Introduction
Glioblastomas (GBMs) are the most common and
aggressive malignant primary brain tumors in humans. GBM accounts
for ∼51% of all primary gliomas (1),
with an annual incidence of 2–3 cases per 100,000 individuals in
Europe and North America (2). GBMs
are usually diagnosed by magnetic resonance imaging, and confirmed
by biopsy (3). Certain genetic
signatures, including TP53, IDH1 or TPEN, are hypothesized to
present potential biomarkers for GBM (4). At present, the standard treatment for
newly diagnosed glioblastoma patients is surgical resection,
followed by a combination of chemotherapy and radiotherapy
(5,6).
The prognosis of patients with glioblastoma is poor, and the median
survival time is 14.6 months (6).
GBMs contain a subpopulation of cancer stem cells (CSCs) that are
able to self-renew in vitro and initiate new tumors in
vivo (7,8). CSCs may also mediate radio- and
chemo-resistance in GBMs (7,8).
Previous studies have hypothesized that the
transmembrane glycoprotein, CD133 (also known as prominin-1), is a
CSC marker in malignant brain tumors (9,10). In
addition, a number of studies have revealed that CD133+
cells, but not CD133− cells, exhibit stem cell-like and
tumor-initiating properties (9,10). In
addition, a number of studies have shown that CD133 closely
correlates with tumor size, a worse prognosis, higher rates of
lymph node metastasis and resistance to adjuvant therapies
(11–13). Therefore, decreasing the expression of
CD133 or exposing the protein to certain antibodies, such as AC133,
may inhibit tumor cell growth, cell motility, spheroid-forming
capacity and tumorigenic ability (14,15).
However, other studies have obtained contradictory results
(16–20). Further controversial results include
inconsistent findings with regard to the prognostic value and
distribution patterns of CD133 (9,10,21–28). These
controversies may be due to the detection limits of currently
available anti-CD133 antibodies (20).
The aim of the present study was to advance
understanding with regard to the significance of CD133 in GBM tumor
biology. Thus, in the current study, novel anti-human CD133
monoclonal antibodies (mAbs) were generated using two recombinant
extracellular domains of human CD133. In addition, the expression
levels of CD133 protein in U87 glioblastoma cells was detected
using the produced antibodies.
Materials and methods
Cell culture and transfection
Human colonic carcinoma Caco-2 cells, human
glioblastoma U87 cells and human embryonic kidney (HEK) 293 cells
were obtained from the American Type Culture Collection (Manassas,
VA, USA). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco Life Technologies, Grand Island, NY, USA)
supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco Life
Technologies), 1% penicillin-streptomycin (MP Biomedicals, Santa
Ana, CA, USA) and 1% L-glutamate (MP Biomedicals). In addition,
mouse myeloma cells, SP2/0 (American Type Culture Collection), were
cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA) supplemented
with 10% FBS. The cell lines were maintained in a humidified
atmosphere of 5% CO2 at 37°C. The standard calcium
phosphate method (29) was used to
transfect HEK 293 cells. The medium was replaced at 4 h
post-transfection and the cells were analyzed at 24–48 h
post-transfection.
Plasmid construction
The cDNA coding CD133 was isolated from the MegaMan
Human Transcriptome Library (Agilent Technologies, Santa Clara, CA,
USA) by polymerase chain reaction (PCR) using forward primer,
5′-aggatcc atggccctcgtactcggct-3′, and reverse primer,
5′-tatcgatttaatgttgtgatgggcttg-3′.
The amino acid sequences of CD133 ectodomain 1
(amino acids 171–420) and CD133 ectodomain 2 (amino acids 507–716)
were selected from the ectodomains of CD133 based on its reported
structure (Fig. 1A) (30). CD133 ectodomains 1 and 2 were
amplified using the following primers: CD133 ectodomain 1 forward,
5′-ccatcgata tga gtc gga aac tgg cag atag-3′, and reverse,
5′-gctctagat tac tga ata gga aga cgc tgag-3′; CD133 ectodomain 2
forward, 5′-ccatcgata tgt gtg aac ctt aca cga gca-3′, and reverse,
5′-gactagttt agt tct gag caa aat cca gag-3′.
