Introduction
Mortality due to lung cancer accounts for 17% of all
cancer deaths, and worldwide, there are ∼1,300,000 mortalities per
year due to this disorder. In 2005 in Japan, lung cancer was
responsible for 19% of all cancer mortalities. The 5-year survival
rate for lung cancer is ∼30%. In total, 80% of lung cancer cases
are non-small cell carcinoma; this can be divided into non-squamous
cell carcinoma and squamous cell carcinoma. Determining a
differential diagnosis between non-squamous and squamous cell
carcinoma is necessary for effective pulmonary cancer therapy
(1). For example, administration of
bevacizumab is contraindicated in patients with squamous cell
carcinoma due to the possibility of massive bleeding from the
lungs, while pemetrexed is only administered for non-squamous cell
carcinoma cases, as it only active against non-squamous cell
carcinoma (2). Therefore, in patients
with lung cancer, precise discrimination between squamous and
non-squamous cell carcinoma is vital. However, precise judgment
with a limited number of tumor cells obtained from a lung biopsy
specimen or in cytology smears is challenging. With biopsy
specimens, accurate diagnosis has been demonstrated with the use of
immunostaining by a number of studies (3), however, diagnosis based on morphological
findings of pap smears is performed in the majority of
laboratories. Furthermore, objective methods have been not
established for differential diagnosis.
With immunostaining, no consensus has been reached
with regard to the use of antibodies, and a companion diagnostic
reagent for determining adaptation is essential for
molecular-targeted medicines (4). For
example, it is necessary to investigate overexpression of the HER-2
protein by immunostaining for adaptation of trastuzumab for mammary
or gastric cancer (5). In such cases,
the modality and directions for use of antibodies are strictly
prescribed (6). Additionally, in lung
cancer, an objective method is required for drug selection
(2). Therefore, it is hypothesized
that the use of immunostaining for differentiation is essential
when tumor samples are limited (3).
In the present study, immunostaining of cytological specimens was
evaluated for its ability to clearly distinguish squamous cell
carcinoma from adenocarcinoma. For the present objective
evaluation, scores for thyroid transcription factor 1 (TTF-1),
napsin A, and CK CAM5.2, adenocarcinoma markers were expressed with
positive values, while those for p40, cytokeratin (CK) 5/6, and
CK5, squamous cell carcinoma markers, were expressed with negative
values. This was performed for the purpose of dividing the subjects
into two groups by immunostaining. A summed score was determined
for each case, and histograms were produced to assess which
combination was the most effective in differentiating squamous cell
carcinoma from adenocarcinoma.
Materials and methods
Samples
Consecutive tumor imprint smears were collected from
70 patients between 2011 and 2012 (49 male, 21 females; mean age,
70 years; age range, 51–86 years) with non-small cell carcinoma of
the lung, who had undergone resection at Tsuchiura Kyodo General
Hospital (Tsuchiura, Japan) from 2011–2012. These were used
consecutively without exception. Histological findings revealed
that the samples comprised 40 adenocarcinomas, 21 squamous cell
carcinomas and 9 samples from other types of non-small cell
carcinoma (4 large cell carcinomas, 1 pleomorphic carcinoma and 4
adenosquamous carcinomas). The study was approved by the ethics
committee of Tsuchiura Kyodo General Hospital and written informed
consent was obtained from the patient or the patient's family.
Immunostaining
Antibodies aganist TTF-1, CK CAM5.2, napsin A, p40,
CK5/6 and CK5 were used (Table I).
Immunostaining was performed using a Novolink Polymer Detection
System (Leica Microsystems; Tokyo Japan) following the appropriate
pretreatment as listed in Table I.
All procedures were performed as according to the manufacturer's
instructions, and the primary antibodies were incubated with the
samples at room temperature for 3 h.
 | Table I.List of antibodies used. |
Table I.
