Introduction
Glioma is one of the most prevalent and aggressive
malignant primary tumors of the central nervous system (CNS),
accounting for 52 and 20% of all cases of brain tissue and
intracranial tumors, respectively (1,2). It is
associated with a poor prognosis, particularly in high grade
tumors, such as glioblastoma multiforme (3). The median life expectancy of patients
with malignant glioma is ∼12 months, with a 5-year survival rate
after diagnosis of <5% (4,5). Although systemic metastases are
relatively rare, the infiltrative nature of glioma cells, which are
able to migrate into the surrounding brain parenchyma, means
achieving total surgical resection is unlikely (6,7).
Considering that complete curative resection and radiotherapy are
not yet attainable, adjuvant chemotherapy is of major importance in
the treatment of malignant gliomas, providing the rationale for the
implementation of novel targeted therapies. Thus, the development
of novel therapeutic approaches to effectively treat gliomas and
increase the positive outcome rate is currently a significant topic
in the field of oncology.
S100 calcium-binding protein B (S100B) is a 20 kDa,
diffusible, Ca+2/Zn+2-p53 binding protein
that has emerged as a critical signaling molecule as it regulates
numerous physiopathological functions including, inflammation,
apoptosis and cell growth (8). S100B
appears to be upregulated in numerous neurodegenerative diseases,
including Alzheimer's and Parkinson's disease, and is known to be
overexpressed in the majority of malignant gliomas (9–11).
Furthermore, the S100B protein has been proposed to significantly
contribute to cancer development by inhibiting the function of
tumor suppressor protein p53 (12,13), and
by stimulating the activity of the mitogenic kinases nuclear
dbf2-related (14) and protein kinase
B (15). Evidence of S100B/p53
crosstalk, and its impact on cell proliferation and survival, has
been the focus of research efforts regarding the development of
inhibitors of the S100B-p53 protein-protein interaction. This
molecular paradigm represents a novel target for the treatment of
the majority of aggressive types of cancer in which S100B protein
is highly expressed, such as melanoma (16). For analogous reasons, direct molecular
targeting of the S100B protein in glioma appears to be an
innovative approach for the development of novel therapeutic
interventions against this form of cancer.
Pentamidine isethionate, an agent that exhibits
antiprotozoal activity and is approved for the treatment of
Pneumocystis cariini pneumonia in the United States, appears
to be a promising candidate for the aforementioned S100B-targeting
of glioma. In addition to its antiprotozoal activity, pentamidine
has been reported to inhibit the S100B-p53 interaction in
vitro in melanoma cells (17).
However, to the best of our knowledge, no data has yet been
determined regarding the possible antiproliferative and
antimigratory effects exerted by pentamidine on glioma cells.
Therefore, the present study used C6 rat glioma cell cultures to
evaluate the in vitro effects of pentamidine on cell
proliferation and survival. C6 cells were utilized as a number of
studies have revealed that the changes in gene expression observed
in the C6 cell line closely resembles those reported in human brain
tumors (18–21). Notably, C6 rat glioma cells possess
the most important features of human gliomas, exhibiting a mutant
p16/cyclin-dependent kinase inhibitor 2a/Ink4a locus (22), high S100B expression levels and no
expression of the p53 protein (23).
Thus, C6 rat glioma cells are ideal candidates for exploring the
activity of novel compounds with anti-glioma activity.
Materials and methods
Materials
Media, substances and reagents for cell cultures
were all purchased from Sigma-Aldrich (St. Louis, MO, USA), unless
otherwise stated. Instruments, reagents and materials for western
blot analysis were obtained from Bio-Rad Laboratories (Milan,
Italy), and pentamidine isethionate was purchased from Tocris
Cookson, Inc. (Ballwin, MO, USA). Monoclonal mouse anti-p53 (cat.
no. sc-393031, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA),
polyclonal rabbit anti-matrix metalloproteinase-2 (MMP-2; cat. no.
ab37150, Abcam, Cambridge, UK), polyclonal mouse anti-β-actin (cat.
no. sc-130656, Santa Cruz Biotechnology Inc.); polyclonal mouse
anti-B-cell lymphoma-2 (Bcl-2)-associated X protein (BAX; cat. no.
ab18210), monoclonal rabbit anti-Bcl-2 (cat. no. ab87435) and
monoclonal rabbit anti-aquaporin 4 (AQP4; cat. no. ab128906)
antibodies were obtained from Abcam (Cambridge, UK); and polyclonal
rabbit anti-mouse IgG from Dako (Glostrup, Denmark).
Cell culture and pentamidine
treatment
C6 rat glioma cells (American Type Culture
Collection, LGC Standards, Middlesex, UK) were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 5%
fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml penicillin, and
100 µg/ml streptomycin in a humidified atmosphere of 5%
CO2 and 95% air at a temperature of 37°C. A total of
1×106 cells/well were plated and incubated for 24 h
under the same conditions as those utilised for the initial
culture. Upon reaching confluence, the cells were washed three
times with phosphate-buffered saline (PBS), detached with
trypsin/EDTA, plated in 10-cm diameter petri dish and allowed to
adhere for 24 h. Subsequently, DMEM was replaced with fresh medium,
and the cells were treated with increasing concentrations of
pentamidine isethionate (0.05, 0.5 and 5 µM) dissolved in ultrapure
and pyrogen-free sterile water at different time points, as
described below. The pentamidine concentrations used in the current
experiments were selected according to the results of a series of
pilot experiments aimed at identifying the lowest effective
concentration (data not shown).