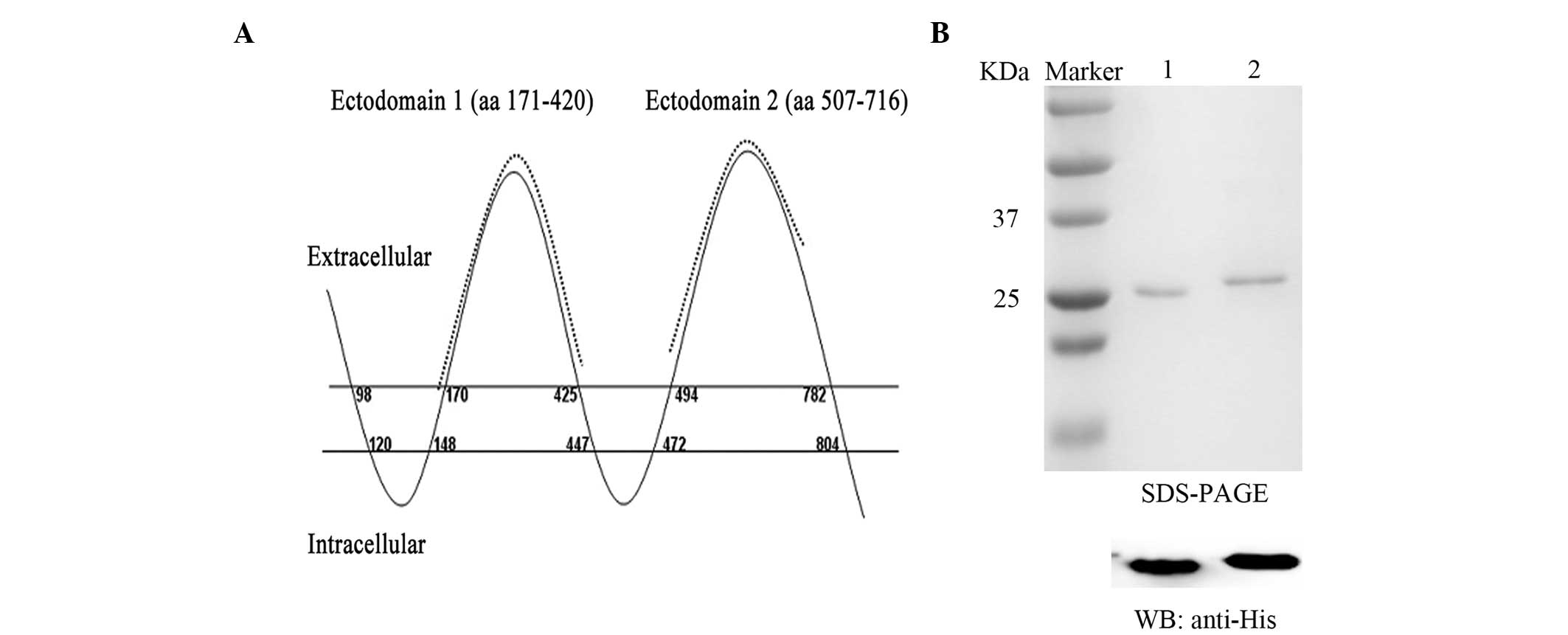 | Figure 1.CD133 antigens used for mAb
production. (A) Topological map of CD133 protein. Recombinant
chimeric CD133 antigens, consisting of aa residues 171–420 and
507–716 (dotted line), were generated. (B) The two antigens, each
tagged by an N-terminal 6xHis-tag, were expressed in E. coli
and purified. The recombinant antigens were further verified by WB
analysis with mouse anti-His mAb. Lane 1, ectodomain 1, Lane 2,
ectodomain 2. CD133, prominin-1; mAb, monoclonal antibody; aa,
amino acid; WB, Western blot. |
PCR was performed in a 50 µl reaction volume,
consisting of 1 µl cDNA template, 10 mM dNTPS (Takara Biotechnology
Co., Ltd., Dalian, China), and 1 U LA Taq DNA polymerase (Takara
Biotechnology Co., Ltd.) under the following conditions: 30 cycles
of 94°C for 30 sec, 98°C for 10 sec, 55°C for 15 sec and 72°C for 1
min, followed by a 10 min extension step at 72°C. All the PCR
products were cloned into a pGEM-T easy vector (Promega
Corporation, Madison, WI, USA). The positive clones were identified
by double restriction enzyme digestion with BamHI/ClaI for full
length CD133, ClaI/XbaI for CD133 ectodomain 1 and ClaI/SpeI for
CD133 ectodomain 2, and sequencing was performed by the Beijing
Genomics Institute (Shenzhen, China). All restriction enzymes were
obtained from New England Biolabs (Ipswich, MA, USA). The DNA
sequences of CD133 ectodomain 1 and CD133 ectodomain 2 were
subsequently inserted into a prokaryotic expression vector, pRSET,
including an N-terminal 6xHis-tag.
Establishment of a stable U87 cell
line expressing full-length CD133
The CD133 full-length cDNA was inserted into a
XhoI/NotI-digested, modified lentiviral vector with
an mCherry reporter gene and a hygromycin resistance gene
(designated as L-mCherry-Hygro-CD133; Addgene Inc., Cambridge, MA,
USA). 293T cells were co-transfected, using the calcium phosphate
method, with the L-mCherry-Hygro-CD133 plasmid, pVPack-vesicular
stomatitis virus G (VSV-G) vector (Addgene, Cambridge, MA, USA)
encoding the VSV-G surface gene, and the psPAX2 vector (Addgene),
which provides the env and gag genes. At 4 h after transfection,
the medium was replaced by fresh DMEM. Next, lentiviral
supernatants were enriched to transduce the U87 cells in the
presence of 10 µg/ml polybrene (Sigma-Aldrich, St. Louis, MO, USA).
At 24 h after transfection, the transduced cells were selected
using 750 µg/ml hygromycin and red fluorescence from the mCherry
reporter gene. The resultant stable cell line was designated as
U87-CD133 overexpression.
CD133 antigen generation
The pRSET vector expressing CD133 ectodomain 1 or 2
was transformed into BL21 (DE3) E. coli. The transformed
bacteria were grown in lysogeny broth medium and were induced by
0.25 mM isopropyl β-D-1-thiogalactopyranoside (MP Biomedicals) for
4 h. Subsequently, the cells were precipitated, resuspended in cell
lysis buffer (50 mM NaH2PO4, 10 mM Tris-HCl,
250 mM NaCl, 5 mg/ml lysozyme; pH 8.0; Takara Biotechnology Co.,
Ltd.) and disrupted by two cycles of sonication on ice at 900 W.
