Introduction
Colorectal cancer is the third most commonly
diagnosed type of cancer worldwide (1). In China, ∼50% of cases of colorectal
cancer arise in the rectum, accounting for >100,000 new
diagnoses of rectal cancer in 2012 (2). Despite the advent of total mesorectal
excision (TME) (3) facilitating major
improvements in the management of locally advanced rectal cancer
(LARC), which is defined as clinical tumor stage (cT)3–4 or
clinical lymph node stage (cN)+, management remains a challenge
following Miles' procedure due to the high recurrence rate and poor
quality of life (4).
Neoadjuvant chemoradiation (CRT) has been
established as the standard treatment strategy for LARC, as it
appears to be associated with improved local control and a higher
rate of sphincter-sparing procedures (5). Pathological tumor response (PTR),
including tumor regression grade (TRG) and downstaging, has been
indicated to be an important prognostic factor for LARC following
CRT (6–8). PTR may be useful to stratify patients
with different prognoses and to tailor surgical treatment
strategies, particularly for sphincter-sparing candidates (9). Therefore, the early and accurate
prediction of PTR is important.
The modified Response Evaluation Criteria in Solid
Tumors (RECIST), based on unidimensional measurements, is currently
considered to be the gold standard for the majority of solid tumors
(10). However, as a traditional
diameter-based method, RECIST is unable to provide data closely
reflecting the actual tumor volume change, as the CRT may induce
apparent fibrosis or inflammation (11,12).
Additionally, the actual tumor volume change was affected by
patient positioning and discrepant scan planes (13). As a canal-shaped organ, rectal cancer
always presents with irregular tumor configurations and non-uniform
treatment-association shrinkage to CRT (14,15).
Furthermore, the RECIST Working Group encourages the development of
novel markers and tools to predict potential therapeutic benefit
for cancer patients (16).
With the advancement in imaging techniques and their
increasing availability in oncological practice, tumor volume
reduction rate (TVRR), which is based on actual tumor volume change
and measured using three-dimensional (3D) region-of-interest (ROI)
magnetic resonance (MR) volumetry, has recently been investigated
(17–19). However, to the best of our knowledge,
all previous TVRR studies involved retrospective analysis, and did
not perform comparisons between TVRR and RECIST.
Therefore, the present study was based on a
prospective randomized trial, and was conducted to determine
whether TVRR is associated with PTR in terms of TRG and
downstaging. In addition, the current study aimed to determine
whether TVRR is superior to RECIST in the evaluation patients with
LARC following CRT.
Patients and methods
Patients
The present study is based on a prospective
randomized trial (www.clinicaltrials.gov; NCT01211210). Between October
2010 and September 2013, 105 primary rectal cancer patients were
treated with pre-operative CRT at the Sixth Affiliated Hospital of
Sun Yat-Sen University (Guangzhou, Guangdong, China). The present
study was conducted in accordance with the Declaration of Helsinki
and approved by the Ethics Committee of the Sixth Affiliated
Hospital of Sun Yat-Sen University. Written informed consent was
obtained from all participants. The inclusion criteria were as
follows: i) Histologically confirmed adenocarcinoma of the rectum;
ii) distal margin of tumor located within 12 cm of the anal verge;
iii) cT3–4 or cN+, evaluated by MR imaging with or without
transrectal ultrasonography; iv) no evidence of distant metastasis;
v) no previous or concurrent malignancy; and vi) the availability
of MR volumetry and contrast-enhanced computed tomography (CT) scan
data.
Treatment strategies
The patients were treated according to the
institutional protocol of Sun Yat-Sen University, as previously
described (20). Pre-operative
radiotherapy of 46 GY in 23 fractions was delivered to the pelvis,
followed by an optional boost of 4 GY in two fractions to the
primary tumor. All patients received 3D conformal radiotherapy with
CT simulation. This three-field treatment plan included a 6-MV
photon posteroanterior field and 15-MV photon opposed lateral
fields.
Concurrent chemotherapy regimens included
5-fluorouracil (5-FU; Xudong Haipu Pharmaceutical Co., Ltd.,
Shanghai, China) alone or a doublet combination of 5-FU and
oxaliplatin (Sanofi, Paris, France). 5-FU was administered
according to a simplified de Gramont regimen [400 mg/m2
intravenous (i.v.) bolus followed by a 46-h protracted i.v.
infusion of 2,400 mg/m2, every two weeks]. Oxaliplatin
was administered at a dose of 85 mg/m2 twice weekly.