Cell proliferation and survival
assays
Cell proliferation was evaluated by performing a
3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT)
assay (24). In brief, C6 cells
(5×104) were plated in 96-well plates and allowed to
adhere for 2 h. After 2 h, DMEM was replaced with fresh medium and
the cells were treated with increasing concentrations pentamidine
(0.05, 0.5 and 5 µM). After 48 h, 25 µl MTT (5 mg/ml MTT in DMEM)
was added to the cells and the mixture was incubated for an
additional 3 h at 37°C. Subsequently, the cells were lysed and the
dark blue crystals were solubilized using a 125-µl solution
containing 50% N,N-dimethylformamide and 20% (w/v) sodium
dodecylsulphate (pH 4.5). The optical density (OD) of each well was
determined using a PerkinElmer, Inc. (Waltham, MA, USA) microplate
spectrophotometer equipped with a 620-nm filter. Cell viability in
response to pentamidine administration was calculated using the
following equation: Cell viability (%) = (ODtreated /
ODcontrol) × 100.
Apoptotic cell staining
A total of 5×105 cells were seeded onto
glass slides, treated with pentamidine (0.5–5 µM) for 2 h, washed
twice with PBS and fixed with paraformaldehyde for 30 min at 4°C.
The C6 cells were stained with Hoechst 33258 for 5 min prior to
analysis by fluorescent microscopy analysis using a Nikon Eclipse
80 microscope (Nikon Instruments, Inc., Amsterdam, Netherlands),
and images were captured at a magnification of ×10 using a
high-resolution digital camera (Nikon Digital Sight DS-U1; Nikon
Instruments, Inc.). Apoptotic cells were characterized by the
specific morphological alterations of condensed nuclei and cell
shrinkage and counted using CellProfiler 2.1.0 software (Broad
Institute, Cambridge, MA, USA).
DNA fragmentation assay
Following treatment with pentamidine, the adherent
and non-adherent C6 cells were harvested, lysed with 400 µl sodium
chloride EDTA buffer (75 mM NaCl and 25 mM EDTA) containing 1%
(w/v) SDS and 2 U/ml proteinase K, and incubated for 2 h at 55°C.
Proteins were precipitated by adding 140 µl 5 M NaCl. After
centrifugation at 11,000 × g for 15 min, DNA in the supernatant was
precipitated by addition of 1×103 ml ethanol and
centrifugation was performed again (15 min; 11,000 × g). After
washing with 70% ethanol (v/v), the DNA was resuspended in
H2O, separated by agarose gel electrophoresis and
stained with ethidium bromide.
Western blot analysis
Protein expression in the C6 cells was evaluated by
performing a western blot analysis. Following treatment with
pentamidine, cells (1×106) were harvested, washed twice
with ice-cold PBS and centrifuged at 180 × g for 10 min at 4°C. The
cell pellet was resuspended in 100 µl ice-cold hypotonic lysis
buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM
phenylmethylsulphonylfluoride, 1.5 µg/ml soybean trypsin inhibitor,
7 µg/ml pepstatin A, 5 µg/ml leupeptin, 0.1 mM benzamidine and 0.5
mM DTT). To lyse the cells, the suspension was rapidly passed
through a syringe needle five to six times prior to centrifugation
for 15 min at 13,000 × g to obtain the cytoplasmic fraction. The
cytosolic fraction proteins were mixed with non-reducing gel
loading buffer (50 mM Tris, 10% SDS, 10% glycerol, 2 mg
bromophenol/ml) at a 1:1 ratio, boiled for 3 min and centrifuged at
10,000 × g for 10 min. The protein concentration was determined
using a Bradford assay and equivalent quantities (100 µg) of each
sample were electrophoresed on a 12% discontinuous polyacrylamide
minigel (25). Subsequently, the
proteins were transferred onto nitrocellulose membranes that had
been saturated by incubation with 10% non-fat dry milk in 1X PBS
overnight at 4°C. Each membrane was incubated with mouse anti-BAX
(dilution, 1:1000), mouse anti-Bcl-2 (dilution, 1:2000), rabbit
anti-MMP-2 (dilution, 1:1000), mouse anti-AQP4 (dilution, 1:5000),
mouse anti-p53, (dilution, 1:1000) or mouse anti-β-actin (dilution,
1:1,000) antibodies for 2 h at room temperature (RT). The membranes
were then incubated with polyclonal rabbit anti-mouse or goat
anti-rabbit IgG coupled to horseradish peroxidase (dilution,
1:2000; cat. nos. P0260 and P0448, respectively; Dako, Glostrup,
Denmark). Immune complexes were revealed using enhanced
chemiluminescence detection reagents (GE Healthcare Life Sciences,
Milan, Italy) and by exposing the membranes to Kodak X-Omat film
(Eastman Kodak Co., Rochester, NY, USA). Protein bands were then
scanned and underwent densitometric analysis using a GS-700 imaging
densitometer (Bio-Rad Laboratories).
