Introduction
Myelodysplastic syndromes (MDSs) are a group of
life-threatening malignancies that primarily afflict the elderly
population. The treatment of elderly patients with intensive
chemotherapy is associated with high rates of treatment-related
morbidity and mortality. The hypermethylation of CpG islands within
promoter regions, and the resultant silencing of tumor-associated
genes, are significant events involved in the pathogenesis of MDS
(1). Previous studies have revealed
that the DNA methyltransferase (DNMT) 1 and 3A enzymes contribute
to aberrant methylation in MDS (2,3).
Therefore, DNMTs have become potential targets for the treatment of
MDS (4–8). Two DNMT-targeted therapies, azacitidine
(AZA) and decitabine [5-aza-2-deoxycytidine (DAC)], have been
approved for clinical use by the Food and Drug administration (FDA)
(1,7).
However, the direct cytotoxic effects upon the hematopoietic system
caused by the approved DNMT inhibitors (DNMTIs), in addition to
their chemical instability, has prompted the development of
alternative therapeutic DNMTIs. The cytidine analogue, zebularine,
is a third nucleoside DNMTI that has been developed. The mode of
action of zebularine is similar to that of DAC, however, zebularine
has an extended half-life compared with AZA and DAC, and can be
selectively incorporated into malignant, rather than normal cells.
The limitation to the development of zebularine is that higher
concentrations of the drug are required to achieve comparable
demethylation results to those observed following treatment with
AZA and DAC (9–11).
In addition to identifying novel nucleoside DNMTIs,
significant efforts have been made to develop
non-nucleoside-targeted therapies that can directly inhibit
individual DNMTs. However, the antisense oligonucleotide-targeting
DNMTI, MG98, demonstrated inconsistent knock-down of DMNT mRNA in
solid and hematopoietic tumors during phase I clinical trials,
which was believed to be the result of inefficient intracellular
MG98 uptake (12). At present,
treatment with DAC alone has not demonstrated any additional
significant survival benefits for high-risk MDS patients compared
with traditional best supportive care (11). However, the use of DAC has been
proposed as a low-intensity therapeutic approach for elderly
patients with high-risk MDS and MDS/acute myeloid leukemia (AML),
who are considered unfit to undergo aggressive chemotherapy
(13). Due to a lack of specificity,
and its incorporation into DNA during DNA synthesis, DAC is able to
induce DNA damage, mutagenesis, cytotoxicity and hypomethylation.
Therefore, low-dose DAC regimens have been investigated in a number
of clinical trials and have been shown to be effective. The results
of the clinical trials demonstrated reduced levels of DAC-induced
toxicity in elderly patients who were unsuitable for high-intensity
induction chemotherapy and consecutive allogeneic hematopoietic
stem cell transplantation (HSCT) (14). In addition, it has been revealed that
low doses of DAC induce significant anti-MDS effects, a safe
toxicity profile and a complete remission rate of 21–39% (14). However, primary and secondary
resistance to low-dose DAC-based regimens is emerging as a
significant clinical issue. The survival rate at relapse following
an initial response to treatment is poor. One study identified that
primary resistance to treatment resulted from the abnormal active
metabolism of DAC, and that secondary resistance was due to the
unbalanced activation of downstream pathways, such as the nuclear
factor-κB (NF-κB) and phosphatidylinositol 3-phosphate/AKT
pathways, which limited the clinical effect of DAC (15).
Artesunate (ART) (Fig.
1), a safe and effective antimalarial drug, is a semi-synthetic
derivative of the drug artemisinin, and is extracted from the
Chinese herb Artemisia annua. Previous studies have
demonstrated that ART inhibits the growth of a number of carcinoma
cell lines, and therefore, may be a potential novel candidate for
the treatment of cancer (16,17). In the present study, the MDS SKM-1
cell line was co-treated with ART and DAC. This regimen was
designed based on the knowledge that cell survival signal
transduction pathways, namely the phosphatidylinositol 3-kinase
(PI3K)-C2α/AKT1 and RELA/NF-κB pathways, are involved in the
secondary resistance to DAC, and that high-risk MDS has the
tendency to transform towards AML (18). A previous study indicated that ART
conferred anticancer and chemosensitive effects by downregulating
the activity of the PI3K/AKT and NF-κB signaling pathways (19). At present, the positive effect of ART
upon the rate of DAC-induced apoptosis and growth inhibition within
high-risk MDS cells is yet to be elucidated. However, ART-DAC
combined therapy may present an alternative strategy for the
treatment of cancer. In the present study, the efficacy of ART in
sensitizing high-risk human MDS cells to DAC, and the intracellular
signaling mechanisms that underlie ART-enhanced DAC-induced
apoptosis and growth inhibition, were investigated.
