Introduction
Lymphoma was one the first hemopathies to be
identified, and at present, lymphoma exhibits incidence rates of
1.39 and 0.84 cases per 100,000 individuals in males and females in
China, respectively (1). In 1985,
Stein et al first identified anaplastic large cell lymphoma
(ALCL), which is characterized by the strong expression of antigen
Ki-1 (2). ALCL is classified as a
non-Hodgkin lymphoma (NHL) derived from peripheral T-cells and is
estimated to account for 2–3% of all lymphoid neoplasms, according
to the World Health Organization (WHO) classification (3,4). ALCL
represents a group of diseases which are heterogeneous with regard
to histology, phenotype, cytogenetics and clinical course and thus,
diagnosis remains difficult (5,6) and the
treatment for ALCL varies considerably in different patients,
involving the CCG-5941, AIEOP LNH-97 and BFM-95 protocols (7–9). The
expression of the anaplastic lymphoma kinase (ALK) protein is the
main characteristic used to classify ALCLs into two different
systemic forms, which included the ALK-positive (ALK+) and
ALK-negative (ALK-) tumors (8–11). ALK-
ALCL is more clinically aggressive and predominantly occurs as
advanced-stage disease in older patients (9–11). It has
been reported that ALK+ ALCLs exhibit a predominance for the
involvement of bone, bone marrow and subcutaneous tissue whereas
ALK- ALCLs are more likely to invade the liver and the
gastrointestinal tract (10).
Although ALCL tends to invade extranodal sites (10), primary involvement of the skeletal
muscle is extremely rare. The present study described the case of a
24-year-old male patient diagnosed with an ALK- ALCL originating in
the left psoas muscle, suggesting that multiple examinations may
help the early recognition and correct diagnosis of ALCL. Written
informed consent was obtained from the patient.
Case report
A 24-year-old male patient was admitted to the Third
Hospital of Hebei Medical University (Shijiazhuang, China) in March
2013 with one-month history of progressively increasing pain in the
lower back, which had been exacerbated for 10 days. The patient had
been initially treated for suspected strained back muscle with no
alleviation of the symptoms. The pain radiated down the front of
the left distal thigh three days before admission. No fever, night
sweats or weight loss were reported, with the exception of the