The protein expression was analyzed by 12% SDS-PAGE using Coomasie
blue (MP Biomedicals) staining and confirmed by western blot
analysis. Recombinant proteins with the 6xHis-tag were purified
using nickel-nitrilotriacetic acid beads.
Immunization and mAb production
All the animal experiments used in the study were
approved by the Institutional Animal Care and Use Committee of
Shaanxi Normal University (Xi'an, China). In total, 20 female
Balb/c mice (age, 6 weeks) were injected subcutaneously with
recombinant protein CD133 ectodomain 1-6xHis or CD133 ectodomain
2-6xHis, which were emulsified in complete Freund's adjuvant
(Sigma-Aldrich). Next, the mice were immunized with the same
recombinant protein three times until a sufficient serum titer
against CD133 was obtained, as analyzed by enzyme-linked
immunosorbent assay (ELISA). Mice with a high serum titer were
selected and boosted a fourth time prior to cell fusion.
The spleen cells of the immunized Balb/c mice were
isolated and fused with SP2/0 myeloma cells using polyethylene
glycol. Following fusion, the cells were cultured in
hypoxanthine-aminopterin-thymidine (HAT) selection medium (Gibco
Life Technologies). The HAT selection medium was refreshed every
2–3 days, and the selection medium was omitted after 6 days.
Following a further 5 days, culture supernatants were screened for
the production of antibodies against CD133 ectodomains 1 and 2
using ELISA.
Western blot analysis
Caco-2, U87 and U87-CD133 overexpression cells were
lysed in radioimmunoprecipitation assay lysis buffer (Shaanxi
Pioneer Biotech Co., Ltd., Xi'an, China). The samples were
subjected to 12% SDS-PAGE and then blotted onto methanol-pretreated
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were incubated with mouse anti-human CD133
ectodomain mAbs (dilution, 1:300) for 90 min at room temperature.
The rabbit anti-human CD133 mAb C24B9 (dilution, 1:1000; cat. no.
3663; Cell Signaling Technology, Danvers, MA, USA), which detects
the full-length CD133 protein in Caco-2 cells (20), was used as a positive control. Next,
the membranes were washed using phosphate-buffered saline
(PBS)/Tween-20 (PBST) and incubated with horseradish peroxidase
(HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) polyclonal
antibody (dilution, 1:10,000; cat. no. ZB-2305; Beijing Zhongshan
Jinqiao Biological Technology Ltd., Beijing, China) or
HRP-conjugated goat anti-rabbit IgG polyclonal antibody (dilution,
1:10,000; cat. no. ZB-2301; Beijing Zhongshan Jinqiao Biological
Technology Ltd.). The membranes were further washed with PBST and
visualized using the SuperSignal West Pico chemiluminescent
substrate (Thermo Fisher Scientific, Hudson, NH, USA).
Fluorescent immunocytochemistry
HEK 293 cells transfected with CD133 full-length
cDNA were fixed with 4% paraformaldehyde in PBS for 15 min,
followed by washing with PBS. The fixed cells were then incubated
with mouse anti-human CD133 ectodomain mAbs (dilution, 1:50) for 2
h at room temperature. Following three washes with PBS, the cells
were incubated with biotin-conjugated goat anti-mouse IgG
polyclonal antibody (dilution, 1:100; cat. no. SA1072; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) for 30 min at 37°C. The
cells were further washed and incubated with streptavidin biotin
complex (SABC)-Cy3 (dilution, 1:100; cat. no. SA1072; Wuhan Boster
Biological Technology, Ltd.) for 15 min at 37°C. Finally, the cells
were briefly washed with PBS and visualized under a Zeiss Axio
Observer Z1 inverted fluorescence microscope (Zeiss, Oberkochen,
Germany).
Immunohistochemistry
U87 cells were fixed with 4% paraformaldehyde in PBS
for 15 min. The cells were subsequently washed using PBS, blocked
in normal horse serum (dilution, 1:200; cat. no. PK-6102; Vector
Laboratories, Burlingame, CA, USA) and incubated with mouse
anti-human CD133 ectodomain mAbs (dilution, 1:50) at 4°C overnight.
After washing with PBS, the cells were incubated with
biotin-conjugated horse anti-mouse IgG polyclonal antibody
(dilution, 1:100; cat. no. PK-6102; Vector Laboratories) for 30 min
at 37°C. The cells were rinsed with PBS prior to incubation with
HRP-conjugated SABC (dilution, 1:100; cat. no. PK-6102; Vector
Laboratories) for 30 min at 37°C. The 3,3′-diaminobenzidine
chromogen (cat. no. ZLI-9017; Beijing Zhongshan Jinqiao Biological
Technology Ltd.) was used for visualization under an inverted
fluorescence microscope (DMIL LED; Leica, Wetzlar, Germany).