Standardized total mesorectal excision (TME) was
scheduled to be performed 6–8 weeks after completion of the
neoadjuvant CRT. Patients were restaged by performing a CT scan of
the chest-abdomen and contrast-enhanced MR imaging of the pelvis.
Creation of a temporary diverting ostomy was at the discretion of
the primary surgeon, however, ostomy takedown was advised following
the completion of all systemic therapy.
MR volumetry and RECIST
evaluation
Two independent radiologists used identical protocol
to perform 3D-ROI MR volumetry for all patients at the initial
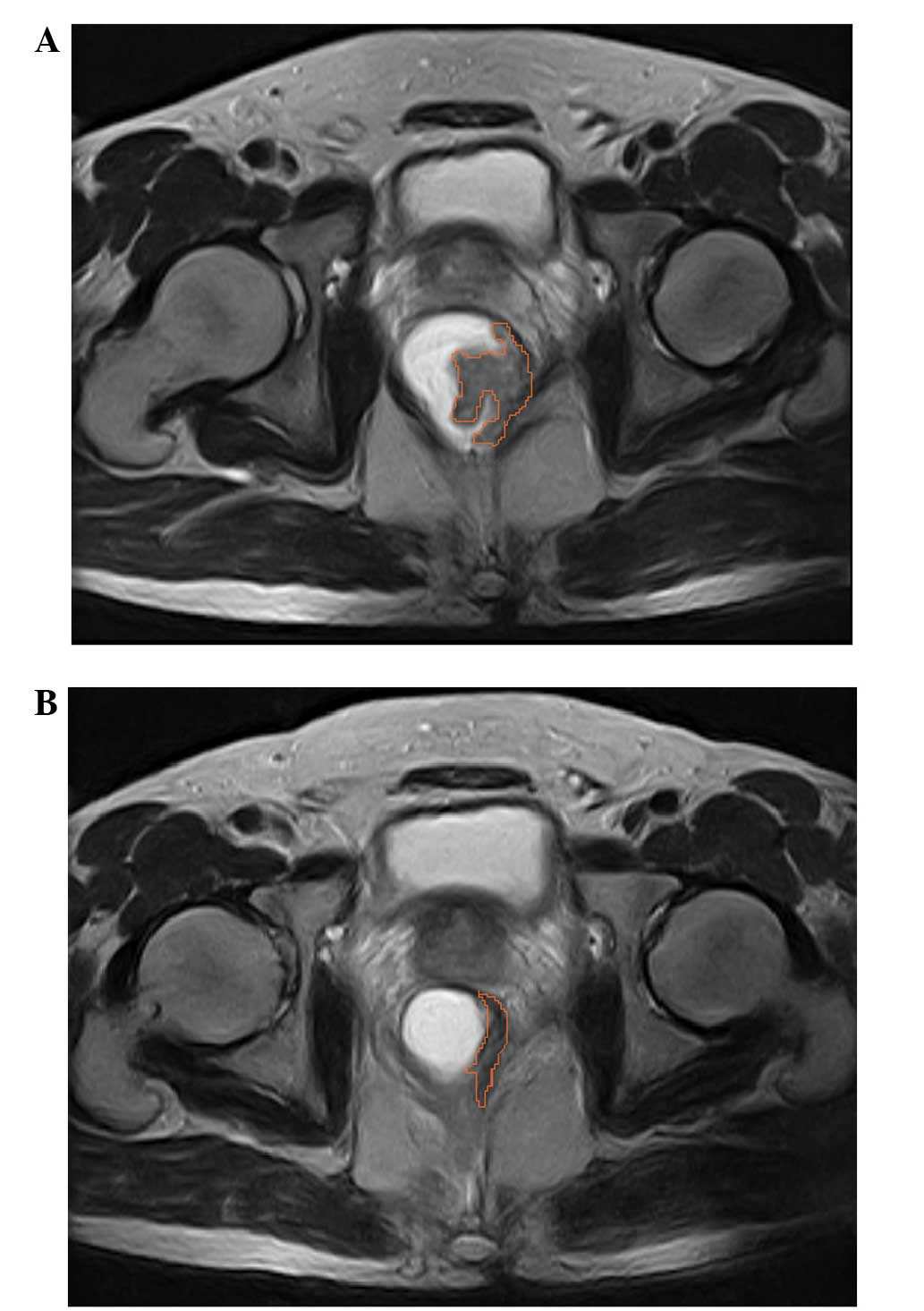
workup and within seven days of undergoing surgery (21). The cross-sectional lesion areas were
measured on axial T2-weighted images by manually tracing the lesion
boundaries. The contour of the cross-sectional lesions were defined
as intermediate signal intensity areas that differed from the
healthy adjacent rectal wall (Fig.