irregular sphincter disturbances. In addition, the patient had no
previous medical history of trauma or cancer. Upon physical
examination, a 15×7 cm elastic hard mass underlying the rib cage
and protruding to the left inguinal region was detected.
Furthermore, cervical, axillary and inguinal lymph node enlargement
was detected. The muscle strength of the left lower limb was
assessed by manual muscle testing according to Medical Research
Council scale (12) and was
determined to be grade 4. Other vital signs were within the normal
limits. Laboratory examinations performed upon admission revealed a
white blood cell count of 3.98×109/l [normal,
(4∼10)×109/l], C-reactive protein level of 18.52 mg/l
(normal, <8 mg/l), platelet count of 121.7×109/l
[normal, (100∼300)×109/l], and lactate dehydrogenase
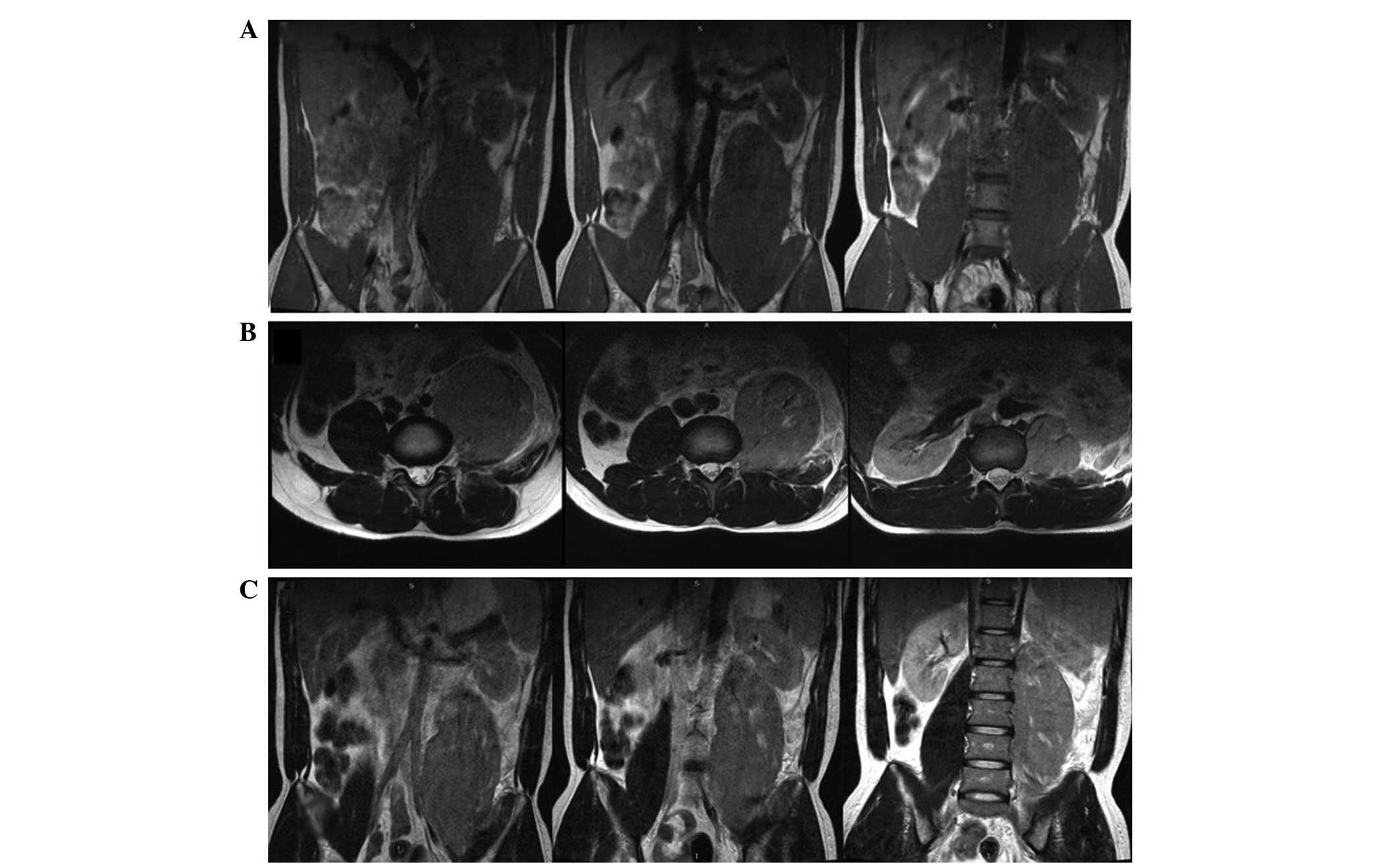
level of 1,958 U/l (normal, 104∼245 U/l) (13). Using magnetic resonance imaging (MRI),
swelling of the psoas muscle was identified (Fig. 1). Radiologically, the mass was
initially considered to be a soft tissue tumor, such as
rhabdomyosarcoma. Surgical resection of the left psoas muscle was
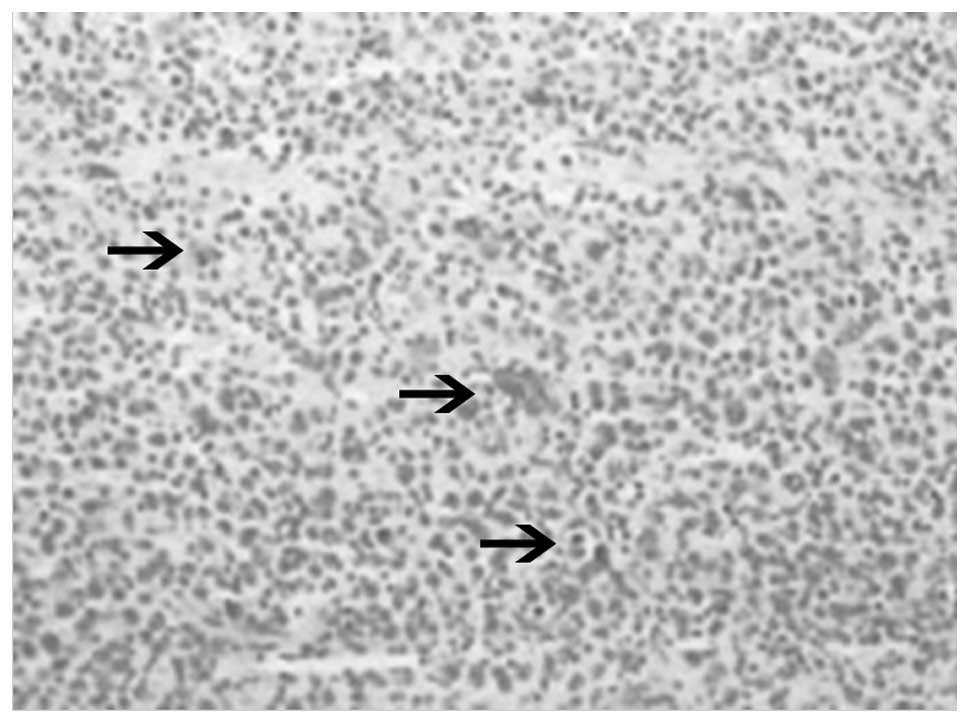
performed, and subsequent histopathological examination revealed
diffuse infiltration of large neoplastic cells with irregular
mitosis (Fig. 2). Immunohistochemical
analysis of the resected tumor was performed and the neoplastic
cells were found to be positive for CD30 and leukocyte common
antigen, whereas they were negative for ALK, CD15, paired box-5,
Epstein-Barr virus (EBV), Melan-A and HMB-45. Therefore, the
patient was diagnosed with ALK- ALCL at stage IV of the disease
according to the Ann Arbor classification (14). Subsequently, three 21-day cycles of
bortezomib (1.3 mg/m2, days 1 and 8) and EPOCH
[etoposide (50 mg/m2, days 1–4), adriamycin (50
mg/m2, days 1–4), vincristine (0.4 mg/m2,
days 1–4), prednisone (60 mg/m2, days 1–5) and
cyclophosphamide (750 mg/m2, day 5)], and one 42-day
cycle of bortezomib (1.3 mg/m2, days 1 and 8) and
hyper-CVAD [cyclophosphamide (300 mg/m2, days 2–4),
epirubicin (16.6 mg/m2, days 5–7), intravenous vindesine
(2 mg, days 5 and 12), dexamethasone (400 mg, days 2–5 and 12–16),
methotrexate (1,000 mg/m2, day 23) and cytarabine (3
g/m2, days 24 and 25)] were administered. The
chemotherapy was complicated by myelosuppression, however, the
patient experienced sustained remission for around 6 months