List of antibodies used.
| Antigen | Clone | Catalog no. | Supplier | Dilution | Pretreatment |
|---|
| TTF-1 | SPT24 | NCL-TTF1 | Leica
Microsystemsa | 150X | Microwave in pH 6.0
buffer |
| CAM5.2 | CAM5.2 | 349205 | Becton
Dickinsona | 1X | Microwave in pH 6.0
buffer |
| Napsin A | TMU-Ad02 | 10221 | IBLb | 50X | Microwave in pH 6.0
buffer |
| p40 | poly | 418101 | Nichirei
Bio-sciencea | 1X | Microwave in pH 6.0
buffer |
| CK5 | XM26 | NCL-L-CK5 | Leica
Microsystemsa | 100X | Microwave in pH 6.0
buffer |
| CK5/6 | D5/16B4 | M7237 | DAKOa | 75X | Autoclave in pH 9.0
buffer |
Scoring for differential
diagnosis
The results of the immunostaining were scored based
on positive area and intensity. The expression level was
semi-quantitatively evaluated using a combination of proportion and
intensity scores from 0–3 as follows: Score 0, negative; score 1,
slightly positive, <10%; score 2, intermediate, ≥10% and ≤90%;
score 3, strongly positive, >90% cells were positively stained.
The scores for TTF-1, napsin A, and CAM5.2 were expressed with
positive values, while those for p40, CK5/6, and CK5 were expressed
with negative values. The score for each case was summed and
evaluated. For example, when a case of adenocarcinoma was scored as
TTF-1=2, CAM5.2=3, p40=0 and CK5=0, the total score would be 5. The
various combinations of CAM5.2, napsin A, CK5/6, and CK5 were
compared with regard to which was the most effective for
determining an accurate diagnosis. Histograms were produced from
