Introduction
The incidence of prostate cancer in many Asian
populations has gradually increased, and almost all prostate
cancers are detected in men aged >50 years. Early prostate
cancer is usually asymptomatic and is typically only detected on
screening examination for prostate specific antigen. The proportion
of patients with metastatic prostate cancer in Asian countries is
higher than that in western countries. A previous study revealed
that the proportion of patients with metastatic prostate cancer in
a Chinese cohort was 32% when compared with 4.2% in an American
cohort (1). Prostate cancer commonly
metastasizes to bone, lung, liver, especially the axial skeleton
and the 5-year survival rate in Singapore-Chinese patients with
metastatic cancer is 33.7% (1).
Solitary rectal metastasis of prostate cancer, however, is
relatively rare. The current study reports a case of rectal
metastasis of a prostate cancer, which was initially incorrectly
diagnosed as rectal cancer.
Case report
A 73-year-old male patient was admitted to the
Department of Colorectal Surgery at Guangdong Gastrointestinal
Hospital, China, due to altered bowel habits with hemafecia which
had persisted for 6 months. The patient denied abdominal pain and
prior episodes of diarrhea and melena. He had no significant
medical background and family history. In 2009 the patient
underwent transurethral resection of the prostate (TURP) due to an
enlarged prostate and prostate cancer was detected in the
postoperative pathological analysis. Following this, the patient
underwent bilateral surgical orchiectomy and oral Casodex (50 mg)
was taken once daily following surgery for 20 months. The Gleason
score of the patient at primary diagnosis was 4+5=9. A
cauliflower-like, half circumferential, firm, non-smooth tumor,
which was 2.5 cm away from the anal verge, was palpable in the 6
o'clock position during the knee-chest position rectal palpation.
The adhesion of the tumor and prostate was noted. Enteroscopy
revealed a 2.5×3.5-cm elevated lesion of thickened mucosa with
ill-defined margins, of which the surface was anabrotic. No