Materials and methods
Chemicals and reagents
ART (Guilin Pharmaceutical Co., Ltd., Shanghai,
China) was dissolved in dimethyl sulfoxide (DMSO) at a
concentration of 20 mg/ml and kept as a stock solution at −20°C.
The caspase 3/7 inhibitor, Ac-DEVD-CHO (Sigma-Aldrich Shanghai
Trading Co., Ltd., Shanghai, China), was dissolved in DMSO at a
concentration of 100 mM. The final concentration of DMSO was kept
below 0.1% throughout the study. DAC (Sigma-Aldrich Shanghai
Trading Co., Ltd.) was dissolved in 0.01 M phosphate-buffered
saline (PBS) at a concentration of 5 mg/ml. Cell counting kit-8
(CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan),
the RPMI 1640 medium and fetal bovine serum (FBS) were from GIBCO
(Carlsbad, CA, USA) and the Annexin V-fluorescein isothiocyanate
(FITC) kit was from Bioseal Biotechnology Co., Ltd. (Beijing,
China). The P1250 total protein extraction kit was obtained from
Applygen Technologies Inc. (Beijing, China), the BCA protein assay
kit was from the Beijing Biosynthesis Biotechnology Co., Ltd.
(Beijing, China), the goat anti-rabbit immunoglobulin G (IgG)
horseradish peroxidase (HRP)-conjugated secondary antibody
(dilution, 1:100) was from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA) and the western chemiluminescent HRP substrate was
from Millipore Corporation (Billerica, MA, USA). The primary
polyclonal rabbit anti-human antibodies (dilution, 1:1,000),
specific to caspase-3 (cat. no. 9664), −8 (cat. no. 4790) and −9
(cat. no. 9502) and apoptosis-inducing factor (AIF) (cat. no.
4642), were obtained from Cell Signaling Technology (Shanghai)
Biological Reagents Co., Ltd., (Shanghai, China), while the primary
poly(ADP-ribose) polymerase (PARP) monoclonal rabbit anti-human
antibody (dilution, 1:1,000) was purchased from Epitomics, Inc.
(cat. no. E78, Zhejiang, China) and the rabbit anti-human GADPH
antibody was from Santa Cruz Biotechnology, Inc. (cat. no.
SC-25778; dilution, 1:1,000). Hoechst 33342, dissolved in 0.01 M
PBS at a concentration of 10 mg/ml, and propidium iodide (PI) were
purchased from Sigma-Aldrich Shanghai Trading Co., Ltd.
Cell culture and treatment
The SKM-1 cells, derived from a patient with
high-risk MDS, were purchased from the Japanese Collection of
Research Bioresources Cell Bank (Osaka, Japan). The cells were
cultured in a flask containing RPMI 1640 medium supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in
a humidified 5% CO2 incubator. In total, two MDS
patients, treated at The Second Hospital of Hebei Medical
University (Shijiazhuang, China), were included in the present
study. Subsequent to obtaining informed consent, mononuclear cells
were isolated from the heparinized bone marrow of the patients by
Ficoll-Hypaque density centrifugation (600 × g, 20 min), and
cultured in RPMI 1640 containing 10% FBS, 2 mM L-glutamine, 100
U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified
5% CO2 incubator. This study was approved by the Medical
Ethics Committee of the Second Hospital of Hebei Medical
University.
