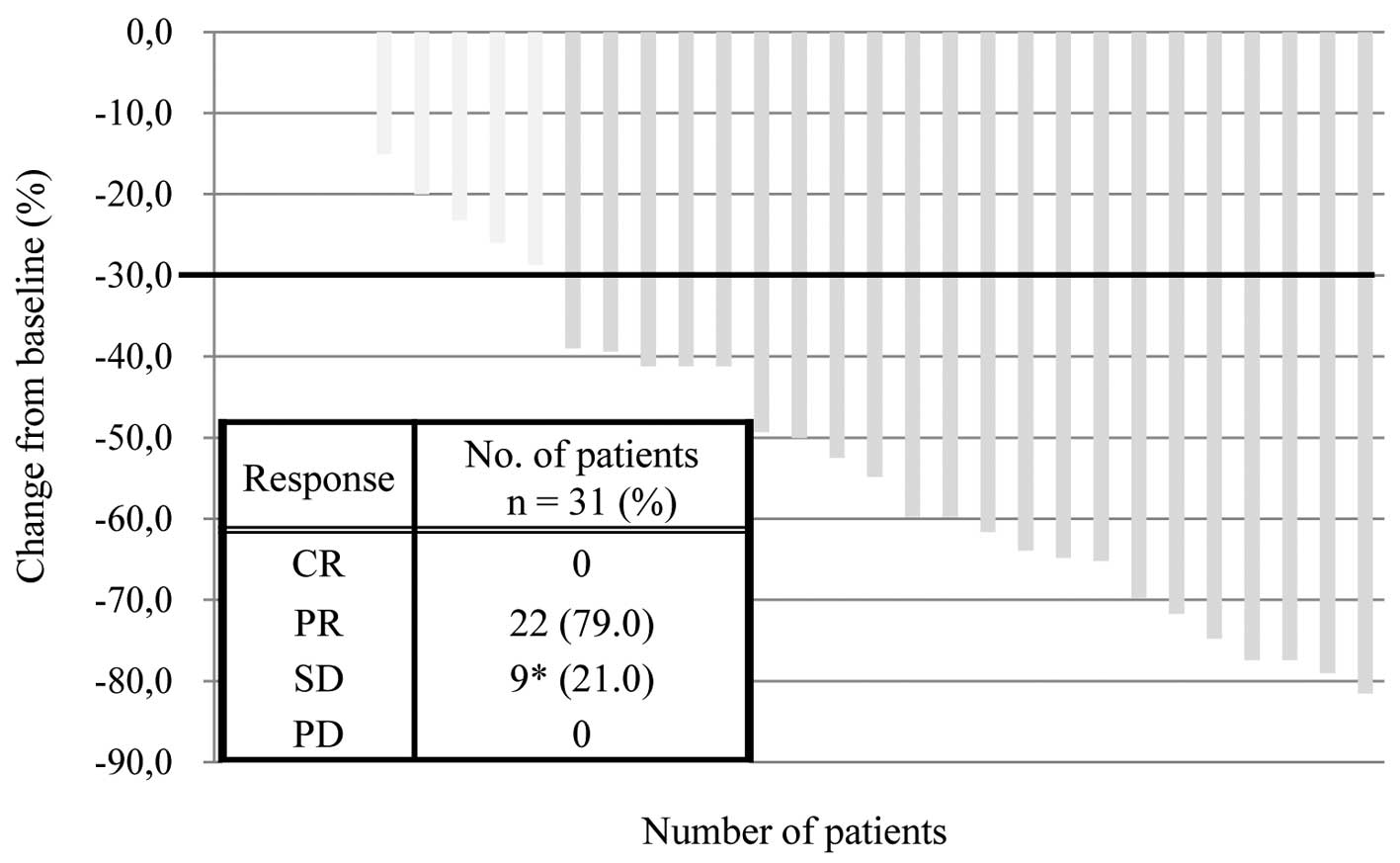
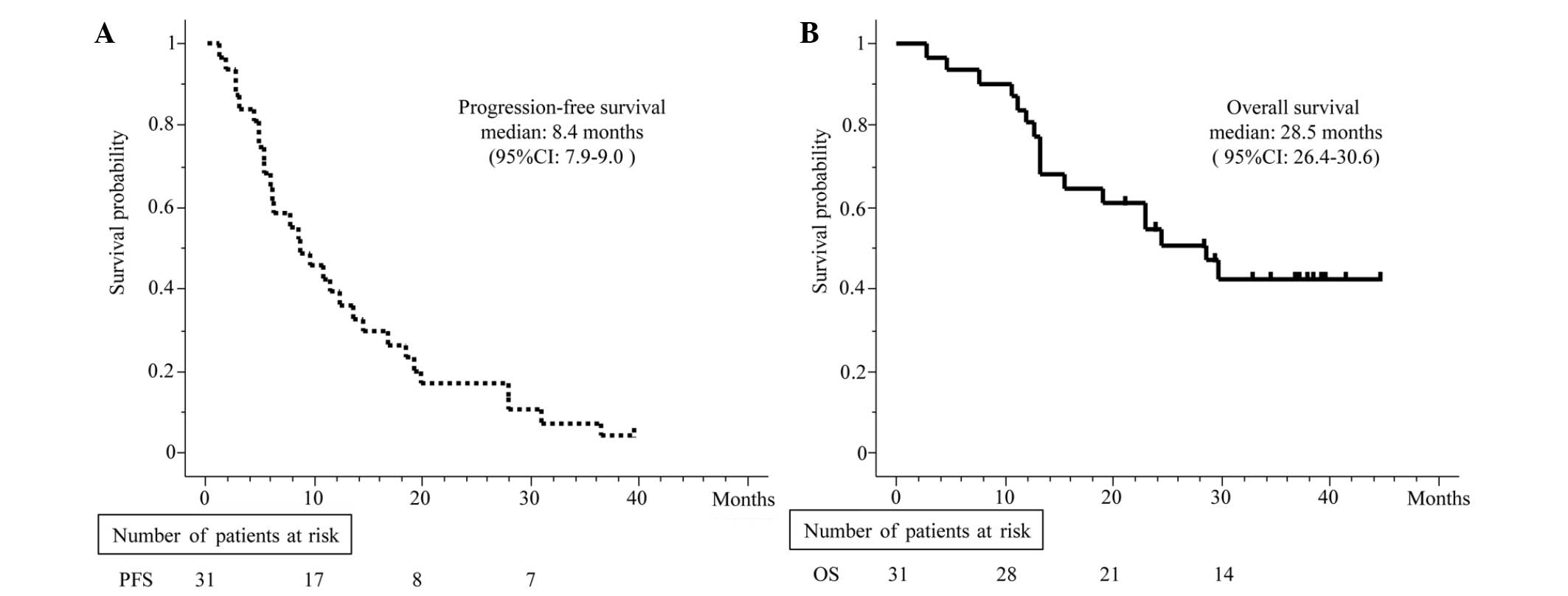
|
1
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor EC, Kuhnt D, Shih C, et al: A
dideazatetrahydrofolate analogue lacking a chiral center at C-6,
N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic
acid, is an inhibitor of thymidylate synthase. J Med Chem.
