion injury (4,5,23).
In four patients in the present study,
post-operative MRI revealed high signal intensity on the T2
weighted images, which may suggest spinal cord edema. Chin et
al used the term ‘white cord syndrome’ to describe this
appearance (4). However, in certain
previous studies, the increased T2-weighted signal intensity was
present even prior to the decompression, and demyelination may also
be a possibility for the clinical relevance, which may reflect on
the various possible causes of the edema and increased signal
intensity (26).
Patients and surgeons should be aware of the
potentially catastrophic results of a seemingly routine tumor
removal for the treatment of intraspinal meningioma with chronic
but severe cord compression. It is necessary to explain the rate of
neurological deterioration and possible post-surgical complications
prior to operative intervention. The majority of the present
patients experienced a progressive neurological deficit recovery
within several weeks to months following surgery, which may aid in
advising patients of the risks of spinal decompression surgery for
the treatment of intraspinal meningioma.
The limitation of the present study is its
retrospective nature, being performed over six years.
Microneurosurgical techniques, intraoperative neuromonitoring and
treatment of spinal cord injury have been considerably more
developed during the follow-up period. These developments may aid
in safely accomplishing tumor removal and avoiding post-operative
complications, therefore exerting an effect on the surgical and
clinical outcome of decompression surgery. Additionally, detailed
neurological examination and surgical findings in certain early
cases was not fully ascertained, since the clinical information was
based on the medical records of the patients. Although 10 cases in
a series appears to be a small number, the present cases constitute
the first reported clinical series reporting atraumatic
neurological deterioration following surgical resection for
intraspinal meningiomas.
The cases presented in the current study highlight
the possibility of a delayed yet severe neurological deterioration
in the absence of direct insult to the spinal cord subsequent to
total removal of intraspinal meningiomas. Ischemia-reperfusion
injury may be one potential etiology for this deterioration. The
present study may aid the improvement of the informed pre-operative
decision making process and merits additional investigation into
the underlying pathophysiology of the present findings.
Acknowledgements
The authors would like to thank all the patients,
physicians and staff who were enrolled in and aided this study.
References
|
1
|
Sandalcioglu IE, Hunold A, Müller O,
Bassiouni H, Stolke D and Asgari S: Spinal meningiomas: Critical
review of 131 surgically treated patients. Eur Spine J.
17:1035–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Setzer M, Vatter H, Marquardt G, Seifert V
and Vrionis FD: Management of spinal meningiomas: Surgicalresults
and a review of the literature. Neurosurg Focus. 23:E142007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambekar S, Sharma M, Kukreja S and Nanda
A: Complications and outcomes of surgery for spinal meningioma: A
Nationwide Inpatient Sample analysis from 2003 to 2010. Clin Neurol
Neurosurg. 118:65–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chin KR, Seale J and Cumming V: White cord
syndrome. of acute tetraplegia after anterior cervicaldecompression
and fusion for chronic spinal cord compression: a case report. Case
Rep Orthop. 2013:6979182013.
|
|
5
|
Lee KS, Shim JJ, Doh JW, Yoon SM, Bae HG
and Yun IG: Transient paraparesis after laminectomy in a patient
with multi-level ossification of the spinal ligament. J Korean Med
Sci. 19:624–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Orchowski J, Bridwell KH and Lenke LG:
Neurological deficit from a purely vascular etiology after
unilateral vessel ligation during anterior thoracolumbar fusion of
the spine. Spine. 30:406–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taher F, Lebl DR, Cammisa FP, Pinter DW,
Sun DY and Girardi FP: Transient neurological deficit following
midthoracic decompression for severe stenosis: A series of three
cases. Eur Spine J. 22:2057–2061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uematsu Y, Tokuhashi Y and Matsuzaki H:
Radiculopathy after laminoplasty of the cervical spine. Spine.
23:2057–2062. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aghakhani N, David P, Parker F, Lacroix C,
Benoudiba F and Tadie M: Intramedullary spinal ependymomas:
Analysis of a consecutive series of 82 adult cases with particular
attention to patients with no preoperative neurological deficit.
Neurosurgery. 62:1279–1285. discussion 1285-12862008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCormick PC, Torres R, Post KD and Stein
BM: Intramedullary ependymoma of the spinal cord. J Neurosurg.
72:523–532. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahn JS, Lee JK and Kim BK: Prognostic
factors that affect the surgical outcome of the laminoplasty in
cervical spondylotic myelopathy. Clin Orthop Surg. 2:98–104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cramer DE, Maher PC, Pettigrew DB and
Kuntz C IV: Major neurologic deficit immediately after adult spinal
surgery: Incidence and etiology over 10 years at a single training
institution. J Spinal Disord Tech. 22:565–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kou J, Fischgrund J, Biddinger A and
Herkowitz H: Risk factors for spinal epidural hematoma after spinal
surgery. Spine. 27:1670–1673. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Keegan JJ: The cause of dissociated motor
loss in the upper extremity with cervical spondylosis. J Neurosurg.
23:528–536. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakaura H, Hosono N, Mukai Y, Ishii T and
Yoshikawa H: C5 palsy after decompression surgery for cervical
myelopathy: Review of the literature. Spine. 28:2447–2451. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ginsburg HH, Shetter AG and Raudzens PA:
Postoperative paraplegia with preserved intraoperative
somatosensory evoked potentials. Case report. J Neurosurg.
63:296–300. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tribus CB: Transient paraparesis: A
complication of the surgical management of Scheuermann's kyphosis
secondary to thoracic stenosis. Spine. 26:1086–1089. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Young WF and Baron E: Acute neurologic
deterioration after surgical treatment for thoracic spinal
stenosis. J Clin Neurosci. 8:129–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasegawa K, Homma T and Chiba Y: Upper
extremity palsy following cervical decompression surgery results
from a transient spinal cord lesion. Spine. 32:E197–E202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamazaki M, Koda M, Okawa A and Aiba A:
Transient paraparesis after laminectomy for thoracic ossification
of the posterior longitudinal ligament and ossification of the
ligamentum flavum. Spinal Cord. 44:130–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiba K, Toyama Y, Matsumoto M, Maruiwa H,
Watanabe M and Hirabayashi K: Segmental motor paralysis after
expansive open-door laminoplasty. Spine. 27:2108–2115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnston CE II, Happel LT Jr, Norris R,
Burke SW, King AG and Roberts JM: Delayed paraplegia complicating
sublaminar segmental spinal instrumentation. J Bone Joint Surg Am.
68:556–563. 1986.PubMed/NCBI
|
|
23
|
Shan LQ, Ma S, Qiu XC, Zhou Y, Zhang Y,
Zheng LH, Ren PC, Wang YC, Fan QY and Ma BA: Hydroxysafflor Yellow
A protects spinal cords from ischemia/reperfusion injury in
rabbits. BMC Neurosci. 11:982010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wisselink W, Money SR, Crockett DE, Nguyen
JH, Becker MO, Farr GH and Hollier LH: Ischemia-reperfusion injury
of the spinal cord: Protective effect of the hydroxyl radical
scavenger dimethylthiourea. J Vasc Surg. 20:444–491. discussion
449-4501994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan PH: Role of oxidants in ischemic
brain damage. Stroke. 27:1124–1129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Seichi A, Takeshita K, Kawaguchi H,
Nakajima S, Akune T and Nakamura K: Postoperative expansion of
intramedullary high-intensity areas on T2-weighted magnetic
resonance imaging after cervical laminoplasty. Spine. 29:1478–1482.
discussion 14822004. View Article : Google Scholar : PubMed/NCBI
|