abnormal findings were revealed via chest and abdomen computed
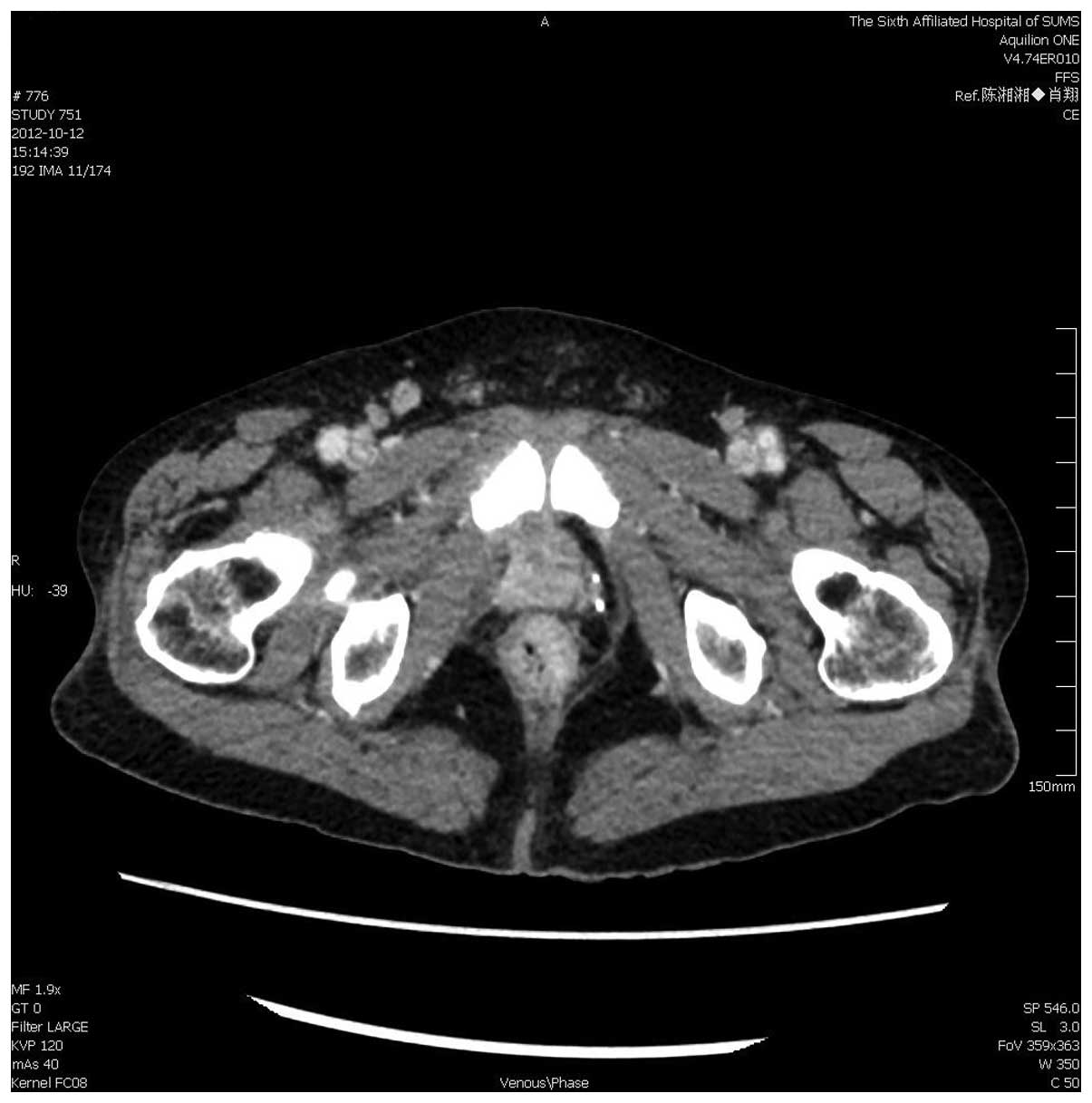
tomography (CT) scanning. Pelvic enhanced CT identified a
thickening of the low rectal wall (Fig.
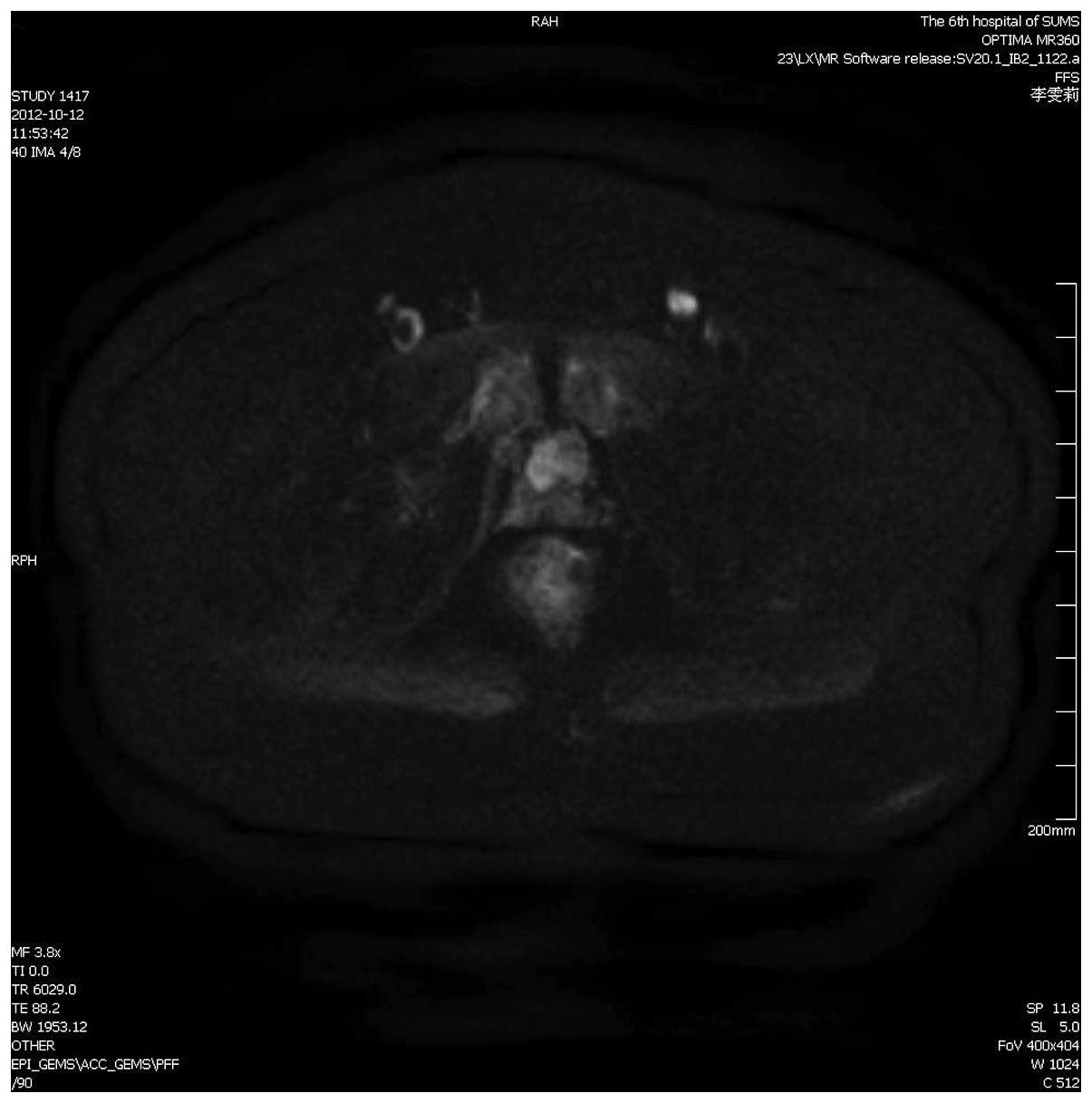
1). Pelvic enhanced magnetic resonance imaging (MRI) revealed
low rectal cancer and a mass located between the prostate and
fundus of the urinary bladder which was considered to be benign and
derived from the prostate, while the prostate was well encapsulated
(Fig. 2). No other sites of lymph
involvement were identified at that time from the chest and abdomen
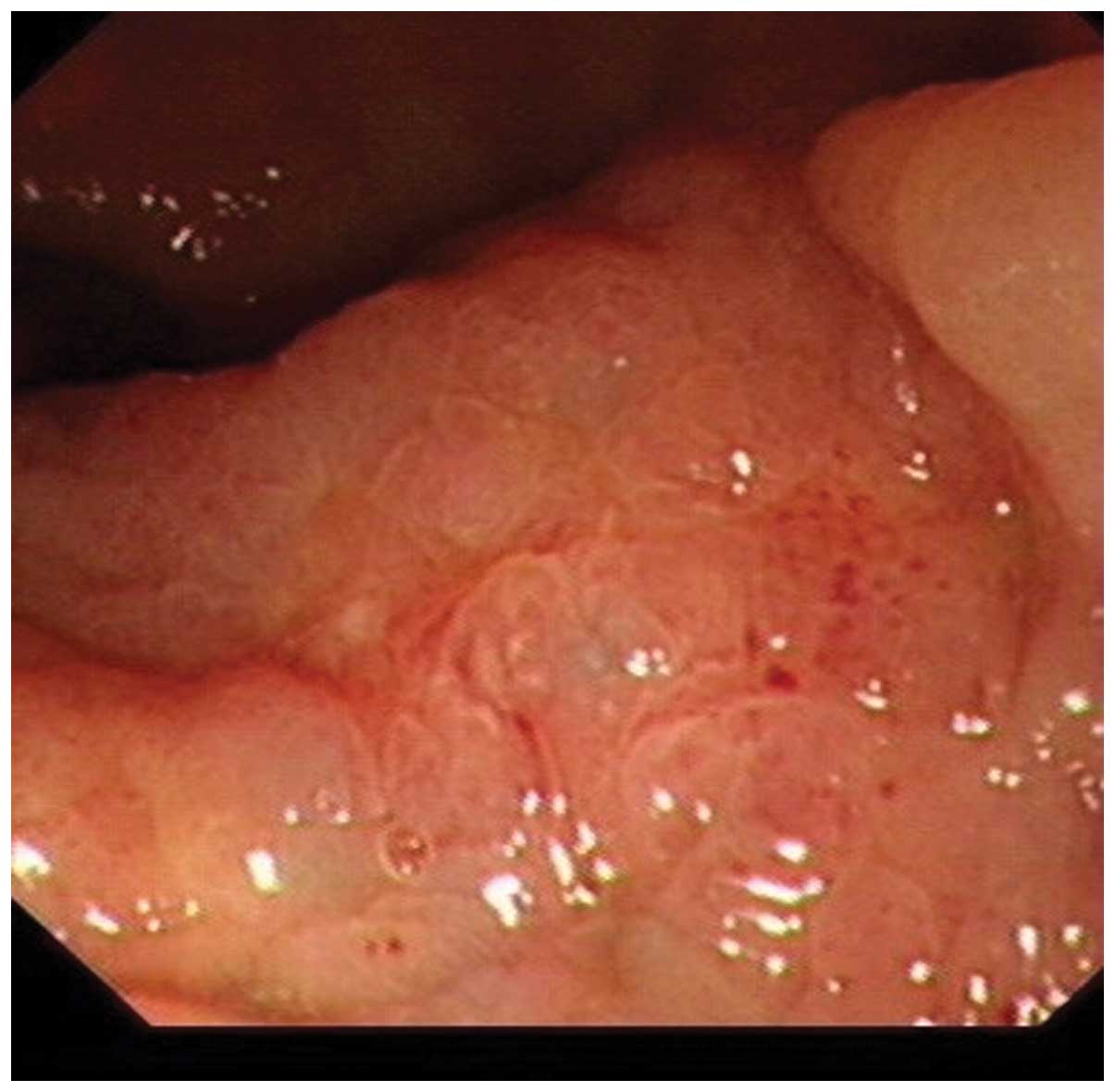
CT and pelvic enhanced CT scans. Enteroscopy revealed a rectal
tumor: a 3.5×3.5-cm in size, firm, poorly defined rectal protruding
lesion with erosion of the surface, which was located 3 cm away
from the anal verge (Fig. 3). An
increased rate of prostate specific antigen (PSA) was identified
following admission. The level of the serum PSA reached 9.387 ng/ml
(normal range, 0–4 ng/ml). No abnormal findings were revealed