all cases, and the mean and median scores were calculated to assess
which combination was able to most effectively differentiate
squamous cell carcinoma from adenocarcinoma. To determine
sensitivity and specificity, a score of >1 was considered to be
positive for adenocarcinoma markers, and a score of <-1 was
considered to be positive for squamous cell carcinoma markers.
Validation with bronchial lavage
fluid
The ability of each combination to precisely
differentiate between adenocarcinoma and squamous cell carcinoma
was examined using preoperative bronchial lavage fluid samples from
19 cases. In each case, the surgery was performed following
bronchial lavage cytology, and a final histological diagnosis was
determined. Smears were produced from the remaining bronchial
lavage fluid, and immunostaining and scoring were conducted as
described previously.
Results
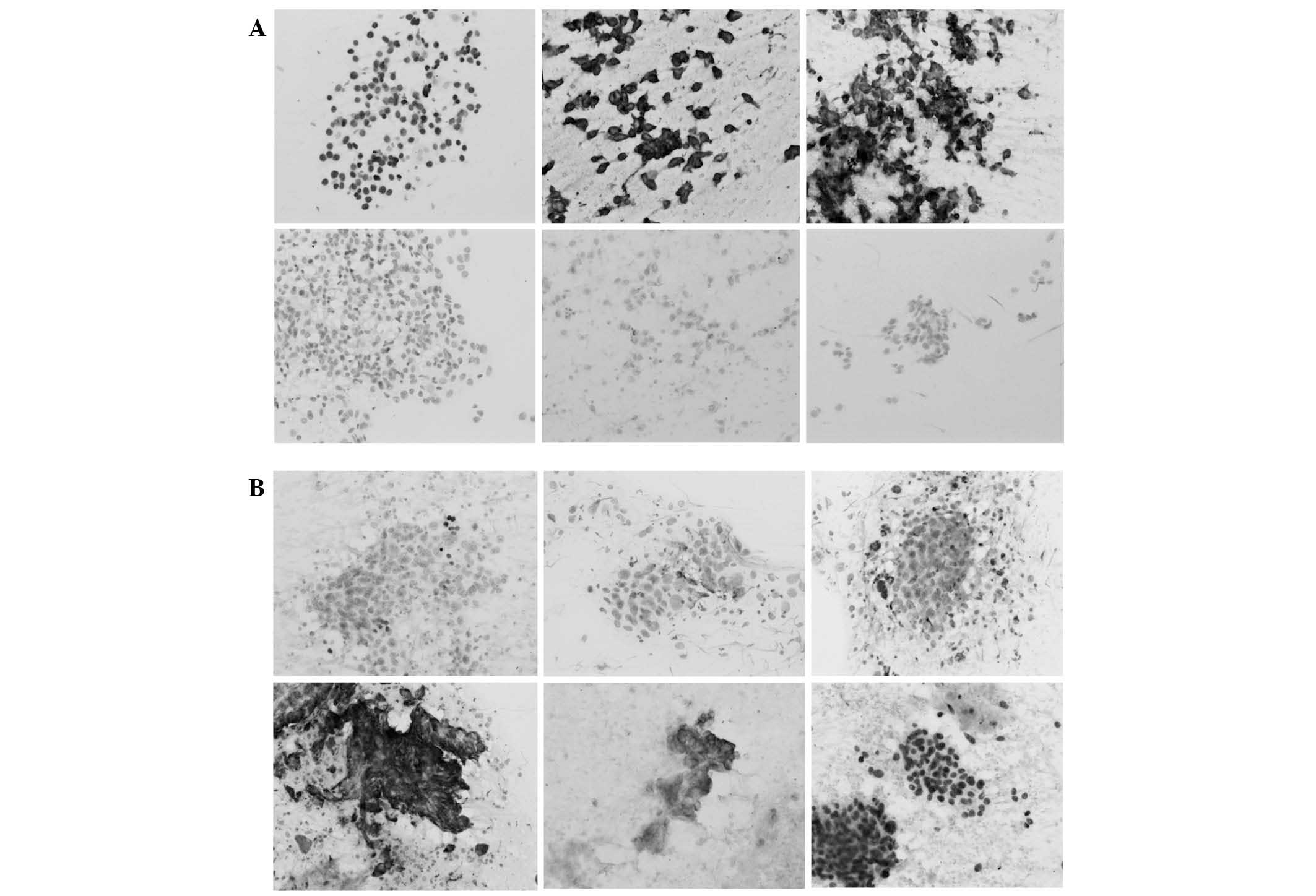
Immunostaining revealed that TTF-1 and p40 reacted
with the tumor cell nuclei, while the remaining antibodies reacted
with the cytoplasm (Fig. 1A and B).
Napsin A reacted strongly to histiocytes in addition to tumor
cells, leading to confusion in the results. Immunostaining results
for sensitivity in adenocarcinomas were 88% for TTF-1, 85% for
napsin A, and 100% for CAM5.2. In squamous cell carcinomas,
sensitivity was 90% for p40, 86% for CK5, and 76% for CK5/6.
Specificity for each of these molecules was determined to be 100%,
95%, 43%, 98%, 100% and 95% respectively. A total of four different