Cell growth inhibition assay
The cell viability, following treatment with or
without DAC and ART, was measured using CCK-8. The SKM-1 and fresh
bone marrow mononuclear cells (BMMNCs) were seeded into a 96-well
plate at a density of 1×104/160 µl per well. Subsequent
to a 4-h incubation, medium containing 10 µl DAC, at a final
concentration of 1, 4, 8, 80 or 1,600 µmol/l, was added to each
well and incubated for a further 30 min. Next, medium containing 10
µl ART, at a final concentration of 1, 5, 10 or 100 µmol/l, was
added to certain wells, and further incubated for 24 h. In total,
20 µl WST-8 solution was added to each well and incubated at 37°C
for 4 h. Optical density (OD) was calculated from 450 nm absorption
(A450) values using an automated enzyme-linked
immunosorbent assay reader (PerkinElmer, Shanghai, China). The cell
viability was determined from the absorbance of soluble formazan
dye generated by living cells. The cell growth inhibition ratio was
calculated as follows: Inhibition ratio = 1 – A450 of
experimental well / A450 of blank control well × 100. In
total, each assay was repeated at least three times. The combined
effects were evaluated using the combination index (CI)
isobologram. The general equation for CI is as follows: CI =
(D)1 / (Dx)1 + (D)2 /
(Dx)2, where CI<1 indicates synergism, CI=1 indicates
an additive effect and CI>1 indicates antagonism (20).
Cell apoptosis assay
The quantitative analysis of cellular apoptosis was
performed using Annexin Ⅴ-FITC/PI double staining. In total,
1.0×105 SKM-1 or BMMN cells were planted in 24-well
plates and exposed to 8 µmol/l DAC and/or 5 µmol/l ART for 24 h.
Next, flow cytometry, with fluorescence channels 1 and 2, was used
to calculate the percentage of apoptotic cells (BD FACS Canto™ II;
BD Biosciences, Franklin Lakes, NJ, USA). The apoptotic morphology
of SKM-1 cells was analyzed by staining the cells with 10 µg/ml of
the fluorescent DNA-binding dye, Hoechst 33342, and 20 µg/ml PI at
37°C for 15 min after culturing in 6-well plates in the
aforementioned manner. Apoptotic cells were identified by the
presence of condensed and fragmented nuclei using fluorescence
microscopy (BX51, Olympus, Tokyo, Japan).
Western blot analysis
The SKM-1 cells, treated as aforementioned, were
harvested, was hed twice with ice-cold PBS and lysed in lysis
buffer containing 50 mM HEPES (pH 7.4), 1% Triton X-100, 2 mM
sodium orthovanadate, 100 mM sodium fluoride, 1 mM EDTA, 1 mM EGTA
and 1 mM phenylmethanesulfonylfluoride. The lysis buffer was
supplemented with proteinase inhibitors; 10 µg/ml aprotinin, 10
µg/ml leupeptin and 100 µg/ml pepstatin, and incubated at 4°C for 1
h. Subsequent to a 15-min 9,500 × g centrifugation at 4°C, the
cellular protein concentration was determined using the BCA protein
assay kit (Beijing Biosynthesis Biotechnology Co., Ltd.). Equal
quantities of total proteins were separated using SDS-PAGE, and
then transferred to a polyvinylidene fluoride membrane
(Sino-American Biotechnology Co. Ltd., Shanghai, China). The
proteins, caspase-3, −8 and −9, and AIF, were detected using the
indicated primary antibodies and the HRP-conjugated secondary
antibody. The signal was detected using the ChemiDoc XRS+ system
with enhanced chemiluminescence, and analyzed by the Image Lab
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunofluorescence assay
The immunofluorescence staining was performed using
paraformaldehyde-fixed cell slices from SKM-1 cells exposed to
either Ac-DEVD-CHO or DMSO. After blocking against non-specific
binding using goat serum, the cells were exposed to the primary
antibodies against caspase-3, −8 and −9, and AIF, followed by
secondary antibody conjugation to the FITC (1:100). The cells were
counterstained using Hoechst 33342 (Beijing Biosea Biotechnology
Co., Ltd., Beijing, China), and then analyzed in images captured by
confocal fluorescence microscopy (TE-2000, Nikon, Tokyo,
Japan).