1). Furthermore, the tumor volumes were automatically
calculated by summing each of the cross-sectional volumes
(multiplying cross-sectional area by section thickness) using the
Advantage Workstation (version 4.0; GE Healthcare, New York, NY,
USA). The mean values determined by the two radiologists were used
as the final volumetry results. In addition, TVRR was calculated as
follows: TVRR = (Vpre-CRT - Vpost-CRT) /
Vpre-CRT × 100, where Vpre-CRT is the pre-CRT
tumor volume and Vpost-CRT is the post-CRT but
pre-surgery tumor volume.
Positive lymph node involvement was defined as the
presence of a lymph node measuring ≥1.0 cm in the smallest
diameter, as observed by MR imaging. T stage was evaluated
according to the method employed by Smith and Brown (22). Complete response (CR; disappearance of
all target lesions), partial response (PR; ≥30% decrease in the sum
of the diameters of the target lesions), progressive disease (PD;
≥20% increase in the sum of the diameters of the target lesions)
and stable disease (SD; insufficient shrinkage to qualify for PR or
insufficient increase to qualify for PD) states were evaluated
according to RECIST, version 1.1 (16). A clinical response was defined as CR
and PR.
Pathological evaluation
Following surgery, pathological evaluation was
performed by an experienced pathologist, according to the
tumor-node-metastasis staging system of the seventh edition of the
American Joint Committee on Cancer (23). Downstaging was defined as
postneoadjuvant therapy (yp)T0–2 and ypN0. Furthermore, TRG was
defined using Ryan's criteria (24),
as follows: Grade 0, no viable cancer cells; grade 1, single cells
or small groups of cancer cells; grade 2, residual cancer outgrown
by fibrosis; grade 3, residual cancer outgrown by fibrosis or no
fibrosis with extensive residual cancer. Regression grading
involved the primary tumor and regional lymph nodes, with a good
TRG defined as TRG grade 0 or 1.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviation or the median (range), whereas categorical
variables are presented as a number (percentage). Comparisons of
TVRR between independent subgroups were performed using a
two-sample t-test or analysis of variance. Comparisons of RECIST
between independent subgroups were performed using Fisher's Exact
test. In addition, receiver operating characteristic (ROC) curves
of TVRR and RECIST were constructed to predict PTR. The ROC curve
was used to determine the optimal cut-off of TVRR for predicting
PTR, and predictive accuracies were quantified and compared using
the area under the ROC curve (AUC) (25). Sensitivities and specificities of TVRR
and RECIST were also calculated. Two-sided P<0.05 was considered
to indicate a statistically significant difference, and SAS
software for Windows (version 9.2; SAS Institute, Cary, NC, USA)
was used for all statistical analyses.
Results
Patient characteristics
A total of 123 patients were enrolled in the present
study, from which 18 patients were excluded due to withdrawal of
informed consent (n=3), protocol deviation (n=3) or receipt of
surgery elsewhere (n=12). The clinical characteristics of the 105
included patients are shown in Table
I. The median age was 56 years (range, 24–73 years), and the
cohort consisted of 73 (69.5%) male patients and 32 (30.5%) female
patients. A total of 84 (80.0%) patients presented with cT3
disease, 72 (68.6%) patients were regional lymph node-positive
prior to treatment and 46 (43.8%) patients had a low lying rectal
tumor. Furthermore, combined chemotherapy was administered to 73
(69.5%) patients. The surgical procedures performed in the present
cohort included a low anterior resection (71 patients; 67.6%), and
Parks' (20 patients; 19.0%) and Miles' (14 patients; 13.3%)
procedures.
 | Table I.Patient characteristics
(n=105)a. |
Table I.