subsequent to discharge. The patient was lost to follow-up.
Discussion
ALK- ALCL, characterized by the strong expression of
CD30 and its aggressive growth, is classified as a provisional
entity of systemic type according to the 2008 WHO classification
(3,4).
However, ALK- ALCL should be distinguished from other ALCL types,
due to the different clinical features, treatment outcomes, and
immunophenotypic and genetic markers used for the diagnosis of the
disease (10).
Systemic ALCLs, among which ALK- ALCLs represent
15–50% of cases, account for 2–3% of NHLs (3,4). ALCL may
involve the extranodal organs, gastrointestinal tract, skin and
central nervous system (15–17). This type of lymphoma rarely arises in
the skeletal muscle, particularly with primary involvement. A
10-year study by Travis et al (18) described a 0.1% rate of primary
lymphomas in the soft tissue and only eight cases with primary
muscle involvement were identified in a review of 7,000 lymphoma
patients. However, a search of PubMed (1950 to August 2013) using
the search terms ‘primar*’ AND ‘anaplastic large cell lymphoma’ AND
‘muscle’, identified only a small number of studies in English
reporting primary skeletal muscle CD30+ ALCL ((Table I)) (19–26). To
the best of our knowledge, the present study is the ninth reported
case in the English literature.
 | Table I.Previously reported cases (n=8) of
anaplastic large cell lymphoma with primary skeletal muscle
involvement. |
Table I.
Previously reported cases (n=8) of
anaplastic large cell lymphoma with primary skeletal muscle
involvement.
| Author (ref.) | Year | ALK
statusa | Age, years | Gender | Site |
|---|
| Chim et al
(19) | 1999 | N/A | 34 | M | Forearm |
| Ishii et al
(20) | 2000 | ALK+ | 11 | F | Right upper arm,
chest wall, left arm, left leg |
| Menon et al
(21) | 2001 | N/A | 10 | F | Upper arm, thigh,
chest wall |
| Liao et al
(22) | 2005 | ALK- | 21 | M | Femoral muscle |
| Recavarren and Yang
(23) | 2009 | ALK- | 83 | F | Psoas muscle |
| Driss et al
(24) | 2009 | ALK+ | 8 | M | Buttock |
| Wu et al
(25) | 2009 | ALK+ | 14 | M | Sarcospinal, lumbar
and femoral muscles |
| Kounami et al
(26) | 2012 | ALK+ | 14 | F | Psoas muscle |
The present study reported the case of a CD30+ and
ALK- ALCL originating in the left psoas of a young adult. No
particular risk factors have been previously identified for ALCL.