Statistical analysis
All data were analyzed using SPSS v.13.0 software
(SPSS, Inc., Chicago, USA) and expressed as the mean ± standard
error of the mean from three independent experiments performed in
triplicate. All data were normally distributed, and analyzed
further by analysis of variance and least significant difference as
a post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
ART enhances DAC-induced cell growth
inhibition
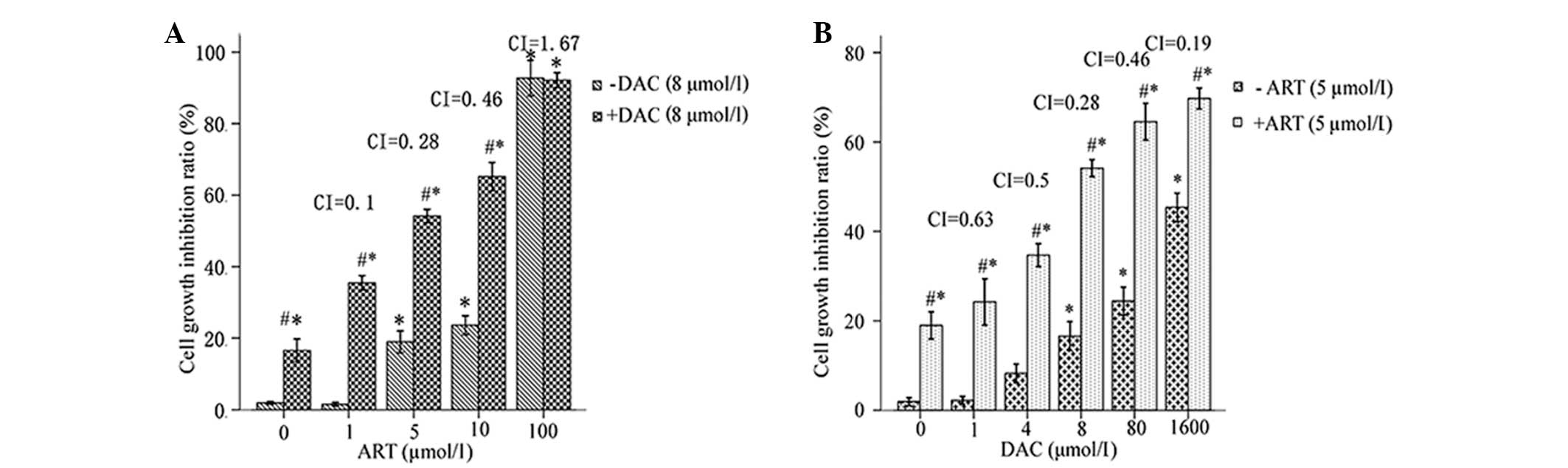
In the preliminary experiment, SKM-1 cellular
cytotoxicity was determined following a 24-h treatment with 0–100
µmol/l ART. A concentration range of 1–5 µM ART demonstrated only
slight toxicity in the SKM-1 cells, as the cell viability was
>80% following treatment (Fig.
1A). In addition, the cytotoxicity of the combination ART-DAC
treatment was investigated. The results revealed that ART induced a
significant positive synergist effect with DAC upon cell growth
inhibition (Fig. 1A). Therefore, ART
at a concentration of 5 µmol/l was selected for further studies. A
24-h treatment with DAC at concentrations of 1–8 µmol/l induced
<20% (16.56±3.21%) of SKM-1 cells to apoptose. This indicated
that the SKM-1 cells were resistant to low-dose DAC-mediated growth
inhibition following 24 h of treatment (Fig. 1B). However, the combination of 5
µmol/l ART with DAC significantly increased the percentage of dead
cells by 14.15–40.62%, depending on the concentration of DAC used
(1–8 µmol/l) (Fig. 1B). In addition,
the enhancement of DAC-induced cell death by ART was
dose-dependent, as revealed by a Spearman's ρ correlation
coefficient of 0.712 (P=0.009). Therefore, a concentration of 5
µmol/l ART combined with 8 µmol/l DAC was selected for further
studies.