Patient characteristics
(n=105)a.
| Characteristic | n (%) |
|---|
| Gender |
|
| Male | 73 (69.5) |
|
Female | 32 (30.5) |
| Distance from the
anal verge, cm |
|
| ≤5 | 46 (43.8) |
|
>5 | 59 (56.2) |
| Histological
grade |
|
| 1 | 39 (37.1) |
| 2 | 53 (50.5) |
| 3 | 13 (12.4) |
| cT
classification |
|
|
cT2 | 2 (1.9) |
|
cT3 | 84 (80.0) |
|
cT4 | 19 (18.1) |
| cN
classification |
|
|
cN- | 33 (31.4) |
|
cN+ | 72 (68.6) |
| Pre-CRT CEA,
ng/ml |
|
|
Normal | 75 (71.4) |
|
Elevated | 30 (28.6) |
| Chemotherapy |
|
| 5-FU
alone | 32 (30.5) |
| 5-FU
plus oxaliplatin | 73 (69.5) |
| Surgical
procedure |
|
|
LAR | 71 (67.6) |
|
Parks' | 20 (19.0) |
|
Miles' | 14 (13.4) |
Association of TVRR and RECIST with
patient characteristics
The mean Vpre-CRT and
Vpost-CRT were 44.82±44.64 cm3 and
18.27±19.04 cm3, respectively, and the mean TVRR was
58.6±24.4%. According to RECIST, 5 (4.8%) patients achieved CR, 44
(41.9%) achieved PR, 55 (52.4%) exhibited SD and only 1 (0.9%)
patient experienced PD. The associations between TVRR and RECIST
and the patient characteristics are presented in Table II. None of the clinical
characteristics investigated were significantly associated with
TVRR or RECIST. For example, patients that received the doublet
chemotherapy regimen exhibited a higher mean TVRR (59.1%) than
those treated with single-agent chemotherapy (57.6%); however, the
difference was not statistically significant (P=0.766).
 | Table II.Association of patient
characteristics with TVRR and RECIST. |
Table II.
Association of patient
characteristics with TVRR and RECIST.
| Characteristic | Patients, n
(%) | TVRR,
%a |
P-valueb | CR+PR, n
(%)c |
P-valued |
|---|
| Gender |
|
| 0.379 |
| 0.403 |
|
Male | 73 (69.5) | 57.2±26.0 |
| 32 (43.8) |
|
|
Female | 32 (30.5) | 61.8±20.1 |
| 17 (53.1) |
|
| Age, years |
|
| 0.239 |
| 0.091 |
|
≤60 | 72 (68.6) | 58.2±20.5 |
| 38 (52.8) |
|
|
>60 | 33 (31.4) | 53.6±29.2 |
| 11 (33.3) |
|
| Distance from the
anal verge, cm |
|
| 0.774 |
| 0.237 |
| ≤5 | 46 (43.8) | 57.9±24.2 |
| 18 (39.1) |
|
|
>5 | 59 (56.2) | 59.2±24.7 |
| 31 (52.5) |
|
| Histological
grade |
|
| 0.652 |
| 0.127 |
| 1 | 39 (37.1) | 60.5±20.4 |
| 23 (59.0) |
|
| 2 | 53 (50.5) | 58.6±28.4 |
| 22 (41.5) |
|
| 3 | 13 (12.4) | 53.2±17.3 |
| 4 (30.8) |
|
| Clinical T
classification |
|
| 0.596 |
| 0.317 |
|
|
cT2-T3 | 86 (81.9) | 58.2±26.1 |
| 38 (44.2) |
|
|
cT4 | 19 (18.1) | 60.6±14.7 |
| 11 (57.9) |
|
| Clinical N
classification |
|
| 0.936 |
| 0.346 |
|
cN- | 33 (31.4) | 58.3±20.9 |
| 15 (45.5) |
|
|
cN+ | 72 (68.6) | 58.8±26.0 |
| 34 (47.2) |
|
| Pre-CRT CEA,
ng/ml |
|
| 0.589 |
| 0.516 |
|
Normal | 75 (71.4) | 59.5±24.9 |
| 37 (49.3) |
|
|
Elevated | 30 (28.6) | 56.6±23.4 |
| 12 (40%) |
|
| Treatment
group |
|
| 0.766 |
| 0.403 |
| 5-FU
alone | 32 (30.5) | 57.6±20.3 |
| 17 (53.1) |
|
| 5-FU
plus oxaliplatin | 73 (69.5) | 59.1±26.1 |
| 32 (43.8) |
|
Association of TVRR and RECIST with
PTR
Following CRT and pathological evaluation, TRG 0, 1,
2 and 3 was identified in 12 (11.5%), 42 (40.0%), 31 (29.5%) and 20
(19.0%) patients, respectively. Additionally, 59 (56.2%) patients
achieved downstaging. Table III
shows the TVRRs and RECIST values according to the PTR findings. The
TVRRs were significantly higher among patients with good TRG (70.2
vs. 46.4%; P<0.001). Additionally, patients with downstaging
exhibited significantly higher TVRRs compared with those without
downstaging (66.5 vs. 48.2%; P<0.001). For RECIST, a clinical
response was more frequently observed in patients with good TRG and
downstaging (P=0.001 and P=0.006, respectively).