Cell proliferation assay
An MTT assay was performed according to a previously
reported procedure (31), with minor
modifications. Briefly, cells in 100 µl medium were seeded in
96-well flat-bottom plates and supplemented with 50 µl mAbs at
various dilutions. Following a three-day incubation period, 15 µl
MTT solution (5 mg MTT/ml of distilled water) were added to each
well, followed by incubation for a further 4 h. Following removal
of the supernatant, 150 µl dimethyl sulfoxide was used to dissolve
the MTT crystals in the wells. Finally, the plates were subjected
to cell viability detection using an ELISA reader (Multiskan EX;
Thermo Fisher Scientific) at a wavelength of 570 nm. Optical
density (OD) at 570 nm [absorbance value at 570nm (OD570)] was
examined to analyze cell proliferation.
Statistical analysis
All data are presented as the mean + standard
deviation (SD) from at least three different experiments. One way
analysis of variance was used to investigate differences between
the experimental and control groups. All statistical analyses were
performed using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Generation and purification of CD133
antigens
CD133 amino acid fragments 171–420 (CD133 ectodomain
1) and 507–716 (CD133 ectodomain 2) were selected as the CD133
antigens. The locations of these two amino acid fragments in the
CD133 protein are illustrated in Fig.
1A. The two CD133 ectodomain antigens, which were tagged by an
N-terminal 6xHis-tag, were expressed in E. coli, purified
and analyzed by SDS-PAGE (Fig. 1B).
The purified recombinant proteins were further verified using
western blot analysis with mouse anti-His mAb (Fig. 1B).
Generation and validation of CD133
mAbs
The purified antigens were injected into Balb/c mice
to generate monoclonal anti-CD133 antibodies using standard
hybridoma technology. ELISA was used to screen cell culture
supernatants from the resulting hybridoma clones for the production
of antibodies against recombinant CD133. At least six positive
stable clones secreting anti-CD133 antibodies were obtained (data
not shown). The positive hybridoma clones were validated by western
blot analysis using prokaryotically-expressed CD133 ectodomain 1
and CD133 ectodomain 2 recombinant proteins. Four hybridoma clones
against CD133 ectodomain 1 and two clones against ectodomain 2 were
identified. HEK 293 cells were transfected with an expression
plasmid encoding the human CD133 full-length cDNA and stained
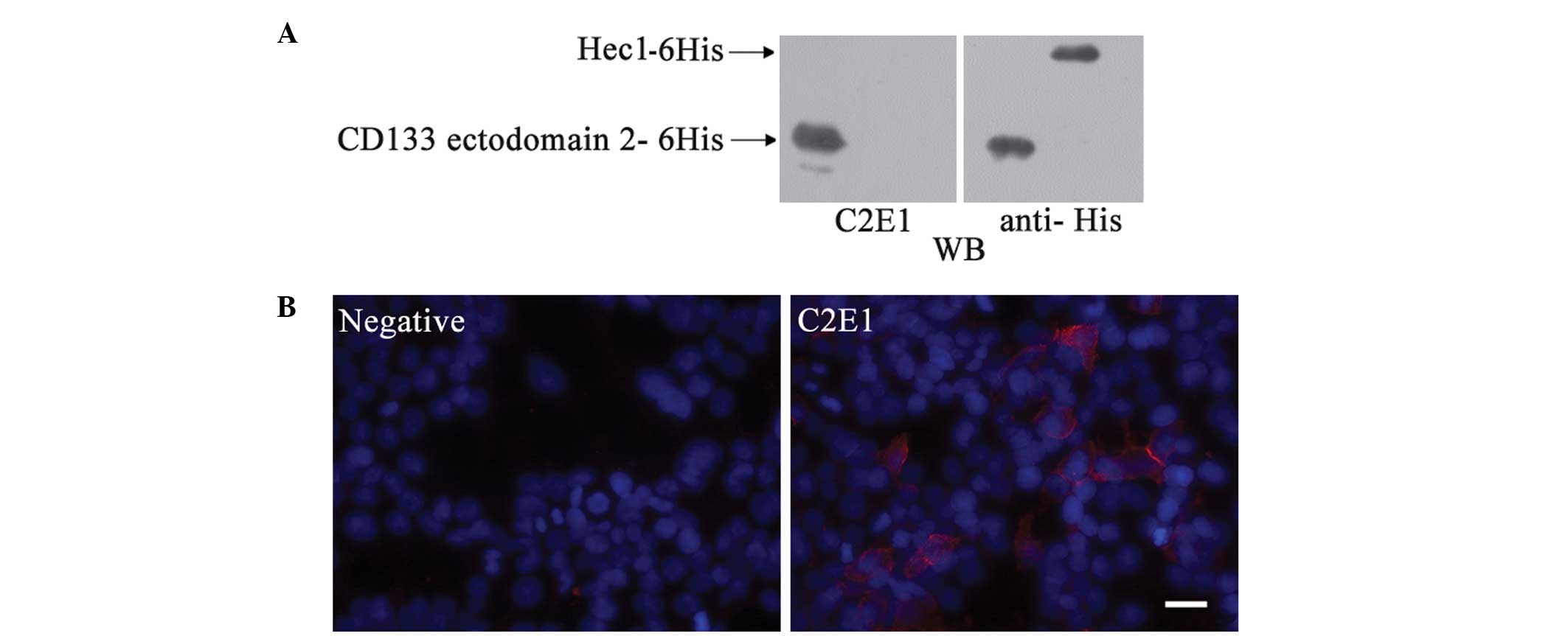
immunohistochemically with different hybridoma clones. One of the
hybridoma clones, C2E1, which recognized the recombinant CD133
ectodomain 2 specifically, exhibited cell membrane and cytoplasmic
staining in the transfected HEK 293 cells (Fig. 2).