Wound healing assay
A wound healing assay using the C6 cells was
performed as described previously, with a number of modifications
(26). Briefly, the C6 cells
(5×105 cells/well) were plated on a six-well plate and
incubated for 24 h in DMEM supplemented with 5% fetal bovine serum
(FBS), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 and
95% air at a temperature of 37°C. The cell layer was scratched
using a 200-µl sterile pipette tip, then cells were washed with PBS
three times and incubated with 0.05, 0.5 and 5 µM pentamidine for
48 h. The cells were washed twice with PBS and fixed with 4%
paraformaldehyde for 30 min. In order to facilitate cell counting,
the nucleus of the C6 cells was stained with Hoechst 33258
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 5 min at RT.
The cells were subsequently washed three times with PBS and images
were captured using a Nikon Eclipse 80 microscope equipped with a
high-resolution digital camera (Nikon Digital Sight DS-U1; Nikon
Instruments, Inc.). The percentage of migration was calculated by
counting the number of cells that had migrated into scratched areas
compared with the number of cells that had remained in the
peripheral areas.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean of n experiments. Statistical analyses were performed
using one-way analysis of variance and multiple comparisons were
performed using a Bonferroni post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of pentamidine on C6 cell
proliferation and apoptosis
The administration of pentamidine (0.05, 0.5 and 5
µM) to C6 cells caused a significant concentration-dependent
decrease in cell viability (58.5±5%, P<0.05; 40.6±7%, P<0.01;
and 13±4%, P<0.001, respectively) compared with the unstimulated
cells (assumed 100% viability; Fig.
1A). In agreement with this data, a Hoechst assay demonstrated
that treatment with pentamidine resulted in a pro-apoptotic effect,
with the apoptotic process detected in cell nuclei 48 h after
treatment (Fig. 1B). Furthermore, the
pentamidine concentration (0.05, 0.5 and 5 µM) was significantly
associated with an increase in the proportion of apoptotic nuclei
(21±5%, P<0.05; 39±3.2%, P<0.01; and 88±4.1%, P<0.001,
respectively) compared with the untreated C6 cells (4.5±1.6%). In
addition, the integrity of the DNA samples extracted from the C6
cells after treatment with pentamidine for 48 h was analyzed using
agarose gel electrophoresis and compared with the DNA samples
obtained from the untreated C6 cells. Qualitative analysis of the
DNA demonstrated that pentamidine treatment (0.05, 0.5 and 5 µM)
increased the amount of smearing on the gel in a
concentration-dependent manner, while DNA obtained from the
untreated cells only travelled a short distance through the gel,
indicating its integrity (Fig. 1C).
Immunoblot analysis of BAX and Bcl-2 determined that pentamidine
treatment (0.05, 0.5 and 5 µM) induced a significant upregulation
in BAX protein expression levels in a concentration-dependent
manner (100%, P<0.05; 453%, P<0.01; and 1000%, P<0.001,
respectively) compared with the untreated cells (Fig. 1D and E). This significant increase in
the pro-apoptotic effector BAX was paralleled to a significant and
concentration-dependent decrease in Bcl-2 protein expression levels
following the treatment of C6 cells with 0.05, 0.5 and 5 µM
pentamidine (-60%, P<0.001; −80.13%, P<0.001; and −95%,
P<0.001, respectively) compared with untreated cells (Fig. 1D and E).