 | Table III.Association of TVRR and RECIST with
PTR. |
Table III.
Association of TVRR and RECIST with
PTR.
| Response | TVRR,
%a |
P-valueb | CR + PR, n
(%)c |
P-valued |
|---|
| TRG |
| <0.001 |
| 0.001 |
|
0–1 | 70.2±15.2 |
| 34 (63.0) |
|
|
2–3 | 46.4±26.4 |
| 15 (29.4) |
|
| Downstaging |
| <0.001 |
| 0.006 |
|
Yes | 66.5±18.0 |
| 35 (58.3) |
|
| No | 48.2±27.9 |
| 14 (31.1) |
|
Predictive values of TVRR and RECIST
for PTR
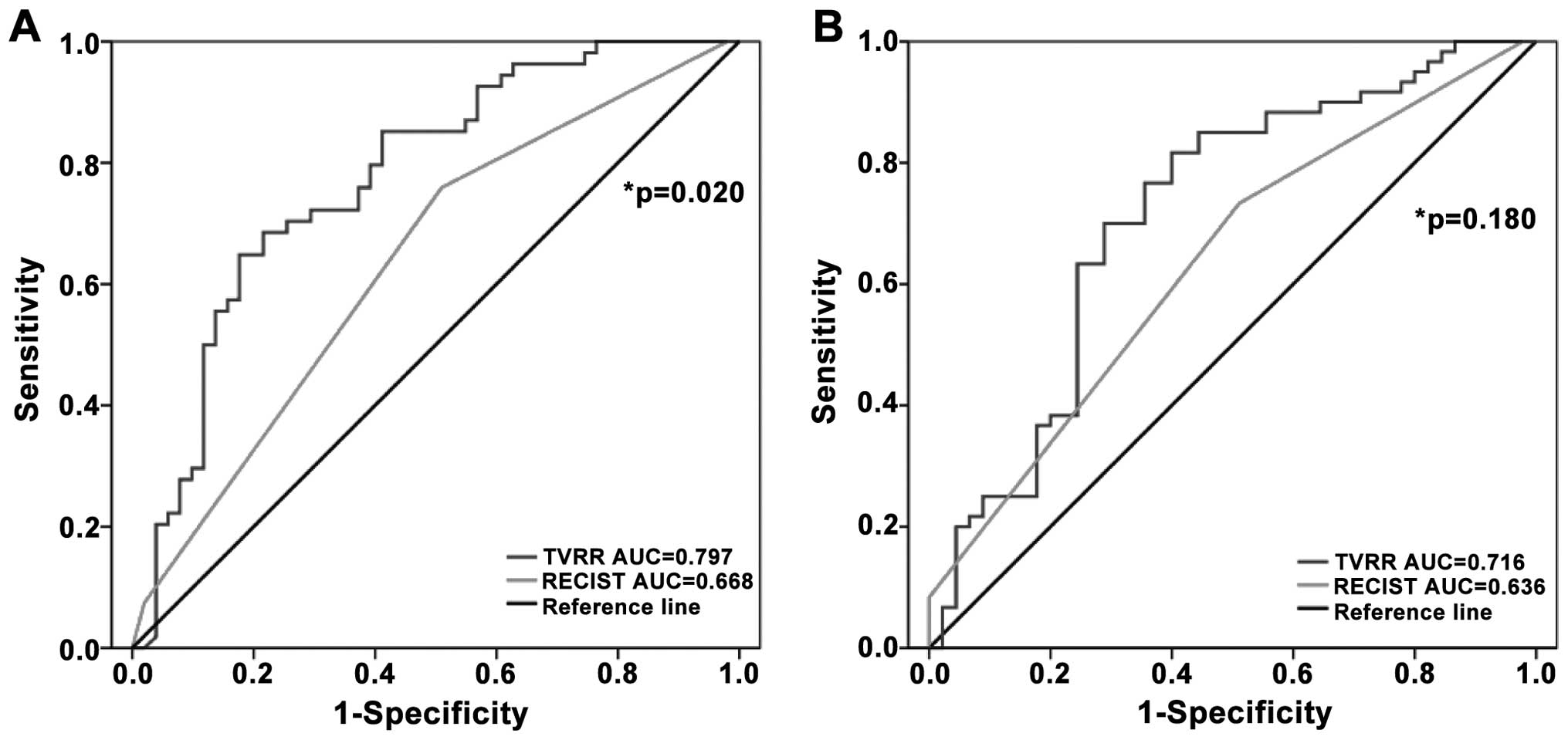
ROC curves of TVRR and RECIST were constructed and
compared (Fig. 2). For the prediction
of TRG, the AUC of TVRR was significantly larger than that of
RECIST (TVRR AUC, 0.797; 95% CI, 0.710–0.885; vs. RECIST AUC,
0.668; 95% CI, 0.577–0.758; P=0.020]. Similarly, the AUC of TVRR
was greater than that of RECIST for downstaging (TVRR AUC, 0.716;
95% CI, 0.612–0.819; vs. RECIST AUC, 0.636; 95% CI, 0.543–0.729);
however, the difference did not reach statistical significance
(P=0.180).
The optimum cut-off for TVRR was determined to be
65% by a trade-off between sensitivity and specificity, thus, ≥65%
TVRR was considered to indicate a clinical response. Using this
optimal cut-off value, TVRR attained higher predictive values
compared with RECIST (Table IV). For
TRG, the sensitivity and specificity of TVRR were 70.4 (95% CI,
56.4–82.0%) and 80.4% (95% CI, 66.9–90.2), respectively. For
downstaging, the sensitivity and specificity of TVRR were 61.7 (95%
CI, 48.2–73.9) and 75.6% (95% CI, 60.5–87.1), respectively.