ART-DAC combination induces an
increased rate of apoptosis of MDS cells
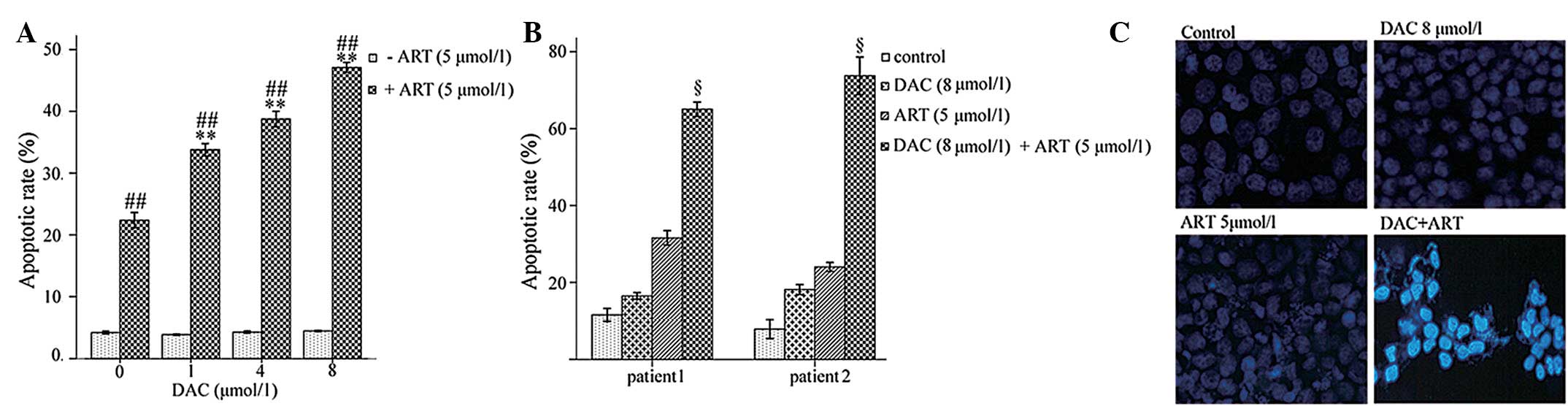
The ART-DAC combination treatment significantly
increased the rate of apoptosis in the SKM-1 cells and the BMMNCs
from high-risk MDS patients compared with DAC or ART alone
(P<0.005; Fig. 2A and B). The
apoptotic cells, which exhibited bright-blue staining and
fragmented nuclei following incubation with Hoechst 33342/PI, are
shown in Fig. 2C. The data suggests
that compared with the single-agent treatment, the ART-DAC
combination markedly enhanced the rate of apoptosis in the SKM-1
cell line and in the primary BMMNCs obtained from the high-risk MDS
patients.
ART-DAC combination enhances the rate
of apoptosis of MDS cells through activation of caspase-3-dependent
and -independent death signals
In order to identify the molecular mechanisms by
which the combined ART-DAC treatment enhanced the rate of apoptosis
in the MDS cells, western blot assays were used to analyze the
activation of the caspases, and the cleavage of PARP in SKM-1 cells
that had been incubated with 8 µmol/l DAC and/or 5 µmol/l ART for
12 h. The results revealed that the combination ART-DAC treatment
significantly increased the activation of caspase-3 and −9, and the
cleavage of PARP compared with DAC or ART alone (Fig. 3A). To confirm the occurrence of
caspase-dependent apoptosis in high-risk MDS cells, the SKM-1 cells
were incubated with 20 µmol/l Ac-DEVD-CHO, a caspase-3 and −7
inhibitor, for 1 h prior to treatment with ART and/or DAC.
Following incubation, it was revealed that Ac-DEVD-CHO abrogated
DAC-induced, but not ART-induced, apoptosis (Fig. 3Ba). Therefore, the results indicated
that DAC and ART induce caspase-dependent and -independent
apoptotic pathways in SKM-1 cells, respectively.
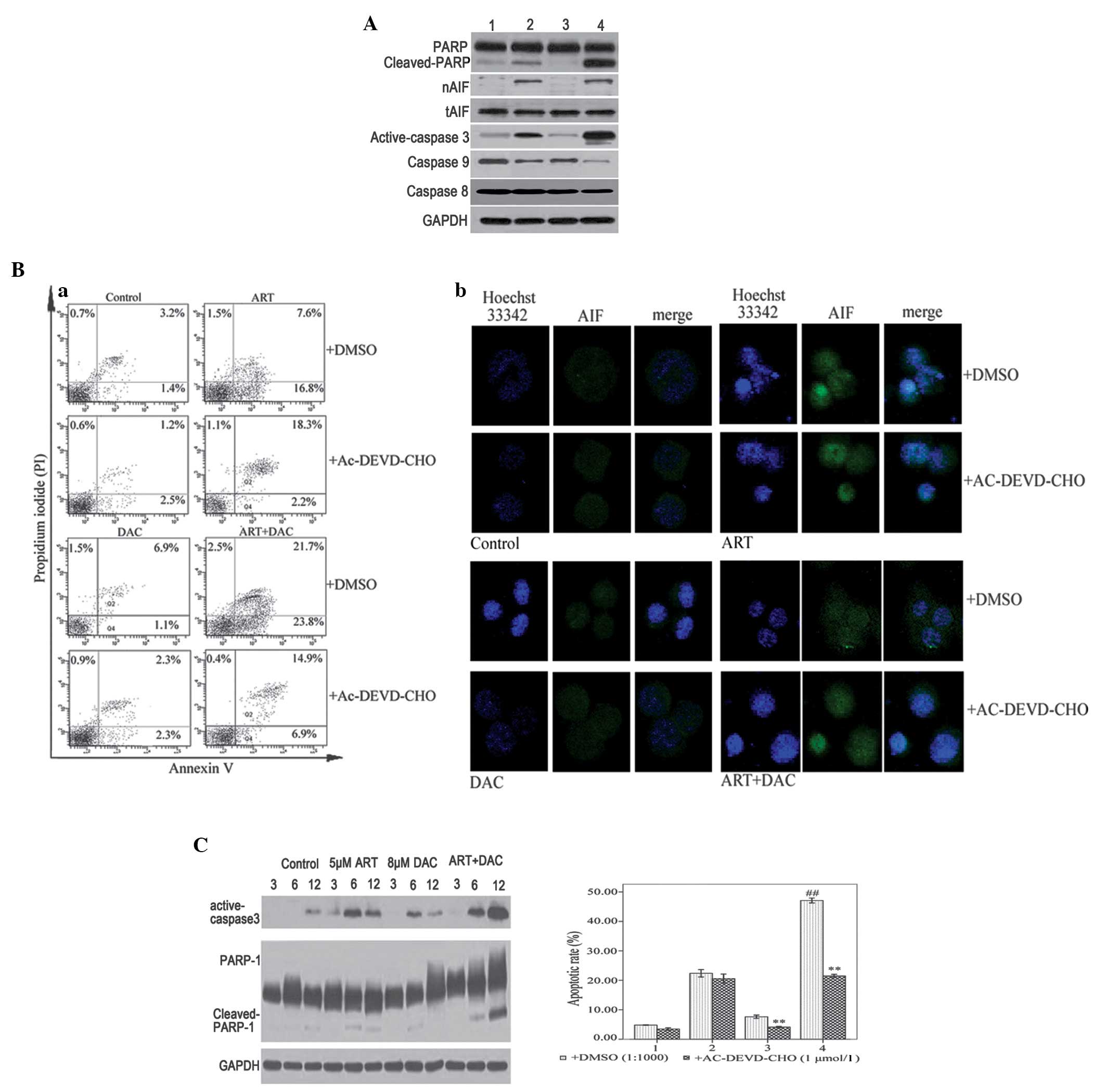 | Figure 3.(A) SKM-1 cells were treated with 8
µmol/l decitabine (DAC) or 0.1% dimethylsulfoxide (DMSO) for 30
min, and then further incubated with 5 µmol/l artesunate (ART) for
12 h. Whole cell lysates or nuclear proteins were analyzed by
western blotting for the apoptotic proteins, caspase-9, −8, and-3,
poly(ADP-ribose) polymerase-1 (PARP-1) and apoptosis-inducing
factor (AIF), with GAPDH as the loading control. Lane 1, control
group; lane 2, ART group; lane 3, DAC group; and lane 4, DAC and