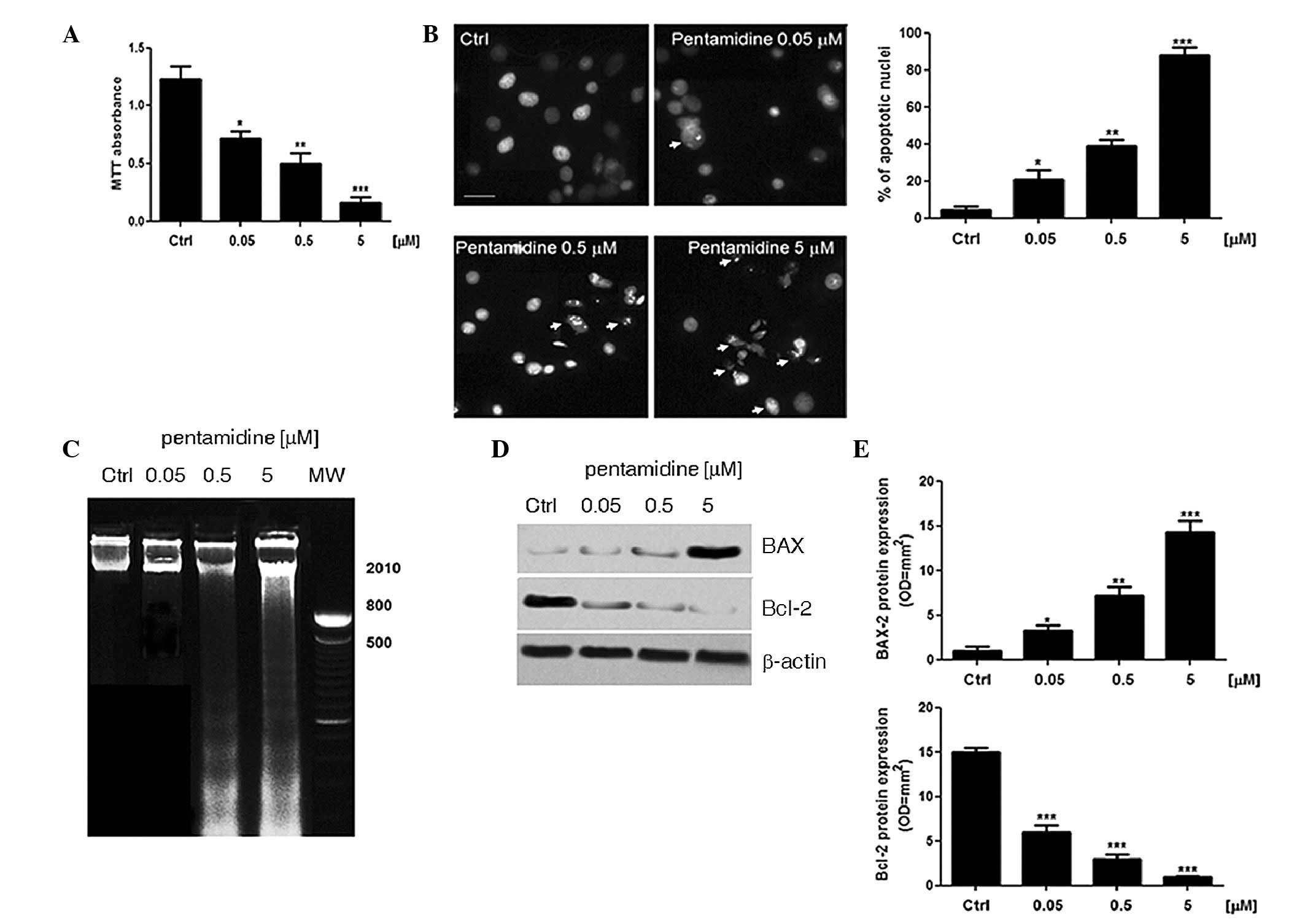 | Figure 1.Pentamidine exerts a pro-apoptotic
effect on cultured C6 rat glioma cells. (A) An MTT absorbance assay
was conducted to determine that pentamidine (0.05, 0.5 and 5 µM)
induces concentration-dependent inhibition of C6 rat glioma cell
proliferation after 48 h. (B) Hoechst staining of C6 rat glioma
cell nuclei in the presence or absence of pentamidine (0.05, 0.5
and 5 µM) and the relative proportion (%) of apoptotic nuclei.
Pentamidine induces a concentration-dependent increase in the
nuclear density of chromatin (arrows) as a marker of apoptosis
(scale bar, 20 µm). (C) Agarose gel electrophoresis of cultured C6
rat glioma cell DNA in the presence or absence of pentamidine
(0.05, 0.5 and 5 µM) for 48 h. The results are representative of
n=3 independent experiments. (D) Western blot analysis of
pro-apoptotic BAX and anti-apoptotic Bcl-2 proteins. Pentamidine
(0.05, 0.5 and 5 µM) induces a concentration-dependent increase in
BAX expression and a parallel decrease in Bcl-2 expression,
demonstrating a clear pro-apoptotic balance in the C6 rat glioma