 | Table IV.Predictive values of TVRR and RECIST
for PTR. |
Table IV.
Predictive values of TVRR and RECIST
for PTR.
| Response | TVRR, % (95%
CI) | RECIST, % (95%
CI) |
|---|
| Downstaging |
|
|
|
Sensitivity | 61.7
(48.2–73.9) | 58.3
(44.9–70.9) |
|
Specificity | 75.6
(60.5–87.1) | 68.9
(53.4–81.8) |
| TRG |
|
|
|
Sensitivity | 70.4
(56.4–82.0) | 63.0
(48.7–75.7) |
|
Specificity | 80.4
(66.9–90.2) | 70.6
(56.2–82.5) |
Discussion
In the present study, the data of 105 cases of LARC
from a prospective randomized trial were analyzed. Compared with
RECIST, TVRR appeared to be a superior method for predicting PTR,
particularly TRG, of LARC patients who had received CRT.
At present, low lying rectal cancers are associated
with relatively high local recurrence rates and a poor quality of
life following sphincter ablation (4,26). In the
current cohort, all patients, excluding one, received complete
resection and 91 (86.7%) patients underwent sphincter-preserving
surgery. These results appear to be an improvement on previous
reports (3,5,27). The
high sphincter-preserving rate observed in the present study may be
associated with the fact that the majority of patients underwent a
novel treatment strategy termed two-stage TME (20). The PTRs of patients in the present
study were consistent with a previous study by Fokas et al
(9); a good TRG was determined in 54
(51.4%) patients and downstaging was observed in 59 (56.2%)
patients, indicating that the novel strategy of two-stage TME was
safe and effective.