ART group. (B) (a) SKM-1 cells were treated with 8 µmol/l DAC or
0.1% DMSO for 30 min, and then further incubated with 5 µmol/l ART
for 12 h. The Annexin V/propidium iodide staining revealed that
compared with single-agent treatment, the combination of ART and
DAC induced an increased rate of apoptosis. The caspase-3/7
inhibitor, Ac-DEVD-CHO, abrogated apoptosis induced by DAC, but not
ART. (B) (b) SKM-1 cells were treated with 8 µmol/l DAC or 0.1%
DMSO for 30 min, and then further incubated with 5 µmol/l ART for 6
h. Laser confocal microscope analysis revealed that ART, but not
DAC, induced AIF transfer to the nucleus. (C) SKM-1 cells were
treated with DAC and/or ART for 3, 6 or 12 h. Western blot analysis
revealed that DAC and ART induced caspase-dependent apoptosis. The
presence of active caspase-3 and cleaved PARP, induced by DAC or
ART alone, was not observed after 12 h. However, the combined
ART-DAC treatment induced a notable activation of caspase-3 and
cleaved PARP, which was observed even after 12 h. **P<0.005
compared with the control group treated with DMSO; and
##P<0.005 compared with the other groups. |
To further investigate the ART-induced
caspase-independent apoptotic pathway, the SKM-1 cells were
incubated with 8 µmol/l DAC and/or 5 µmol/l ART for 6 h following a
1-h pre-treatment with either DMSO (1:1,000) or 20 µmol/l
Ac-DEVD-CHO. Following incubation, laser confocal microscopy
analysis revealed that ART, but not DAC, induced AIF release from
the mitochondria to the cytosol and into the nucleus. Furthermore,
pre-treatment with AC-DEVD-CHO was unable to inhibit the release
and transfer of AIF (Fig. 3Bb). The
results indicated that ART, but not DAC, induced
caspase-independent apoptosis via AIF transfer from the
mitochondria to the nucleus. Furthermore, ART and DAC were able to
induce caspase-dependent apoptosis, but the active caspase-3 and
cleaved PARP, induced by DAC or ART, were not observable 12 h
later. The cells treated with the ART-DAC combination demonstrated
the most significant activity of caspase-3 and cleaved PARP
(Fig. 3C). The activation of two
apoptotic signaling pathways supported the hypothesis that ART and
DAC induce apoptosis via two distinct signaling cascades. The
combined ART-DAC treatment significantly enhanced the rate of
apoptosis in the SKM-1 cells compared with ART or DAC alone.