cells. (E) Relative quantification of immunoreactive bands of Bcl-2
and BAX proteins (arbitrary units). Results are expressed as the
mean ± standard error of the mean of n=5 experiments performed in
triplicate. *P<0.05; **P<0.01; and ***P<0.001 vs. ctrl
cells. MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium
bromide; ctrl, control; BAX, B-cell lymphoma-2-associated X
protein; Bcl-2, B-cell lymphoma-2. |
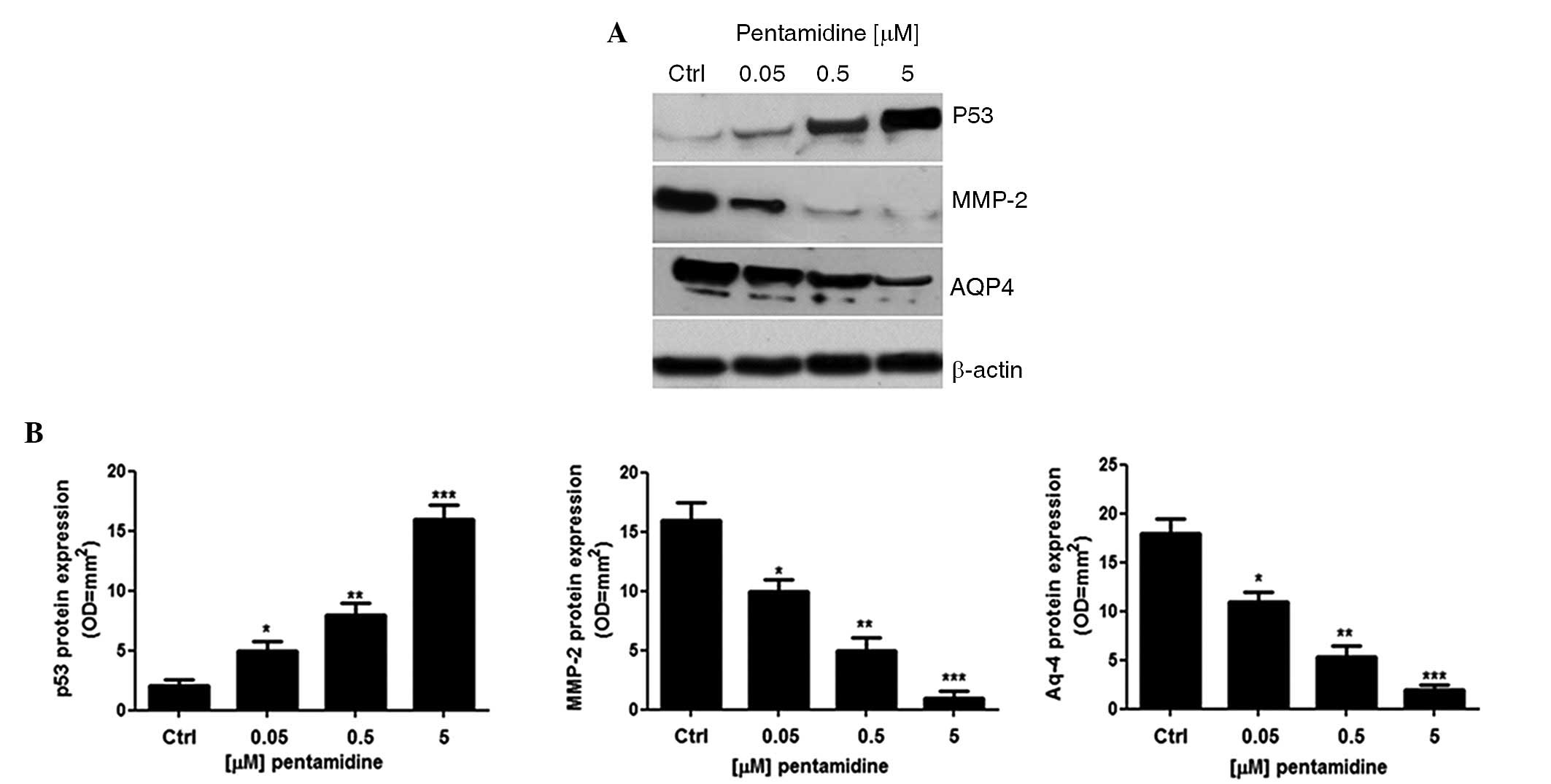
Effect of pentamidine on p53, MMP-2
and AQP-4 protein expression levels
Compared with the untreated cells, incubation of C6
cells with pentamidine induced a significant and concentration
dependent upregulation of p53 protein expression (681±87.5%,
P<0.05; 1244±94.3%, P<0.01; and 2244±111%, P<0.001,
respectively; Fig. 2A and B). In line
with this, the expression of MMP-2 (42±2.3%, P<0.05; 71±2.5%,
P<0.01; and 95.8±3.3%, P<0.001, respectively) and AQP4
(38±2.5%, P<0.05; 69±2.6%, P<0.01; and 88±3.0%, P<0.001,
respectively) were significantly lower in pentamidine-treated cells
compared with untreated cells.