At present, no convincing evidence exists suggesting that any
viruses, including EBV and the human T-cell leukaemia/lymphoma
virus family, may result in the development of NHL in humans.
However, a previous study has demonstrated that T-cell ALCL risk
was increased for patients with psoriasis and celiac disease
suggesting that autoimmune disorders may lead to the development of
this lymphoma (27).
At presentation, the possible diagnoses may include
a broad spectrum of tumors, such as neuroectodermal tumor or
sarcoma. Therefore, recognizing the MRI features of ALCL may
promote the identification between primary skeletal muscle lymphoma
and other soft-tissue neoplasms. The initial manifestations are
typically abnormal muscle signal intensity and muscle enlargement.
Generally, the neoplasms have slightly increased signal intensities
compared with normal muscles on T1-weighted images and intermediate
signal intensities compared with fat tissues on T2-weighted images
(28). In addition, fine-needle
aspiration (FNA) biopsy is normally conducted. However, considering
the variable degree of cellular pleomorphism and number of
anaplastic cells on the smears, the characteristic hallmark cells
of ALCL are not always identified in a FNA biopsy sample, and an
accurate diagnosis of ALCL is difficult. Therefore, performing
ancillary tests, including immunohistochemical and flow cytometric
analyses, may assist the diagnosis of ALCL (29). All the aforementioned examinations may
help to confirm the diagnosis in order to initiate an appropriate
chemotherapy regimen.
In conclusion, the present study represents a rare
case of ALCL, primarily involving the skeletal muscle. This disease
should be considered when establishing the diagnosis of a
hematogenous disease in cases where the patient presents diffuse
muscle swelling. The presence of a soft tissue mass on MRI scans,
as well as the results of a FNA biopsy and immunohistochemical
analysis of the mass, may help the early recognition and correct
diagnosis of ALCL, allowing for the appropriate treatment strategy
to be initiated.
Acknowledgements
The authors would like to thank Dr Shuaishuai Wu
(Department of Orthopedic Center, Third Hospital of Hebei Medical
University, Shijiazhuang, China) for the collection of the
data.
References
|
1
|
Lu ZY and Zhong NS: Internal
MedicineLymphoma. 7th. People's Medical Publishing House; Beijing:
pp. 6172009
|
|
2
|
Stein H, Mason DY, Gerdes J, et al: The
expression of the Hodgkin's disease associated antigen Ki-1 in
reactive and neoplastic lymphoid tissue: evidence that
Reed-Sternberg cells and histiocytic malignancies are derived from
activated lymphoid cells. Blood. 66:848–858. 1985.PubMed/NCBI
|
|
3
|
Delsol G, Jaffe ES and Falini B:
Anaplastic large cell lymphoma (ALCL), ALK-positiveWHO
Classification of Tumours of Haematopoietic and Lymphoid Tissues.
4th. Swerdlow SH, Campo E, Harris NL, et al: IARC; Lyon: pp.
312–316. 2008
|
|
4
|
Mason DY, Harris NL, Delsol G, et al:
Anaplastic large cell lymphoma, ALK-negativeWHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 4th. Swerdlow SH,
Campo E, Harris NL, et al: IARC; Lyon: pp. 317–319. 2008
|
|
5
|
Kadin ME: Primary Ki-1-positive anaplastic
large-cell lymphoma: a distinct clinicopathologic entity. Ann
Oncol. 5:(Suppl 1). S25–S30. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tilly H, Gaulard P, Lepage E, et al:
Primary anaplastic large-cell lymphoma in adults: clinical
presentation, immunophenotype, and outcome. Blood. 90:3727–3734.
1997.PubMed/NCBI
|
|
7
|
Williams DM, Hobson R, Imeson J, Gerrard
M, McCarthy K and Pinkerton CR: United Kingdom Children's Cancer
Study Group: Anaplastic large cell lymphoma in childhood: analysis
of 72 patients treated on the United Kingdom Children's Cancer
Study Group chemotherapy regimens. Br J Haematol. 117:812–820.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lowe EJ, Sposto R, Perkins SL, Gross TG,
Finlay J, Zwick D and Abromowitch M: Children's Cancer Group Study
5941: Intensive chemotherapy for systemic anaplastic large cell
lymphoma in children and adolescents: final results of Children's
Cancer Group Study 5941. Pediatr Blood Cancer. 52:335–339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pillon M, Gregucci F, Lombardi A, et al:
NHL-Committee of the Italian Association of Pediatric Hematology
and Oncology (AIEOP): Results of AIEOP LNH-97 protocol for the
treatment of anaplastic large cell lymphoma of childhood. Pediatr
Blood Cancer. 59:828–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Savage KJ, Harris NL, Vose JM, et al:
International Peripheral T-Cell Lymphoma Project: ALK-anaplastic
large-cell lymphoma is clinically and immunophenotypically
different from both ALK+ ALCL and peripheral T-cell lymphoma, not
otherwise specified: report from the International Peripheral
T-Cell Lymphoma Project. Blood. 111:5496–5504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falini B, Bigerna B, Fizzotti M, et al:
ALK expression defines a distinct group of T/null lymphomas (‘ALK
lymphomas’) with a wide morphological spectrum. Am J Pathol.