In the present study, 3D ROI MR volumetry was used
to determine TVRR. The mean Vpre-CRT was 44.82±44.64
cm3 and the mean Vpost-CRT was 18.27±19.04
cm3. Previous studies (14,15,17) have
reported a mean Vpre-CRT range of 19.0–58.0
cm3 and a mean Vpost-CRT range of 6.0–20.0
cm3. This implies that 3D ROI MR volumetry is a reliable
and reproducible method in clinical practice, and may be suitable
for wide application with the availability of MR and a computer
station.
The results of the present study identified no
significant association between patient demographics and TVRR and
RECIST. In agreement, a previously conducted large-scale
retrospective study (28) identified
no significant associations, and demonstrated that TVRR and RECIST
were independent clinical methods for assessing treatment efficacy.
Furthermore, TVRR and RECIST were significantly associated with
PTR, including TRG, and downstaging in the current analysis. This
is consistent with previous studies (17,18,28,29),
and confirms that the two parameters are effective and
reliable.
To the best of our knowledge, the present data
indicates for the first time that volumetric-based TVRR is more
accurate than diameter-based RECIST in predicting a good TRG. In
agreement with findings from previous studies, which ranged from 45
to 70% (17,18,28), a
TVRR cut-off value of 65% was selected in the present study. All
previous studies were retrospective, while the current data was
prospectively collected. Therefore, the current results should be
carefully interpreted and the cut-off value of 65% should be
assessed by additional follow-up, including long-term outcome.
Volumetric measurement requires more labor and time compared with
diameter measurement. However, in the present study, each rectal
volumetric examination took ∼15 min compared with the 30 min
reported by Nougaret et al (17). Furthermore, semi-automated volumetry
using 3D MR, which appears to be more feasible, accurate and
reproducible, requires additional investigation.
Valentini et al (8) reported that patients that downstaged
(ypT0-T2) following pre-operative CRT exhibited five-year local
control rates of 83–100% and overall survival rates of 81–91%.
These rates are similar to those of cT1-T2 patients treated with
conservative surgery alone. Nougaret et al (17) reported that patients with a TVRR of
≥70% exhibited significantly longer DFS times (hazard ratio, 13.70;
95% CI, 3.98–31.93; P<0.001) and that TVRR was an independent
prognostic parameter for DFS (P=0.003). However, the overall
survival and relapse-free survival data of the present study have
yet to mature. Therefore, this data require analysis at a later
date to clarify the preliminary results.
In conclusion, the present analysis compared TVRR
determined by 3D ROI MR volumetry with RECIST for predicting PTR
following CRT and demonstrated the superiority of TVRR. The TVRR
cut-off value of 65% is a parameter that can easily be used as a
surrogate for clinical response to predict TRG. Thus, confirmation
of TVRR as a parameter for predicting PTR and long-term outcome in
rectal cancer is warranted.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zhang S, et al: Annual
report on status of cancer in China, 2010. Chin J Cancer Res.
26:48–58. 2014.PubMed/NCBI
|
|
3
|
Maurer CA, Renzulli P, Kull C, et al: The
impact of the introduction of total mesorectal excision on local
recurrence rate and survival in rectal cancer: long-term results.
Ann Surg Oncol. 18:1899–1906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiltink LM, Chen TY, Nout RA, et al:
Health-related quality of life 14 years after preoperative
short-term radiotherapy and total mesorectal excision for rectal
cancer: report of a multicenter randomised trial. Eur J Cancer.
50:2390–2398. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sauer R, Liersch T, Merkel S, et al:
Preoperative versus postoperative chemoradiotherapy for locally
advanced rectal cancer: results of the German CAO/ARO/AIO-94
randomized phase III trial after a median follow-up of 11 years. J
Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sannier A, Lefèvre JH, Panis Y, et al:
Pathological prognostic factors in locally advanced rectal
carcinoma after neoadjuvant radiochemotherapy: analysis of 113
cases. Histopathology. 65:623–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhadda AS, Bessell EM, Scholefield J, et
al: Mandard tumour regression grade, perineural invasion,
circumferential resection margin and post-chemoradiation nodal
status strongly predict outcome in locally advanced rectal cancer
treated with preoperative chemoradiotherapy. Clin Oncol (R Coll
Radiol). 26:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valentini V, Coco C, Picciocchi A, et al:
Does downstaging predict improved outcome after preoperative
chemoradiation for extraperitoneal locally advanced rectal cancer?