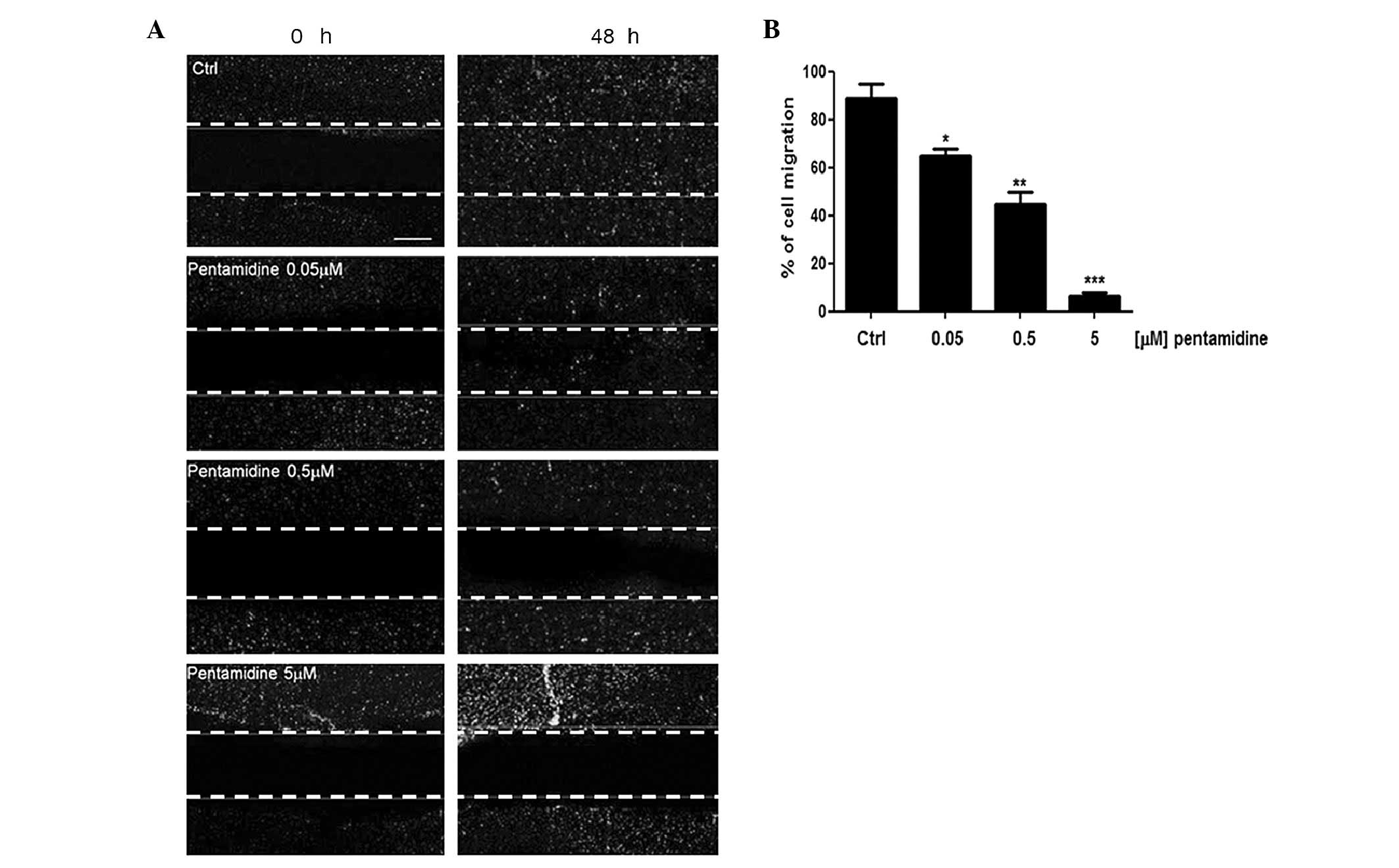
Effect of pentamidine on C6 cell
migration in vitro
Malignant gliomas are characterized by aberrant
proliferative activity, migration and invasion. The wound healing
assay was used to evaluate the putative effect of pentamidine
treatment on C6 cell migration. As indicated in Fig. 3A, while untreated C6 cells were able
to invade and fully recolonize the scratched area within 48 h, the
migration of cells treated with 0.05, 0.5 and 5 µM pentamidine was
significantly impaired in a concentration-dependent manner
(88±4.2%, P<0.05; 64±2%, P<0.01; and 42±3.1%, P<0.001,
respectively), with the distance between the borders of the wound
significantly different compared to that measured in the untreated
cells. Furthermore, at a concentration of 5 µM, pentamidine caused
an almost complete absence of migration (Fig. 3B).
Discussion
Despite the aggressive surgical and adjuvant
treatments currently used for the management of malignant glioma,
few advances have been made in determining the optimal therapeutic
approach to this disease (27).
However, the results of the present study demonstrate that
pentamidine significantly decreases C6 rat glioma cell
proliferation, exerting a pro-apoptotic effect and, thus,
highlighting pentamidine as a possible therapeutic agent in the
treatment of malignant glioma.
Anti-apoptotic Bcl-2 protein, and pro-apoptotic BAX
protein are two well-characterized signaling molecules that exhibit
opposing functions and expression levels, with their ratio
profoundly influencing the rate of cell apoptosis and survival
(28). The activity of Bcl-2, a
potent inhibitor of cell death, has been extensively described in
the resistance to numerous anticancer chemotherapeutic agents, as
well as in cancer development. By contrast, BAX protein is known to
induce apoptosis in various cell lines (29). In concurrence with the
antiproliferative activity of pentamidine described in a number of
other cell types, including cultured human melanoma cells (16,17,30,31),
the results of the present study indicate that pentamidine
treatment dose-dependently increases the BAX/Bcl-2 ratio,
demonstrating the pro-apoptotic role of this antiprotozoal agent in
cultured glioma cells. The pro-apoptotic effect displayed by
pentamidine appears to be directly associated with the inhibition
of the S100B-p53 protein-protein interaction, resulting in a marked
restoration of wild-type p53 protein function. This effect is
considered to be the pivotal mechanism of the anticancer effect of
pentamidine (17,30,31). Among
the various factors involved in the acquisition of invasive
capacities by tumor cells, MMP-2 and AQP4 have emerged as critical
markers of glioma cell migration. MMP-2 belongs to a large family
of extracellular matrix degrading enzymes, reported to be
associated with tumor invasion (32),
while AQP4 is a member of the water channel aquaporins (AQPs) that
correlate with tumor progression and angiogenesis (33). At least 13 AQPs have been identified
in mammals and are expressed by various cell types, including
epithelium and endothelium cells (34); among these, AQP4 has a key role in
glial cell migration (35). For
example, it has been reported that AQP4 is significantly
upregulated in glioblastoma compared with low grade gliomas and
healthy brain tissue (35).