combinations, using either CAM5.2 or napsin A, and either CK5/6 or
CK5, were assessed, and the mean and median were calculated in
order to determine which combination provided the most precise
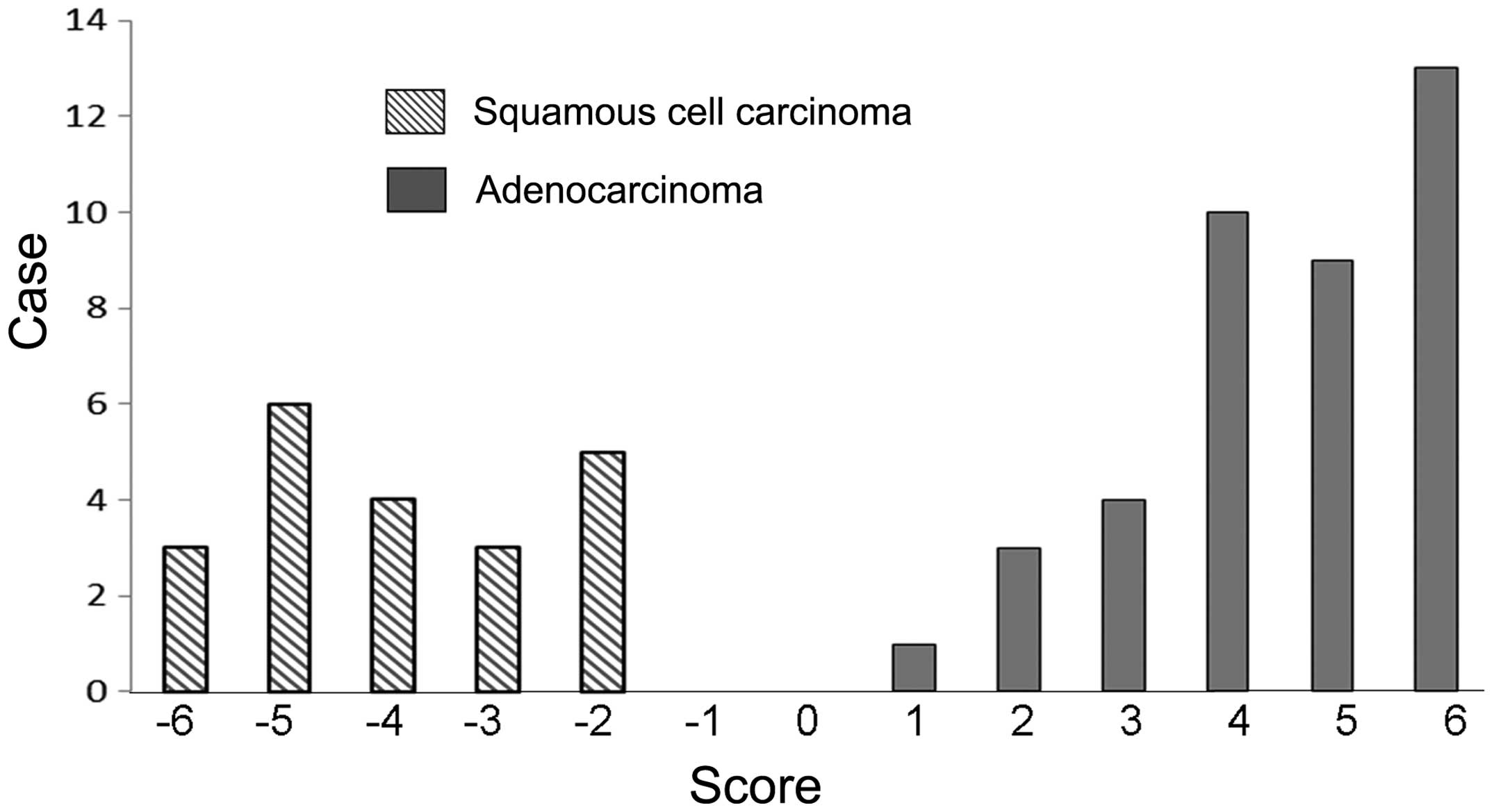
differentiation between the tissue types (Table II). The combination that exhibited
the greatest difference between adenocarcinoma and squamous cell
carcinoma was TTF-1+napsin A (mean, 4.55) and p40+CK5 (mean,
−3.95); this combination was considered to possess the strongest
judgment ability (Fig. 2). Using this
combination, all adenocarcinomas scored >1 and all squamous cell
carcinomas scored <-2. The majority of cases of other types of
non-small cell carcinomas, including large cell carcinoma, were
distributed between scores of 2 and −1 (Table III).
 | Table II.Mean and mode scores obtained using
each combination of antibodies. |
Table II.
Mean and mode scores obtained using
each combination of antibodies.
|
| Adenocarcinoma | Squamous cell
carcinoma | Adenocarcinoma minus
squamous cell carcinoma |
|---|
|
|
|
|
|
|---|
| Combination | Mean | Mode | Mean | Mode | Mean | Mode |
|---|
| TTF-1, napsin A, p40,
CK5 | 4.55 | 6 | -3.95 | -5 | 8.5 | 11 |
| TTF-1, CAM5.2, p40,
CK5/6 | 4.4 | 4 | -3.1 | -3 | 7.5 | 7 |
| TTF-1, CAM5.2, p40,
CK5 | 4.88 | 6 | -3.1 | -3 | 7.98 | 9 |
| TTF-1, napsin A, p40,
CK5/6 | 4.08 | 4 | -3.91 | -4 | 7.99 | 8 |
 | Table III.Score of large cell carcinomas and
adenosquamous carcinomas using antibodies against TTF-1, napsin A,
p40 and CK5. |
Table III.