throughout the laboratory tests with the exception of the PSA
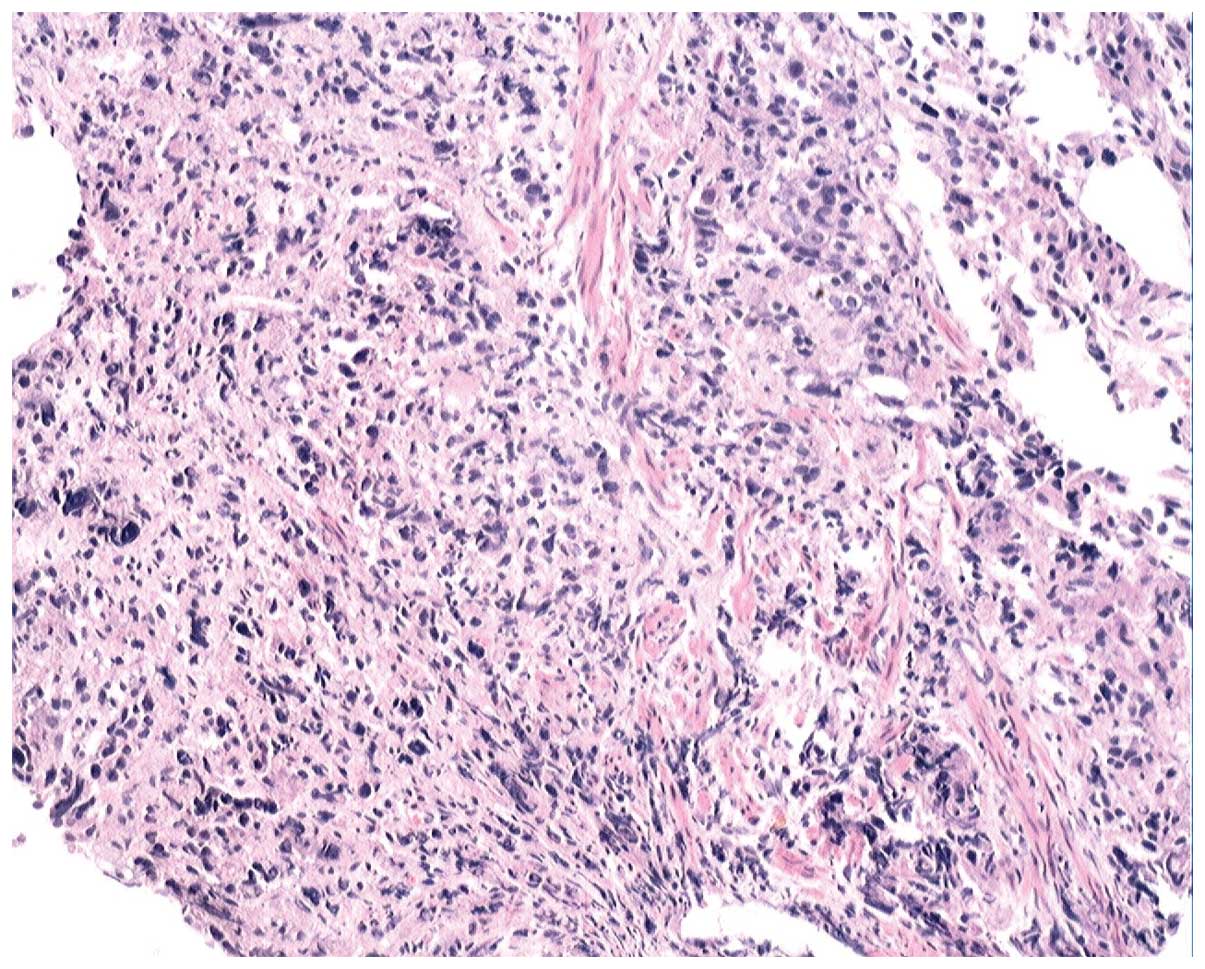
level. Rectoscopic biopsies were performed and revealed a poorly
differentiated adenocarcinoma (Fig.
4). The immunohistochemical analysis of the biopsy specimen
revealed PSA(+), CK20(-), CDX2(-), Villin(+) and P504S(+). From the
overall clinical presentation, preceding history and
immunohistochemical analysis, the view that the rectal
adenocarcinoma was derived from the prostate was still favored.
Finally, it was confirmed that the patient's rectal neoplasm was
derived from the prostate by the results and his clinical
manifestation. Taking the noted adhesion of the tumor and prostate
into consideration, we advised the patient to undergo neoadjuvant
chemoradiotherapy prior to surgery. Neoadjuvant chemoradiotherapy
is considered to achieve notable downsizing of the tumor which
benefits surgery. Written informed consent was obtained from the
patient and this study was approved by the ethics committee of The
Sixth Affiliated Hospital of Sun Yat-Sen University (Guangzhou,
China).
Discussion
Colorectal cancer is the third most common type of
cancer worldwide. The incidence of the colorectal cancer is high,
particularly in China where the crude rate reached
29.44/105 in 2012. In China, colorectal cancer is the
fourth most common carcinoma (2).
Small colorectal neoplasm is often asymptomatic. Occult blood in
the stool may be the only symptom. As the size of the lesion grows,
certain other symptoms including a change in stool caliber,
tenesmus, constipation or obstruction may occur. The lesions may
produce abdominal cramps. Constitutional symptoms, including weight
loss, anorexia and fatigue, are common. The most frequent
presenting symptoms are constipation, abdominal pain, rectal
bleeding and diarrhea; symptoms which are identical to those
observed with carcinoma of the rectum. Prostate cancer is the most
common male genitourinary tract malignancy, usually occurring after
the age of 60 years. The treatment for metastatic disease is
hormonal ablation, as most prostate cancers are androgen-sensitive.