Furthermore, AQP4 knockdown in rat and human cells has been
associated with decreased cell migration and invasion, indicating
that AQP4 may be involved in glioma malignancy (35). In addition to previous studies
describing the antiproliferative effect of pentamidine, the current
preliminary data demonstrated that pentamidine treatment caused a
profound inhibition of AQP-4 and MMP-2 proteins when compared with
untreated C6 cells, resulting in a concentration-dependent
inhibition of cell migration rate in vitro. Although the
results of the present study are limited by its in vitro
approach, the inhibition of the S100B-p53 crosstalk induced by
pentamidine may represent a promising pharmacological tool to
increase the suppression of glioma cell malignancy. In particular,
inhibition of the S100B-p53 interaction appeared to induced a
significant pro-apoptotic effect as well as a reduction in the
migratory capability of C6 rat glioma cells. As previously stated,
surgical resection of glioma is limited due to the high rate of
local relapse (35), which may be
dependent on MMP-2 and AQP4 expression. Thus, we hypothesize that
since pentamidine inhibits the expression of these proteins, it may
reduce the risk of local recurrences of glioma.
Agents that are able to induce apoptosis as well as
inhibit migration may expand the spectrum of possible
pharmacological treatment strategies for cancer, in particular
malignant glioma. Thus, pentamidine and other S100B-p53 inhibitors
are promising compounds for the treatment of this highly malignant
form of cancer. A phase II trial (clinicaltrials.gov; no. NCT00729807) investigating the
effect of pentamidine in relapsed or refractory melanoma is
currently under evaluation (17).
From a translational perspective, pentamidine only exhibits minimal
crossing of the blood brain barrier. Therefore, studies have been
conducted that aimed to increase the passage of pentamidine into
the CNS by modifying its structure prior to its introduction into
clinical practice (36,37). However, it has been established that
pentamidine is slowly delivered to the CNS via a complex process
involving multiple transporters, such as P-glycoprotein and
multidrug resistance-associated protein (MRP) transporters
(37). In particular, the interaction
of [3H] pentamidine with P-glycoprotein and MRP has been
proposed as a possible strategy to improve the delivery of
pentamidine to the CNS (36).
Therefore, pentamidine analogues that are able to block the
S100B-p53 protein-protein interaction are promising compounds for
restoring p53 expression levels in patients with malignant melanoma
and other types of cancer that overexpress S100B protein (38).
In conclusion, although the present study is an
in vitro preliminary report and requires confirmation in
vivo, the results pave the way for the development of novel
compounds that may potentially impact on the future treatment
strategies of glial cell-originating tumors.
Acknowledgements
This study was partially supported by Regione
Campania (grant. no. L.R. n 5/2001/2008).
References
|
1
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crocetti E, Trama A, Stiller C, Caldarella
A, Soffietti R, Jaal J, et al: RARECARE Working Group: Epidemiology
of glial and non-glial brain tumours in Europe. Eur J Cancer.
48:1532–1542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Pavlidis N and Jelic S: ESMO
Guidelines Task Force: ESMO Minimum Clinical Recommendations for
diagnosis, treatment and follow-up of malignant glioma. Ann Oncol.
16:(Suppl 1). i64–i65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giese A, Rief MD, Loo MA and Berens ME:
Determinants of human astrocytoma migration. Cancer Res.
54:3897–3904. 1994.PubMed/NCBI
|
|
7
|
Louis DN, Pomeroy SL and Cairncross JG:
Focus on central nervous system neoplasia. Cancer Cell. 1:125–128.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donato R, Sorci G, Riuzzi F, et al:
S100B's double life: intracellular regulator and extracellular
signal. Biochim Biophys Acta. 1793:1008–1022. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chaves ML, Camozzato AL, Ferreira ED, et
al: Serum levels of S100B and NSE proteins in Alzheimer's disease
patients. J Neuroinflammation. 7:62010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sathe K, Maetzler W, Lang JD, et al: S100B
is increased in Parkinson's disease and ablation protects against
MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain.
135:3336–3347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weide B, Richter S, Büttner P, et al:
Serum S100B, lactate dehydrogenase and brain metastasis are
prognostic factors in patients with distant melanoma metastasis and
systemic therapy. PLoS One. 8:e816242013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Yang Q, Yan Z, Markowitz J, Wilder
PT, Carrier F and Weber DJ: Inhibiting S100B restores p53 levels in
primary malignant melanoma cancer cells. J Biol Chem.