Score of large cell carcinomas and
adenosquamous carcinomas using antibodies against TTF-1, napsin A,
p40 and CK5.
|
|
| Score |
|---|
|
|
|
|
|---|
| Case | Histology | TTF-1 | Napsin A | p40 | CK5 | Total |
|---|
| 1 | Large cell
carcinoma | 0 | 0 | 0 | 1 | -1 |
| 2 | Large cell
carcinoma | 0 | 0 | 0 | 1 | -1 |
| 3 | Large cell
carcinoma | 0 | 0 | 0 | 2 | -2 |
| 4 | Large cell
carcinoma | 0 | 2 | 3 | 0 | -1 |
| 5 | Pleomorphic
carcinoma | 0 | 1 | 0 | 2 | -1 |
| 6 | Adenosquamous
carcinoma | 1 | 0 | 0 | 0 | 1 |
| 7 | Adenosquamous
carcinoma | 1 | 1 | 0 | 1 | 1 |
| 8 | Adenosquamous
carcinoma | 1 | 2 | 1 | 1 | 1 |
| 9 | Adenosquamous
carcinoma | 3 | 1 | 0 | 0 | 4 |
The scoring system was subsequently utilized in the
same manner using preoperative bronchial lavage fluid samples
obtained from 19 cases in order to determine whether this
combination was effective. Of the 19 cases, 12 exhibited a score of
>1 and were determined to be adenocarcinoma, while the remaining
7 cases exhibited scores of <-2 and were therefore determined to
be squamous cell carcinoma. The results were consistent with the
final postoperative histological diagnosis in all cases.
Discussion
In the present study, immunostaining of cytological
specimens was assessed with regard to its ability to differentiate
squamous cell carcinoma from adenocarcinoma specimens. The
differential diagnosis of non-squamous and squamous cell carcinoma
is necessary for effective pulmonary cancer therapy specific to the
tumor subtype, and a number of studies have been conducted by
various institutions with this aim (3,7).
Investigations of micro-tissue array sections of lung cancer have
shown the value of TTF-1, napsin A, p63 and CK5/6 (3). Yanagita et al (7) reported findings obtained using a
cocktail of TTF-1, napsin A, p63, and CK14 antibodies. In this
report, the combination of antibodies was useful fordifferential
diagnosis; in particular, TTF-1 and napsin A were effective in
determining a differential diagnosis. However, to date, no
consensus has been reached with regard to the optimum method for
the differential diagnosis of lung carcinomas.
TTF-1 is a marker of lung adenocarcinoma and is
routinely used for the pathological diagnosis of metastasizing
pulmonary adenocarcinoma (8,9). We have previously reported that TTF-1,
which reacts in the nucleus, is useful for observations of
cytological specimens as the reactions in the nucleus of tumor may
identify the cytology of specimen (10).
The expression of napsin A has been reported to be
high in lung adenocarcinoma samples (11), while an additional study noted that a
large quantity of tissue array samples, with the exception of lung
and renal cancer specimens, had extremely low expression (12). Furthermore, the napsin A expression
rate was observed to be higher than that of TTF-1 in lung
adenocarcinomas. However, the use of napsin A requires particular
attention in smears due to its strong expression in histiocytes
(13). Shibuki et al (14) also reported that TTF-1 and napsin A
were beneficial for improving the diagnostic accuracy of lung
adenocarcinoma and squamous cell carcinoma with cytological
specimens. In the present study, we distinguish adenocarcinoma
cells from histiocytes by reaction of TTF-1 and/or morphological
findings.
p63 has generally been used as a marker of squamous
cell carcinoma (15), and is
considered to be useful and highly sensitive marker for routine
pathological diagnosis, as the vast majority of squamous cell
carcinoma specimens are positive for this antigen (16). Furthermore, its use is advantageous
with cytological specimens due to its expression in the nucleus
(17). p63 is expressed in poorly
differentiated adenocarcinomas, and p40, an isoform of p63, has
also been investigated (18). As p40
does not react in the majority of adenocarcinomas, in contrast with
p63, it has been proposed to be a specific marker of squamous cell
carcinoma (18).