Discussion
With respect to drug resistance, MDS is considered a
significantly challenging disease to treat. This is due, in part,
to the heterogeneity of the malignancy and the failure of current
therapies to sustain durable remissions. In clinical practice,
cases of relapsed/refractory MDS prove difficult to treat.
Therefore, novel therapeutic strategies that target MDS are
required. At present, the only approved curative treatment for
patients with MDS is HSCT. However, this approach is associated
with high treatment-related morbidity and mortality. Other FDA
approved treatments, namely DAC and AZA, have been used in
low-intensity MDS therapeutic regimens. However, due to untargeted
DNA damage and mutagenesis, and the occurrence of primary and
secondary resistance, DAC and AZA have demonstrated limited
clinical responses (12,15). The future of MDS-targeted therapies
may therefore require combinations of novel drugs. The original use
of ART was as an effective anti-malaria agent, however, the drug
has also demonstrated potent effects against certain malignant
diseases. Previous preclinical data has revealed that ART is active
in vitro and in vivo in cases of multiple myeloma and
leukemia (21,22). The present study identified that ART
activated caspase-dependent and caspase-independent mitochondrial
pathways. Furthermore, the study revealed that the AIF-mediated
caspase-independent pathway was the primary cascade responsible for
the initiation of apoptosis in the SKM-1 cells. The molecular
mechanism by which DAC initiated apoptosis in SKM-1 cells was
revealed to be associated with the activation of caspases-9 and −3,
and with the cleavage of PARP (Fig.
3). In addition, the effect of the ART-DAC combination
treatment upon high-risk MDS cells was analyzed in vitro in
order to identify the underlying therapeutic mechanisms. These
mechanisms may then provide the basis for future clinical trials
that examine cases of relapsed or refractory MDS. The results
revealed that the combination of ART and DAC inhibited the growth
of the SKM-1 cell line in vitro (Fig. 1). Furthermore, it was identified that
compared with single-agent treatment, the combined treatment
significantly enhanced the rate of apoptosis in the SKM-1 and
primary high-risk MDS cells in vitro (Fig. 2A and C). In addition, the combination
of ART and DAC significantly enhanced the effects of apoptosis in
the cells obtained from patients with MDS (Fig. 2B). The western blot analysis confirmed
that compared with ART or DAC alone, the combined ART-DAC treatment
activated caspase-9 and −3, and cleaved PARP more potently. The
pre-treatment of cells with the caspase-3 and −7 inhibitor,
Ac-DEVD-CHO, abrogated DAC-induced, but not ART-induced, apoptosis
(Fig. 3A and B). Furthermore,
following a 12-h incubation with ART, but not DAC, it was revealed
that AIF was released from the mitochondria and was transited to
the nucleus (Fig. 3C). The
ART-induced apoptosis of the SKM-1 cells was identified to be
primarily caspase-independent, and therefore, not completely
inhibited by the caspase-3 and −7 inhibitor, Ac-DEVD-CHO. The
results of the present study support the hypothesis that the
combination of ART and DAC induces apoptosis in MDS cells via two
distinct signaling cascades. This additive response is consistent
with the observation that the ART-DAC combination treatment
activated a caspase-dependent and -independent mitochondrial
pathway.