279:34071–34077. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rustandi RR, Baldisseri DM and Weber DJ:
Structure of the negative regulatory domain of p53 bound to
S100B(betabeta). Nat Struct Biol. 7:570–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Millward TA, Heizmann CW, Schäfer BW and
Hemmings BA: Calcium regulation of Ndr protein kinase mediated by
S100 calcium-binding proteins. EMBO J. 17:5913–5922. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arcuri C, Bianchi R, Brozzi F and Donato
R: S100B increases proliferation in PC12 neuronal cells and reduces
their responsiveness to nerve growth factor via Akt activation. J
Biol Chem. 280:4402–4414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hartman KG, McKnight LE, Liriano MA and
Weber DJ: The evolution of S100B inhibitors for the treatment of
malignant melanoma. Future Med Chem. 5:97–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith J, Stewart BJ, Glaysher S, Peregrin
K, Knight LA, Weber DJ and Cree IA: The effect of pentamidine on
melanoma ex vivo. Anticancer Drugs. 21:181–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sibenaller ZA, Etame AB, Ali MM, Barua M,
Braun TA, Casavant TL and Ryken TC: Genetic characterization of
commonly used glioma cell lines in the rat animal model system.
Neurosurg Focus. 19:E12005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amberger VR, Hensel T, Ogata N and Schwab
ME: Spreading and migration of human glioma and rat C6 cells on
central nervous system myelin in vitro is correlated with tumor
malignancy and involves a metalloproteolytic activity. Cancer Res.
58:149–158. 1998.PubMed/NCBI
|
|
20
|
Gunnersen JM, Spirkoska V, Smith PE, Danks
RA and Tan SS: Growth and migration markers of rat C6 glioma cells
identified by serial analysis of gene expression. Glia. 32:146–154.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grobben B, DE Deyn PP and Slegers H: Rat
C6 glioma as experimental model system for the study of
glioblastoma growth and invasion. Cell Tissue Res. 310:257–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schlegel J, Piontek G, Kersting M,
Schuermann M, Kappler R, Scherthan H, et al: The p16/Cdkn2a/Ink4a
gene is frequently deleted in nitrosourea-induced rat glial tumors.
Pathobiology. 67:202–206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Asai A, Miyagi Y, Hashimoto H, Lee SH,
Mishima K, Sugiyama A, et al: Modulation of tumor immunogenicity of
rat glioma cells by s-Myc expression: eradication of rat gliomas in
vivo. Cell Growth Differ. 11:1153–1158. 1994.
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renault-Mihara F, Beuvon F, Iturrioz X,
Canton B, De Bouard S, Léonard N, et al: Phosphoprotein enriched in
astrocytes-15 kDa expression inhibits astrocyte migration by a
protein kinase C delta-dependent mechanism. Mol Biol Cell.
17:5141–5152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rieger L, Weller M, Bornemann A, Schabet
M, Dichgans J and Meyermann R: BCL-2 family protein expression in
human malignant glioma: a clinical-pathological correlative study.
J Neurol Sci. 155:68–75. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paul-Samojedny M, Kokocińska D, Samojedny
A, Mazurek U, Partyka R, Lorenz Z and Wilczok T: Expression of cell
survival/death genes: Bcl-2 and Bax at the rate of colon cancer
prognosis. Biochim Biophys Acta. 1741:25–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pathak MK, Dhawan D, Lindner DJ, Borden
EC, Farver C and Yi T: Pentamidine is an inhibitor of PRL
phosphatases with anticancer activity. Mol Cancer Ther.
1:1255–1264. 2002.PubMed/NCBI
|
|
31
|
Zimmer DB, Lapidus RG and Weber DJ: In
vivo screening of S100B inhibitors for melanoma therapy. Methods
Mol Biol. 963:303–317. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du R, Petritsch C, Lu K, Liu P, Haller A,
Ganss R, et al: Matrix metalloproteinase-2 regulates vascular
patterning and growth affecting tumor cell survival and invasion in
GBM. Neuro Oncol. 10:254–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ding T, Zhou Y, Sun K, Jiang W, Li W, Liu
X, et al: Knockdown a water channel protein, aquaporin-4, induced
glioblastoma cell apoptosis. PLoS One. 8:e667512013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Verkman AS: Aquaporins in clinical
medicine. Annu Rev Med. 63:303–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giese A: Glioma invasion-pattern of
dissemination by mechanisms of invasion and surgical intervention,
pattern of gene expression and its regulatory control by
tumorsuppressor p53 and proto-oncogene ETS-1. Acta Neurochir Suppl.
88:153–162. 2003.PubMed/NCBI
|
|
36
|
Sanderson L, Dogruel M, Rodgers J, et al:
Pentamidine movement across the murine blood-brain and
blood-cerebrospinal fluid barriers: effect of trypanosome
infection, combination therapy, P-glycoprotein, and multidrug
resistance-associated protein. J Pharmacol Exp Ther. 329:967–977.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kotthaus J, Kotthaus J, Schade D, et al:
New prodrugs of the antiprotozoal drug pentamidine. ChemMedChem.
9:2233–2242. 2011. View Article : Google Scholar
|
|
38
|
Raseroka BH and Ormerod WE: The
trypanocidal effect of drugs in different parts of the brain. Trans
R Soc Trop Med Hyg. 80:634–641. 1986. View Article : Google Scholar : PubMed/NCBI
|