High-molecular weight CK has also been used as a
marker of squamous cell carcinoma, and may be detected by the
antibody clone 34βE12, which recognizes CK subtypes 1, 5, 10, and
14. However, this antibody only reacts in approximately one-third
of adenocarcinomas, dependent upon the extent of histological
differentiation (10). Thus,
antibodies against CK5/6 and CK5, which exhibit no reaction in the
vast majority of adenocarcinomas, are currently used for the
detection of high-molecular weight CK. In particular, CK5 has been
demonstrated to be a reliable marker of triple-negative breast
carcinoma (19). In a study of lung
cancer, Sethi et al (20)
described its utility in diagnosis using cell blocks produced from
fine needle aspiration specimens. In this report, both sensitivity
and specificity for CK5 for squamous cell carcinoma were 100%.
As our pilot study revealed that the expression of
p40 was more specific for squamous cell carcinoma compared with
p63, p40 was used in the present experiments (21). The value of TTF-1 as a distinct marker
of adenocarcinomas, using immunocytochemistry, has been
demonstrated in a number of reports (10,14). In
the present study, the expression of p40 and TTF-1 was investigated
in conjunction with the expression of CAM5.2, napsin A, CK5/6 and
CK5, a combination that has been shown to exhibit effective
diagnostic properties (3,10). As the results based on conventional
tests of sensitivity and specificity were considered to be
ineffective in determining a differential diagnosis, a new method
of scoring using objective values was evaluated. The results
indicated that the optimal combination comprised the antibodies
against TTF-1, napsin A, p40, and CK5. In addition, all six markers
were assessed for their diagnostic value when used simultaneously,
however, this reduced the judgment ability (data not shown). As the
four selected antibodies are utilized in similar antigen retrieval
methods, transcription of smears is unnecessary, and double
immunostaining is easily conducted. This method demonstrated
complete judgment ability in a prospective examination using
preoperative bronchial lavage fluid, thus it is proposed to be a
beneficial technique for specimens with a limited number of tumor
cells.
The four antibodies used in the present study were
able to distinguish even poorly differentiated cases of lung
carcinoma. However, certain challenges were evident. For example,
the score was reduced in cases of mucinous adenocarcinoma that were
negative for TTF-1. It may be useful to examine new markers,
including mucin 5B, in this regard (22). In addition, the investigation of
specific markers for large cell carcinoma is required. Histological
findings and revisions of other methods are necessary to
discriminate cases with values between 1 and −2 based on the
scoring system developed in the current study.
In conclusion, the present study assessed
immunostaining scoring as a means to differentiate between squamous
cell carcinoma and adenocarcinoma specimens, in order to determine
the most effective combination of antibodies. Antibodies against
TTF-1, napsin A, p40, and CK5 provided the best combination.
Although numerical values obtained with the present method are
unable to differentiate all cases, it may be useful to create an
index to aid with diagnosis when a limited number of tumor cells
are available, as the majority of adenocarcinoma samples had scores
>2, and the majority of squamous cell carcinoma samples scored
<-2.
References
|
1
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–40. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whithaus K, Fukuoka J, Prihoda TJ and
Jagirdar J: Evaluation of napsin A, cytokeratin 5/6, p63, and
thyroid transcription factor 1 in adenocarcinoma versus squamous
cell carcinoma of the lung. Arch Pathol Lab Med. 136:155–162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker RA, Handy A, Pinder SE, et al:
Current issues in diagnostic breast pathology. J Clin Pathol.