Novel anti-CD133 mAb detects CD133
expression in the glioblastoma U87 cell line
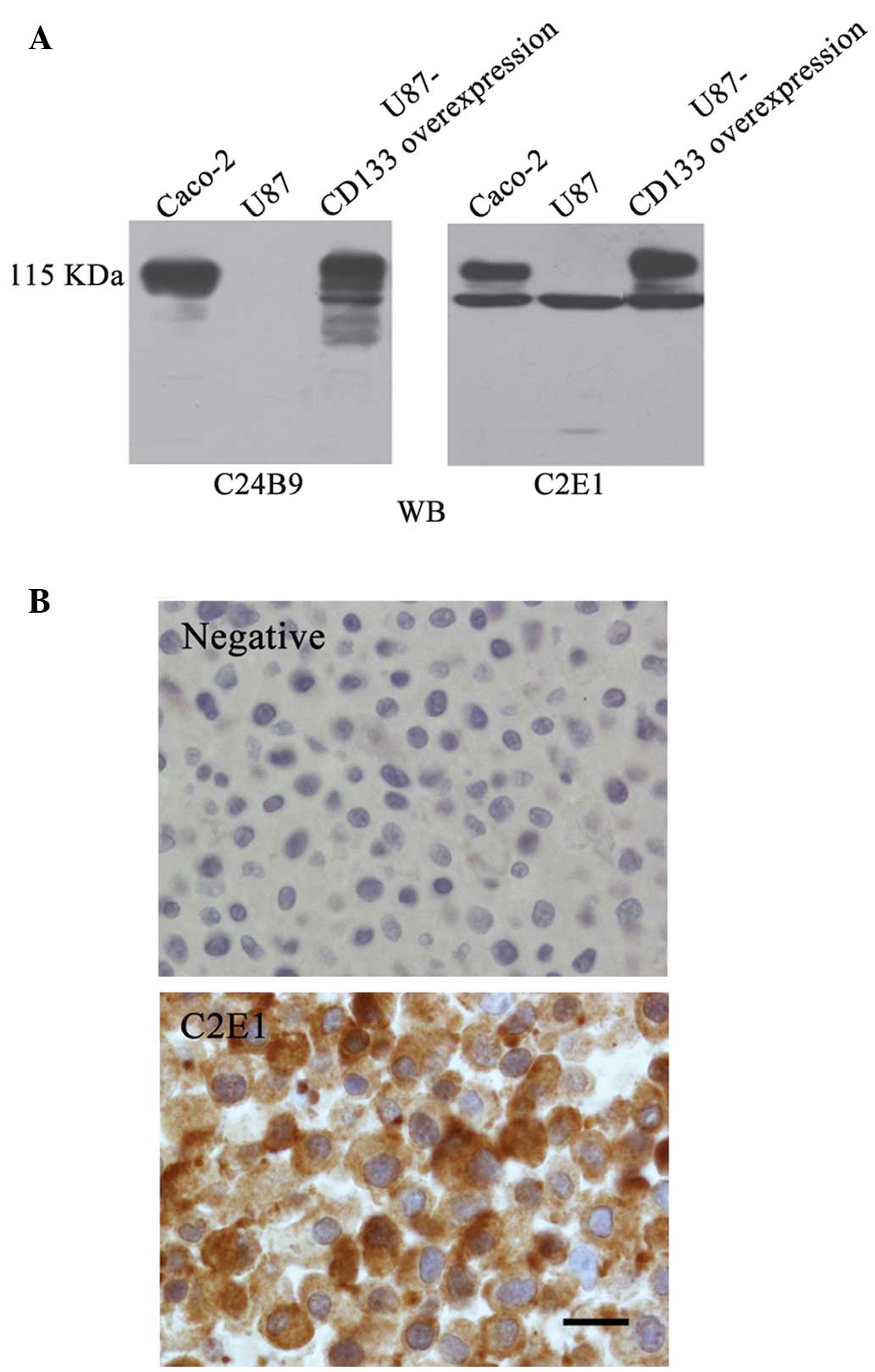
CD133 expression in U87 cells was screened with each
of the obtained anti-CD133 mAbs. The CD133+ colorectal
adenocarcinoma cell line, Caco-2, was used as a positive control
and the commercially available anti-CD133 mAb, C24B9, was used as a
positive control. Similar to C24B9, all the hybridoma clones
detected a 115 kDa glycosylated full-length CD133 protein in Caco-2
cells, but were unable to detect a considerable CD133 expression in
U87 cells, with the exception of C2E1 (Fig. 3A). In the C2E1 clones, a 95 kDa band
was revealed in Caco-2 and U87 cells, in addition to the 115 kDa
band in Caco-2 cells (Fig. 3A). A
stable U87 cell line expressing full-length CD133 (termed as
U87-CD133 overexpression) was established by infecting native U87
cells with CD133-expressing lentivirus, followed by selection.