153:875–886. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Medical Research Council, . Aids to the
examination of the peripheral nervous systemMemorandum no. 45. Her
Majesty's Stationery Office; London: 1976
|
|
13
|
Chen WB and Pan X: Laboratory diagnosisIn:
Diagnostics. 7th. People's Medical Publishing House; Beijing: pp.
25–440. 2009
|
|
14
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin's
Disease Staging Classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
15
|
Zheng W, Song Y, Lin N, Tu M, Liu W and
Zhu J: Primary gastrointestinal mantle lymphoma with massive
bleeding: a case report and literature review. Chin J Cancer Res.
25:250–253. 2013.PubMed/NCBI
|
|
16
|
Alaibac M, Bordignon M, Pennelli N, Aversa
S, Fornasa CV and Chiarion V: Primary subcutaneous B-cell lymphoma:
case report and literature review. Acta Derm Venereol. 88:151–154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao RN, Mishra D, Agrawal P and Kumar R:
Primary B-cell central nervous system lymphoma involving fourth
ventricle: A rare case report with review of literature. Neurol
India. 61:450–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Travis WD, Banks PM and Reiman HM: Primary
extranodal soft tissue lymphoma of the extremities. Am J Surg
Pathol. 11:359–366. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chim CS, Choy C and Liang R: Primary
anaplastic large cell lymphoma of skeletal muscle presenting with
compartment syndrome. Leuk Lymphoma. 33:601–605. 1999.PubMed/NCBI
|
|
20
|
Ishii E, Honda K, Nakagawa A, Urago K and
Oshima K: Primary CD30/Ki-1 positive anaplastic large cell lymphoma
of skeletal muscle with der(17)t(1;17)(q11;p11). Cancer Genet
Cytogenet. 122:116–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Menon BS, Maziah W, Samarendra M and Toha
A: Pathological case of the month. Ki-1-positive anaplastic large
cell lymphoma involving muscle. Arch Pediatr Adolesc Med.
155:411–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao WP, Dai MS, Hsu LF and Yao NS:
Anaplastic large-cell lymphoma primarily infiltrating femoral
muscles. Ann Hematol. 84:764–766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Recavarren RA and Yang J: Cytomorphologic
features of primary anaplastic large cell lymphoma of the psoas
muscle: a case report and literature review. Diagn Cytopathol.
38:208–212. 2010.PubMed/NCBI
|
|
24
|
Driss M, Abbes I, Mrad K, et al: Primary
CD30/ALK-1 positive anaplastic large cell lymphoma of the skeletal
muscle in a child. Pathologica. 101:97–100. 2009.PubMed/NCBI
|
|
25
|
Wu L, Wang Y, Fu SL, Huang L, Tongji FC
and Qi JY: Anaplastic large cell lymphoma with primary involvement
of skeletal muscle: a rare case report and review of the
literature. Pediatr Hematol Oncol. 26:142–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kounami S, Shibuta K, Yoshiyama M, et al:
Primary anaplastic large cell lymphoma of the psoas muscle: a case
report and literature review. Acta Haematol. 127:186–188. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Derringer GA, Thompson LD, Frommelt RA,
Bijwaard KE, Heffess CS and Abbondanzo SL: Malignant lymphoma of
the thyroid gland: a clinicopathologic study of 108 cases. Am J
Surg Pathol. 24:623–639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chun CW, Jee WH, Park HJ, et al: MRI
features of skeletal muscle lymphoma. AJR. Am J Roentgenol.
195:1355–1360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hudacko R, Rapkiewicz A, Berman RS and
Simsir A: ALK-negative anaplastic large cell lymphoma mimicking a
soft tissue sarcoma. J Cytol. 28:230–233. 2011. View Article : Google Scholar : PubMed/NCBI
|