65:771–785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–12. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennacchia I, Carbone A, Di Cerbo A, et
al: 2013 ASCO/CAP updated guidelines for human epidermal growth
factor receptor 2 testing: Impact on routine practice. Breast. Feb
19–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yanagita E, Imagawa N, Ohbayashi C and
Itoh T: Rapid multiplex immunohistochemistry using the 4-antibody
cocktail YANA-4 in differentiating primary adenocarcinoma from
squamous cell carcinoma of the lung. Appl Immunohistochem Mol
Morphol. 19:509–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ng WK, Chow JC and Ng PK: Thyroid
transcription factor-1 is highly sensitive and specific in
differentiating metastatic pulmonary from extrapulmonary
adenocarcinoma in effusion fluid cytology specimens. Cancer.
96:43–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yatabe Y, Mitsudomi T and Takahashi T:
TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol.
26:767–773. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikeda S, Funakoshi N, Suzuki K, et al: A
study of double immunohistochemical staining for differential
diagnosis of adenocarcinoma and squamous cell carcinoma in
respiratory cytological smears. J Jpn Clin Cytol. 42:5–9. 2003.[(In
Japanese)]. View Article : Google Scholar
|
|
11
|
Hirano T, Auer G, Maeda M, et al: Human
tissue distribution of TA02, which is homologous with a new type of
aspartic proteinase, napsin A. Jpn J Cancer Res. 91:1015–1021.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Turner BM, Cagle PT, Sainz IM, et al:
Napsin A, a new marker for lung adenocarcinoma, is complementary
and more sensitive and specific than thyroid transcription factor 1
in the differential diagnosis of primary pulmonary carcinoma:
Evaluation of 1674 cases by tissue microarray. Arch Pathol Lab Med.
136:163–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kakizaki Y, Nakagawa M, Honda T and Ishii
E: Immunohistochemical study using anti-Napsin A antibody for
primary lung adenocarcinoma. J Jpn Clin Cytol. 46:120–121.
2007.[(In Japanese)].
|
|
14
|
Shibuki Y, Tsuta K, Nomoto K, et al:
Immunocytochemical study of specific immunohistochemical markers
for primary lung adenocarcinoma: Surfactant apoprotein A, Napsin A,
Thyroid transcription factor-1. J Jpn Clin Cytol. 45:6–11.
2006.[(In Japanese)]. View Article : Google Scholar
|
|
15
|
Kim DH and Kwon MS: Role of fine needle
aspiration cytology, cell block preparation and CD63, P63 and CD56
immunostaining in classifying the specific tumor type of the lung.
Acta Cytol. 54:55–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uke M, Rekhi B, Ajit D and Jambhekar NA:
The use of p63 as an effective immunomarker in the diagnosis of
pulmonary squamous cell carcinomas on de-stained bronchial lavage
cytological smears. Cytopathology. 21:56–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aikawa E, Kawahara A, Hattori S, et al:
Comparison of the expression levels of napsin A, thyroid
transcription factor-1, and p63 in nonsmall cell lung cancer using
cytocentrifuged bronchial brushings. Cancer Cytopathol.
119:335–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bishop JA, Teruya-Feldstein J, Westra WH,
et al: p40 (ΔNp63) is superior to p63 for the diagnosis of
pulmonary squamous cell carcinoma. Mod Pathol. 25:405–415. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhargava R, Beriwal S, McManus K and Dabbs
DJ: CK5 is more sensitive than CK5/6 in identifying the
‘basal-like’ phenotype of breast carcinoma. Am J Clin Pathol.
130:724–730. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sethi S, Geng L, Shidham VB, et al: Dual
color multiplex TTF-1 + Napsin A and p63 + CK5 immunostaining for
subcategorizing of poorly differentiated pulmonary non-small
carcinomas into adenocarcinoma and squamous cell carcinoma in fine
needle aspiration specimens. Cytojournal. 9:102012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nonaka D: A study of ΔNp63 expression in
lung non-small cell carcinomas. Am J Surg Pathol. 36:895–9. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Wang X, Ao M, et al: Aberrant
Mucin5B expression in lung adenocarcinomas detected by iTRAQ
labeling quantitative proteomics and immunohistochemistry. Clin
Proteomics. 10:152013. View Article : Google Scholar : PubMed/NCBI
|