Western blot analysis with C2E1 and C24B9 demonstrated glycosylated
full-length CD133 bands in the U87-CD133 overexpression cell line
(Fig. 3A). Cellular distribution of
endogenous CD133 expression in the native U87 cells was evaluated
by immunohistochemical staining with C2E1, which revealed a
cytoplasmic distribution pattern (Fig.
3B).
In vitro biological effects of the
novel anti-CD133 mAb on cancer cells
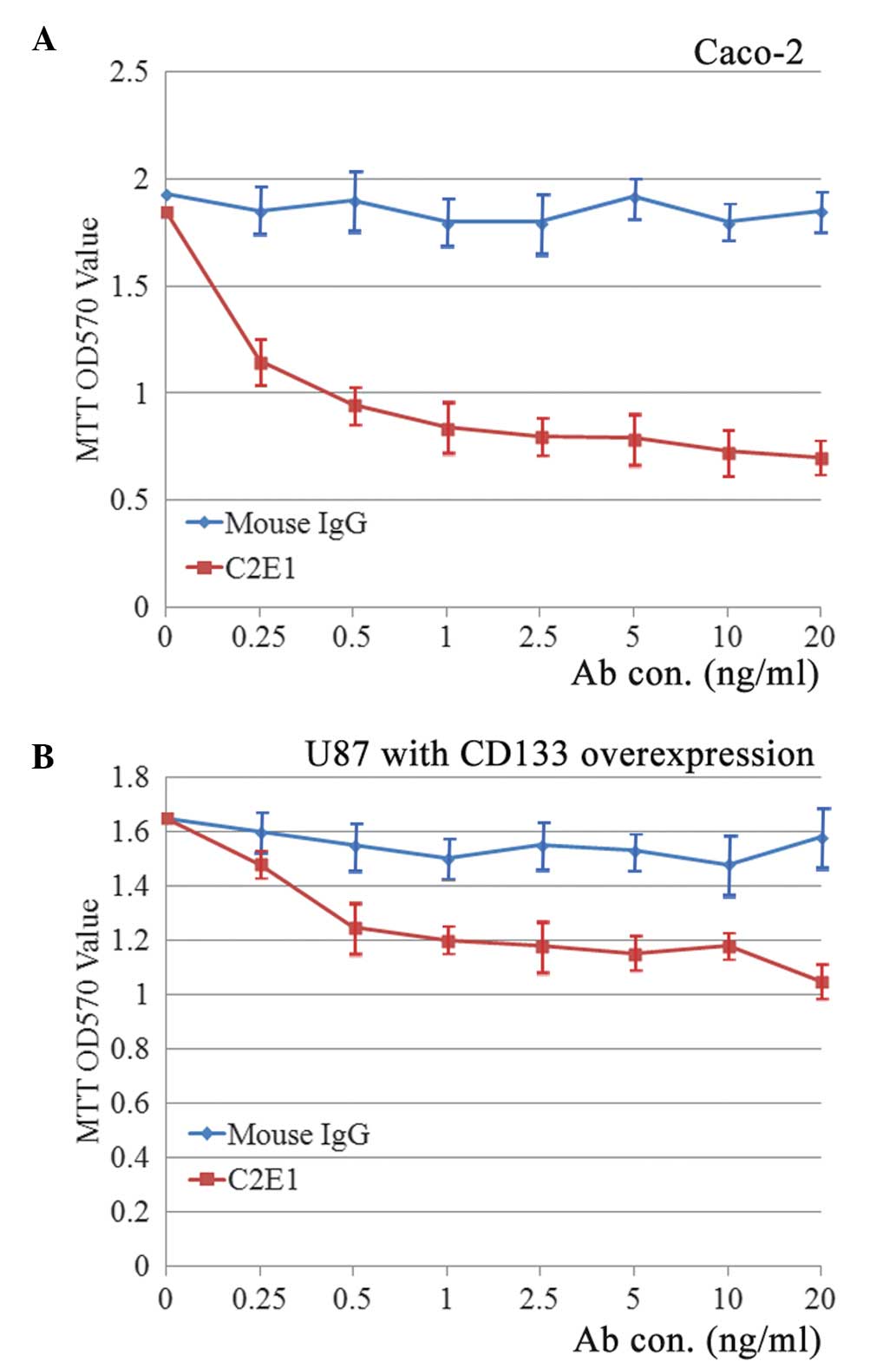
To investigate whether the C2E1 antibody exhibits a
biological activity (for instance, neutralization of the membranous
CD133 protein), the Caco-2, native U87 and U87-CD133 overexpression
cells were incubated with increasing concentrations of C2E1 for 72
h. An MTT assay revealed that incubation with C2E1 inhibited the
proliferation of Caco-2 cells in a dose-dependent manner (Fig. 4A). The inhibitory rate was calculated
using the following formula: Inhibitory rate (%) = (OD570 value of
normal mouse IgG - OD570 value of C2E1)/(OD570 value of normal
mouse IgG) × 100. The highest inhibitory rate was 63.3±2.93%
(P<0.01), which was observed when the antibody concentration
reached 20 ng/ml. However, C2E1 had no effect on the proliferation
of U87 cells (data not shown), which may be due to the
predominantly cytoplasmic distribution of CD133 protein in native
U87 cells. As predicted, U87-CD133 overexpression cells responded
to the cell growth inhibitory effect of C2E1 (Fig. 4B). The highest inhibitory rate was
33.3±4.73% (P<0.05), which was observed when the antibody
concentration reached 20 ng/ml. Notably, the cell proliferation
rate of untreated U87-CD133 overexpression cells was similar to
that of native U87 cells.