A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys.
53:664–674. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fokas E, Liersch T, Fietkau R, et al:
Tumor regression grading after preoperative chemoradiotherapy for
locally advanced rectal carcinoma revisited: updated results of the
CAO/ARO/AIO-94 Trial. J Clin Oncol. 32:1554–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Curvo-Semedo L, Lambregts DM, Maas M, et
al: Rectal cancer: assessment of complete response to preoperative
combined radiation therapy with chemotherapy-conventional MR
volumetry versus diffusion-weighted MR imaging. Radiology.
260:734–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CC, Lee RC, Lin JK, et al: How
accurate is magnetic resonance imaging in restaging rectal cancer
in patients receiving preoperative combined chemoradiotherapy. Dis
Colon Rectum. 48:722–728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang JZ, Mayr NA, Zhang D, et al:
Sequential magnetic resonance imaging of cervical cancer: the
predictive value of absolute tumor volume and regression ratio
measured before, during, and after radiation therapy. Cancer.
116:5093–5101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torkzad M, Lindholm J, Martling A and
Blomqvist L: Retrospective measurement of different size parameters
of non-radiated rectal cancer on MR images and pathology slides and
their comparison. Eur Radiol. 13:2271–2277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mayr NA, Yuh WT, Taoka T, et al: Serial
therapy-induced changes in tumor shape in cervical cancer and their
impact on assessing tumor volume and treatment response. AJR Am J
Roentgenol. 187:65–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang JH, Kim YC, Kim H, et al: Tumor
volume changes assessed by three-dimensional magnetic resonance
volumetry in rectal cancer patients after preoperative
chemoradiation: the impact of the volume reduction ratio on the
prediction of pathologic complete response. Int J Radiat Oncol Biol
Phys. 76:1018–1025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nougaret S, Rouanet P, Molinari N, et al:
MR volumetric measurement of low rectal cancer helps predict tumor
response and outcome after combined chemotherapy and radiation
therapy. Radiology. 263:409–418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yeo SG, Kim DY, Park JW, et al: Tumor
volume reduction rate after preoperative chemoradiotherapy as a
prognostic factor in locally advanced rectal cancer. Int J Radiat
Oncol Biol Phys. 82:e193–e199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Wang J, Deng Y, Wu X and Wang L:
Neoadjuvant therapy followed by local excision and two-stage total
mesorectal excision: a new strategy for sphincter preservation in
locally advanced ultra-low rectal cancer. Gastroenterol Rep (Oxf).
2:37–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barbaro B, Vitale R, Leccisotti L, et al:
Restaging locally advanced rectal cancer with MR imaging after
chemoradiation therapy. Radiographics. 30:699–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith N and Brown G: Preoperative staging
of rectal cancer. Acta Oncol. 47:20–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobin LH and Compton CC: TNM seventh
edition: what's new, what's changed: communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryan R, Gibbons D, Hyland JM, et al:
Pathological response following long-course neoadjuvant
chemoradiotherapy for locally advanced rectal cancer.
Histopathology. 47:141–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: a nonparametric approach.
Biometrics. 44:837–845. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
den Dulk M, Putter H, Collette L, et al:
The abdominoperineal resection itself is associated with an adverse
outcome: the European experience based on a pooled analysis of five
European randomised clinical trials on rectal cancer. Eur J Cancer.
45:1175–1183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bosset JF, Collette L, Calais G, et al:
Chemotherapy with preoperative radiotherapy in rectal cancer. N
Engl J Med. 355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeo SG, Kim DY, Kim TH, et al: Tumor
volume reduction rate measured by magnetic resonance volumetry
correlated with pathologic tumor response of preoperative
chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys.
78:164–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park IJ, You YN, Agarwal A, et al:
Neoadjuvant treatment response as an early response indicator for
patients with rectal cancer. J Clin Oncol. 30:1770–1776. 2012.
View Article : Google Scholar : PubMed/NCBI
|