Methods of androgen ablation include surgical and pharmacological
options. Bilateral surgical orchiectomy is the gold standard for
ablating testosterone production. Prostate cancer commonly
metastasizes to the bone, in particular the axial skeleton.
Peripheral bone metastasis of prostate adenocarcinoma is even more
uncommon (3). Digestive tract
metastasis of the prostate adenocarcinoma is relatively rare, but
may occur in the esophagus (4),
stomach (5), small intestine
(6) and other locations. Although
prostate cancer is one of the most commonly encountered
malignancies in clinical practice, it is extremely unusual for
prostate cancer to metastasize to the small bowel, colon and rectum
(7). Studies relating to solitary
rectal metastasis are uncommon (8,9). Herein we
report a case of solitary rectal metastasis of prostate
adenocarcinoma, for which a differential diagnosis was required.
This type of case had not been previously reported in the
literature. Prostate carcinoma involving the rectum occasionally
presents with rectal obstructive symptoms and an annular
constricting lesion of the rectum (10) and rectal bleeding, or as a rectal
ulcer (11). Prostate cancer is a
slowly growing neoplasm that is easily missed during its early
stages. Patients not previously diagnosed with prostatic
adenocarcinoma may present initially with metastases (12). Rectal infiltration takes the form of
an anterior rectal mass with or without ulceration in 52% of cases,
an annular stricture in 45%, and separate metastasis in 3%. In 40%
of patients, a preceding history of prostatic adenocarcinoma was
elicited at the time of gastrointestinal presentation, while in 60%
it was not elicited (13). Our
patient had a preceding one-year history of prostate adenocarcinoma
prior to admission to our department.
Prostatic adenocarcinoma spreading to the rectum
takes place by various routes, including direct invasion and
distant metastasis. For our patient, a contiguity invasion was
excluded due to the fact that the prostatic adenocarcinoma was
encapsulated. It is extremely rare for prostate cancer to
metastasize to nearby organs, including the rectum. Autopsy studies
have indicated that rectal involvement by prostatic adenocarcinoma
occurs in 4% of patients (13).
Prostate cancer metastasizing to colorectal tissue may occur
through at least four potential routes. The first is an
implantation of prostate cancer cells due to transrectal biopsy of
the prostate. Prostate cancer cells can spread through needle
biopsy, by seeding into perirectal or rectal tissue along the
needle biopsy (14). Our patient had
never undergone transrectal biopsy of the prostate; therefore this
situation could be excluded. The second route is through the
lymphatic channels, since the prostate and rectum share certain
lymphatic drainage to groups of pelvic lymph nodes (15). Only one case has been reported to
support this. Histopathological examination of the resected rectal
tissue of that patient using the step section method revealed a
number of cancer cells in the intramural lymphatic duct (10). Prostate adenocarcinoma metastasizing
to the rectum by way of lymphatic flow is directly evident. The
fact that the prostate and rectum share certain lymphatic drainage
to groups of pelvic lymph nodes also demonstrates the rare solitary
prostate metastasis of colorectal carcinoma (16,17).
However, a retrospective study also supports this view, with a
conclusion that 5 of 112 (4.5%) rectal adenocarcinoma patients were
identified as having metastatic prostate adenocarcinoma within the
positive perirectal lymph nodes (15). The third route is hematogenous
metastasis, which is also possible in the case of our patient, as
he had previously undergone TURP, during which bleeding is
relatively common. The fourth type, with subserosal metastatic
implant of the proximal sigmoid, may occasionally be encountered
(16).
Although the PSA may not be at a high level in all
patients with prostatic adenocarcinoma (17), the elevated rate of serum PSA
indicated a recurrence of prostate adenocarcinoma. This suggests
that the monitoring of the PSA rate is a significant element of
follow-up after resection of the prostate.
Discriminating between primary rectal carcinoma and
prostate carcinoma metastasis to the rectum is of obvious
significance due to the different treatments and prognoses
involved. The significance of the correlation between urinary and
gastrointestinal symptoms in detecting prostatic neoplasms in older
male patients should be emphasized. Careful immunohistochemical
examination of specimens may prevent inappropriate surgical
interventions. Immunohistochemical inspection is an essential tool
in distinguishing the origin of a lymph node metastasis,
particularly in cases when the histology does not appear typical of
rectal carcinoma (18).