Discussion
U87, a commonly-used commercial cell line for
glioblastoma research, exhibits the behaviors and features of CSCs
(32,33). However, previous studies have reported
that the U87 cell line does not express the CSC marker, CD133, upon
employing standard anti-CD133 antibodies, including AC133, 293C3
(34) or W6B3C1 (35). To the best of our knowledge, in the
current study, an anti-human CD133 mAb was constructed that
detected a 95 kDa endogenous CD133 protein with a cytoplasmic
distribution pattern in U87 cells for the first time.
A number of studies have reported inconsistent
immunolabeling of CSCs upon using different CD133 antibodies. This
inconsistency may be associated with the glycosylation status and
variants of the CD133 glycoprotein (36,37). Tumor
stem cells may change the glycosylation status of CD133 upon
differentiation (28,36,38).
Antibodies that recognize the glycosylated epitopes may therefore
detect only a limited subset of CD133+ cells. In
addition, ≥28 alternatively spliced CD133 variants have been
identified (39–41). As different CD133 antibodies do not
recognize all the splice variants, this may explain the
inconsistencies in immunolabeling and varying protein sizes in
different studies (20).
In the present study, the novel mAb C2E1 recognized
a CD133 protein product of U87 cells with a molecular weight of 95
kDa, which has a similar size to the non-glycosylated full-length
CD133. However, the 95 kDa CD133 in U87 cells may be a CD133
variant rather than the non-glycosylated full-length CD133, since
the latter should be detected by the ‘pan’-C24B9 antibody (20). Furthermore, the cytoplasmic
distribution of CD133 in U87 cells revealed by C2E1 immunostaining
indicated that this protein is not likely to be glycosylated.
Whether this novel CD133 antigen plays a role in the
stem cell behavior of U87 cells, or whether it is present in other
CSCs, remains unclear. CD133 analysis must not focus on specific
glycosylated epitopes, but instead cover all the CD133 variants.
The inclusion of different CD133 variants may produce more
consistent results in the study of the CD133 biology in CSCs
(20). C2E1, the novel anti-CD133 mAb
that was produced in the current study, is able to recognize the 95
kDa CD133 variant and also bind full-length glycosylated CD133.
Therefore, this novel mAb may be a valuable tool for the study of
CD133 as a CSC marker.
An important advance in cancer therapy is the
development of targeted agents that are able to neutralize the CSC
response to circulating growth factors. The results of the current
study indicated that incubation with C2E1 may inhibit proliferation
in Caco-2 and U87-CD133 overexpression cells, indicating that C2E1
exerts a neutralizing effect on CD133+ cells; this may
be of significance in the development of cancer therapies.
In conclusion, the novel anti-CD133 mAb, C2E1,
indicated that endogenous CD133 expression is present in
glioblastoma U87 cells. This mAb is able to bind cell surface CD133
and inhibit the proliferation of tumor cells.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities (grant nos. GK201301010 and
GK201104004), the Innovation Funds of Graduate Programs, Shaanxi
Normal University (grant no. 2011CXB006) and research grants to
Haibin Xia and Xiaojing Zheng from the National Natural Science
Foundation of China (grant nos. 81272543, 81471772 and
81301957).
References
|
1
|
Ostrom QT, Gittleman H, Liao P, et al:
CBTRUS statistical report: primary brain and central nervous system
tumors diagnosed in the United States in 2007–2011. Neuro Oncol.
16:(Suppl 4). iv1–iv63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia A, De Angelis G and Capocaccia
R: Estimation and projections of cancer prevalence from cancer
registry data. Stat Med. 21:3511–3526. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M: Novel diagnostic and therapeutic
approaches to malignant glioma. Swiss Med Wkly.
141:w132102011.PubMed/NCBI
|
|
4
|
McNamara MG, Sahebjam S and Mason WP:
Emerging biomarkers in glioblastoma. Cancers (Basel). 5:1103–1119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Mason WP, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumour and Radiation Oncology Groups; National Cancer Institute of
Canada Clinical Trials Group: Effects of radiotherapy with
concomitant and adjuvant temozolomide versus radiotherapy alone on
survival in glioblastoma in a randomised phase III study: 5-year
analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stupp R, Mason WP, van den Bent MJ, et al:
European Organisation for Research and Treatment of Cancer Brain
Tumor and Radiotherapy Groups; National Cancer Institute of Canada
Clinical Trials Group: Radiotherapy plus concomitant and adjuvant
temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
11
|
Lim SH, Jang J, Park JO, et al:
CD133-positive tumor cell content is a predictor of early
recurrence in colorectal cancer. J Gastrointest Oncol. 5:447–456.
2014.PubMed/NCBI
|
|
12
|
Mizugaki H, Sakakibara-Konishi J, Kikuchi
J, et al: CD133 expression: a potential prognostic marker for
non-small cell lung cancers. Int J Clin Oncol. 19:254–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashimoto K, Aoyagi K, Isobe T, et al:
Expression of CD133 in the cytoplasm is associated with cancer
progression and poor prognosis in gastric cancer. Gastric Cancer.