35:4450–4454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schultz RM, Patel VF, Worzalla JF and Shih
C: Role of thymidylate synthase in the antitumor activity of the
multitargeted antifolate, LY231514. Anticancer Res. 19:437–443.
1999.PubMed/NCBI
|
|
5
|
Shih C, Habeck LL, Mendelsohn LG, Chen VJ
and Schultz RM: Multiple folate enzyme inhibition: mechanism of a
novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme
Regul. 38:135–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, et al: Phase III study comparing cisplatin plus
gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive
patients with advanced-stage non-small-cell lung cancer. J Clin
Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grønberg BH, Bremnes RM, Fløtten O, et al:
Phase III study by the Norwegian lung cancer study group:
pemetrexed plus carboplatin compared with gemcitabine plus
carboplatin as first-line chemotherapy in advanced non-small-cell
lung cancer. J Clin Oncol. 27:3217–3224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29:(Suppl 16). 10–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seto T, Higashiyama M, Funai H, et al:
Prognostic value of expression of vascular endothelial growth
factor and its flt-1 and KDR receptors in stage I non-small-cell
lung cancer. Lung Cancer. 53:91–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gray R, Bhattacharya S, Bowden C, Miller K
and Comis RL: Independent review of E2100: a phase III trial of
bevacizumab plus paclitaxel versus paclitaxel in women with
metastatic breast cancer. J Clin Oncol. 27:4966–4972. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Escudier B, Pluzanska A, Koralewski P, et
al: AVOREN Trial Investigators: Bevacizumab plus interferon alfa-2a
for treatment of metastatic renal cell carcinoma: a randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman HS, Prados MD, Wen PY, et al:
Bevacizumab alone and in combination with irinotecan in recurrent
glioblastoma. J Clin Oncol. 27:4733–4740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson DH, Fehrenbacher L, Novotny WF, et
al: Randomized phase II trial comparing bevacizumab plus
carboplatin and paclitaxel with carboplatin and paclitaxel alone in
previously untreated locally advanced or metastatic non-small-cell
lung cancer. J Clin Oncol. 22:2184–2191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niho S, Kunitoh H, Nokihara H, et al:
JO19907 Study Group: Randomized phase II study of first-line
carboplatin-paclitaxel with or without bevacizumab in Japanese
patients with advanced non-squamous non-small-cell lung cancer.
Lung Cancer. 76:362–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barlesi F, Gervais R, Lena H, et al:
Pemetrexed and cisplatin as first-line chemotherapy for advanced
non-small-cell lung cancer (NSCLC) with asymptomatic inoperable
brain metastases: a multicenter phase II trial (GFPC 07–01). Ann
Oncol. 22:2466–2470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Common Terminology Criteria for Adverse
Events (CTCAE). v.4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmJanuary.
2014
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
William WN Jr, Kies MS, Fossella FV, et
al: Phase 2 study of carboplatin, docetaxel and bevacizumab as
frontline treatment for advanced nonsmall-cell lung cancer. Cancer.
116:2401–2408. 2010.PubMed/NCBI
|
|
25
|
Clément-Duchêne C, Krupitskaya Y, Ganjoo
K, et al: A phase II first-line study of gemcitabine, carboplatin
and bevacizumab in advanced stage nonsquamous non-small cell lung
cancer. J Thorac Oncol. 5:1821–1825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paz-Ares L, de Marinis F, Dediu M, et al:
Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mok TS, Hsia TC, Tsai CM, et al: Efficacy
of bevacizumab with cisplatin and gemcitabine in Asian patients
with advanced or recurrent non-squamous non-small cell lung cancer
who have not received prior chemotherapy: a substudy of the Avastin
in Lung trial. Asia Pac J Clin Oncol. 7:(Suppl 2). 4–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Besse B, Lasserre SF, Compton P, Huang J,
Augustus S and Rohr UP: Bevacizumab safety in patients with central
nervous system metastases. Clin Cancer Res. 16:269–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|