Acknowledgements
This study was financially supported by grants from
the National Natural Science Foundation of China (grant nos.
81100255 and 81370480), and the New Star of Zhujiang Science and
Technology Foundation (grant no. 2013J2200023).
References
|
1
|
Ito K: Prostate cancer in Asian men. Nat
Rev Urol. 11:197–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu J and Chen N: Current status of rectal
cancer treatment in China. Colorectal Dis. 5:1345–1350. 2013.
View Article : Google Scholar
|
|
3
|
Reigman HI and Stokkel MP: Peripheral bone
metastases in prostate cancer: a rare localization at initial
presentation. Clin Nucl Med. 29:335–336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura T, Mohri H, Shimazaki M, et al:
Esophageal metastasis from prostate cancer: diagnostic use of
reverse transcriptase-polymerase chain reaction for
prostate-specific antigen. J Gastroenterol. 32:236–240. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larkin JO, Collins CG, Martin ST, et al:
Paraesophageal lymph node metastasis from prostatic adenocarcinoma
in a patient with esophageal squamous carcinoma. Dis Esophagus.
18:124–126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malhi-Chowla N, Wolfsen HC, Menke D and
Woodward TA: Prostate cancer metastasizing to the small bowel. J
Clin Gastroenterol. 32:439–440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lebret T and Mejean A: Rare locations of
metastases from prostate cancer. Prog Urol. 18 Suppl 7:357–364.
2008.[(In French)]. View Article : Google Scholar
|
|
8
|
Venara A, Thibaudeau E, Lebdai S, et al:
Rectal metastasis of prostate cancer: about a case. J Clin Med Res.
2:137–139. 2010.PubMed/NCBI
|
|
9
|
Abbas TO, Al-Naimi AR, Yakoob RA, Al-Bozom
IA and Alobaidly AM: Prostate cancer metastases to the rectum: a
case report. World J Surg Oncol. 9:562011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morita T, Meguro N, Tomooka Y, et al:
Rectal metastasis of prostatic cancer causing annular stricture: a
case report. Hinyokika Kiyo. 37:295–298. 1991.[(In Japanese)].
PubMed/NCBI
|
|
11
|
Wadhwa P, Mandal AK, Singh SK, Goswami AK,
Sharma SC, Joshi K, et al: Primary transitional cell carcinoma of
the prostate presenting as a rectal ulcer. Urol Int. 72:176–177.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo CC, Pisters LL and Troncoso P:
Prostate cancer invading the rectum: a clinicopathological study of
18 cases. Pathology. 41:539–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bowrey DJ, Otter MI and Billings PJ:
Rectal infiltration by prostatic adenocarcinoma: report on six
patients and review of the literature. Ann R Coll Surg Engl.
85:382–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vaghefi H, Magi-Galluzzi C and Klein EA:
Local recurrence of prostate cancer in rectal submucosa after
transrectal needle biopsy and radical prostatectomy. Urology.
66:8812005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murray SK, Breau RH, Guha AK and Gupta R:
Spread of prostate carcinoma to the perirectal lymph node basin:
analysis of 112 rectal resections over a 10-year span for primary
rectal adenocarcinoma. Am J Surg Pathol. 28:1154–1162. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gengler L, Baer J and Finby N: Rectal and
sigmoid involvement secondary to carcinoma of the prostate. Am J
Roentgenol Radium Ther Nucl Med. 125:910–917. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gallee MP, Visser-de JongE, van der Korput
JA, van der Kwast TH, ten Kate FJ, Schroeder FH, et al: Variation
of prostate-specific antigen expression in different tumour growth
patterns present in prostatectomy specimens. Urol Res. 18:181–187.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lane Z, Epstein JI, Ayub S and Netto GJ:
Prostatic adenocarcinoma in colorectal biopsy: clinical and
pathologic features. Hum Pathol. 39:543–549. 2008. View Article : Google Scholar : PubMed/NCBI
|