17:97–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rappa G, Fodstad O and Lorico A: The stem
cell-associated antigen CD133 (Prominin-1) is a molecular
therapeutic target for metastatic melanoma. Stem Cells.
26:3008–3017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CH, Chiou SH, Chou CP, et al:
Photothermolysis of glioblastoma stem-like cells targeted by carbon
nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine.
7:69–79. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clément V, Marino D, Cudalbu C, et al:
Marker-independent identification of glioma-initiating cells. Nat
Methods. 7:224–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clément V, Dutoit V, Marino D, Dietrich PY
and Radovanovic I: Limits of CD133 as a marker of glioma
self-renewing cells. Int J Cancer. 125:244–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang J, Sakariassen PØ, Tsinkalovsky O, et
al: CD133 negative glioma cells form tumors in nude rats and give
rise to CD133 positive cells. Int J Cancer. 122:761–768. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beier D, Hau P, Proescholdt M, et al:
CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show
differential growth characteristics and molecular profiles. Cancer
Res. 67:4010–4015. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hermansen SK, Christensen KG, Jensen SS
and Kristensen BW: Inconsistent immunohistochemical expression
patterns of four different CD133 antibody clones in glioblastoma. J
Histochem Cytochem. 59:391–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeppernick F, Ahmadi R, Campos B, et al:
Stem cell marker CD133 affects clinical outcome in glioma patients.
Clin Cancer Res. 14:123–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Immervoll H, Hoem D, Sakariassen PØ,
Steffensen OJ and Molven A: Expression of the ‘stem cell marker’
CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC
Cancer. 8:482008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thon N, Damianoff K, Hegermann J, et al:
Presence of pluripotent CD133+ cells correlates with
malignancy of gliomas. Mol Cell Neurosci. 43:51–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beier D, Wischhusen J, Dietmaier W, et al:
CD133 expression and cancer stem cells predict prognosis in
high-grade oligodendroglial tumors. Brain Pathol. 18:370–377. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pallini R, Ricci-Vitiani L, Banna GL, et
al: Cancer stem cell analysis and clinical outcome in patients with
glioblastoma multiforme. Clin Cancer Res. 14:8205–8212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Christensen K, Schrøder HD and Kristensen
BW: CD133 identifies perivascular niches in grade II-IV
astrocytomas. J Neurooncol. 90:157–170. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bidlingmaier S, Zhu X and Liu B: The
utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J Mol Med (Berl). 86:1025–1032. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Florek M, Haase M, Marzesco AM, et al:
Prominin-1/CD133, a neural and hematopoietic stem cell marker, is
expressed in adult human differentiated cells and certain types of
kidney cancer. Cell Tissue Res. 319:15–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jordan M, Schallhorn A and Wurm FM:
Transfecting mammalian cells: optimization of critical parameters
affecting calcium-phosphate precipitate formation. Nucleic Acids
Res. 24:596–601. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shmelkov SV, St Clair R, Lyden D and Rafii
S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 37:715–719.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Plumb JA, Milroy R and Kaye SB: Effects of
the pH dependence of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium
bromide-formazan absorption on chemosensitivity determined by a
novel tetrazolium-based assay. Cancer Res. 49:4435–4440.
1989.PubMed/NCBI
|
|
32
|
Campos B and Herold-Mende CC: Insight into
the complex regulation of CD133 in glioma. Int J Cancer.
128:501–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu A, Oh S, Wiesner SM, et al: Persistence
of CD133+ cells in human and mouse glioma cell lines:
detailed characterization of GL261 glioma cells with cancer stem
cell-like properties. Stem Cells Dev. 17:173–184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Platet N, Liu SY, Atifi ME, et al:
Influence of oxygen tension on CD133 phenotype in human glioma cell
cultures. Cancer Lett. 258:286–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Christensen K, Aaberg-Jessen C, Andersen
C, Goplen D, Bjerkvig R and Kristensen BW: Immunohistochemical
expression of stem cell, endothelial cell, and chemosensitivity
markers in primary glioma spheroids cultured in serum-containing
and serum-free medium. Neurosurgery. 66:933–947. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Corbeil D, Röper K, Hellwig A, et al: The
human AC133 hematopoietic stem cell antigen is also expressed in
epithelial cells and targeted to plasma membrane protrusions. J
Biol Chem. 275:5512–5520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miraglia S, Godfrey W, Yin AH, et al: A
novel five-transmembrane hematopoietic stem cell antigen:
isolation, characterization, and molecular cloning. Blood.
90:5013–5021. 1997.PubMed/NCBI
|
|
38
|
Mizrak D, Brittan M and Alison M: CD133:
molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fargeas CA, Huttner WB and Corbeil D:
Nomenclature of prominin-1 (CD133) splice variants - an update.
Tissue Antigens. 69:602–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jaszai J, Fargeas CA, Florek M, Huttner WB
and Corbeil D: Focus on molecules: prominin-1 (CD133). Exp Eye Res.
85:585–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fargeas CA, Joester A, Missol-Kolka E,
Hellwig A, Huttner WB and Corbeil D: Identification of novel
Prominin-1/CD133 splice variants with alternative C-termini and
their expression in epididymis and testis. J Cell Sci.
117:4301–4311. 2004. View Article : Google Scholar : PubMed/NCBI
|