In conclusion, the results of the present study
indicated that compared with single-agent therapy, the combination
of ART and DAC exhibited an increased efficacy against MDS cells
in vitro. The combined treatment may confer increased
antitumor activity and overcome specific cases of cellular
resistance and/or identify novel anti-apoptotic mechanisms.
Considering the results from the present study, future studies may
be performed that address these issues and investigate the
therapeutic effects of combined treatment approaches in patients
with relapsed or refractory MDS. Additional studies are required to
affirm these results, and to provide an improved understanding of
the mechanisms, interactions and cellular targets that are involved
in the signaling pathways targeted by drugs such as ART and
DAC.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Hebei Province (no. C2008001097). The present
study was supported by the Medical Science Research Project of
Hebei Province(no. 20130186). The authors would like to thank the
Department of Cardiology, Second Hospital of Hebei Medical
University for providing assistance with the western blot
analysis.
References
|
1
|
Fenaux P, Mufti GJ, Hellstrom-Lindberg E,
et al: Efficacy of azacitidine compared with that of conventional
care regimens in the treatment of higher-risk myelodysplastic
syndromes: a randomised, open-label, phase III study. Lancet Oncol.
10:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stankov K, Bogdanovic G, Kojic V, et al:
Expression analysis of genes involved in epigenetic regulation and
apoptosis in human malignant haematopoietic cell lines treated with
5-azacytidine. J BUON. 16:116–122. 2011.PubMed/NCBI
|
|
3
|
Saunthararajah Y, Triozzi P, Rini B, et
al: p53-independent, normal stem cell sparing epigenetic
differentiation therapy for myeloid and other malignancies. Semin
Oncol. 39:97–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin J, Yao DM, Qian J, et al: Recurrent
DNMT3A R882 mutations in Chinese patients with acute myeloid
leukemia and myelodysplastic syndrome. PLoS One. 6:e269062011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walter MJ, Ding L, Shen D, et al:
Recurrent DNMT3A mutations in patients with myelodysplastic
syndromes. Leukemia. 25:1153–1158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shih AH, Abdel-Wahab O, Patel JP and
Levine RL: The role of mutations in epigenetic regulators in
myeloid malignancies. Nat Rev Cancer. 12:599–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faderl S, Garcia-Manero G, Jabbour E, et
al: A randomized study of 2 dose levels of intravenous clofarabine
in the treatment of patients with higher-risk myelodysplastic
syndrome. Cancer. 118:722–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piekarz RL and Bates SE: Epigenetic
modifiers: basic understanding and clinical development. Clin
Cancer Res. 15:3918–3926. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng JC, Matsen CB, Gonzales FA, et al:
Inhibition of DNA methylation and reactivation of silenced genes by
zebularine. J Natl Cancer Inst. 95:399–409. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Robak T: New nucleoside analogs for
patients with hematological malignancies. Expert Opin Investig
Drugs. 20:343–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flotho C, Claus R, Batz C, et al: The DNA
methyltransferase inhibitors azacitidine, decitabine and zebularine
exert differential effects on cancer gene expression in acute
myeloid leukemia cells. Leukemia. 23:1019–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gotze K, Platzbecker U, Giagounidis A, et
al: Azacitidine for treatment of patients with myelodysplastic
syndromes (MDS): practical recommendations of the German MDS Study
Group. Ann Hematol. 89:841–850. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klisovic RB, Stock W, Cataland S, et al: A
phase I biological study of MG98, an oligodeoxynucleotide antisense
to DNA methyltransferase 1, in patients with high-risk
myelodysplasia and acute myeloid leukemia. Clin Cancer Res.
14:2444–2449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang X, Lay F, Han H and Jones PA:
Targeting DNA methylation for epigenetic therapy. Trends Pharmacol
Sci. 31:536–546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin T, Castoro R, El Ahdab S, et al:
Mechanisms of resistance to decitabine in the myelodysplastic
syndrome. PLoS One. 6:e233722011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mercer AE, Copple IM, Maggs JL, O'Neill PM
and Park BK: The role of heme and the mitochondrion in the chemical
and molecular mechanisms of mammalian cell death induced by the
artemisinin antimalarials. J Biol Chem. 286:987–996. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondo A, Yamashita T, Tamura H, et al:
Interferon-gamma and tumor necrosis factor-alpha induce an
immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB
activation in blasts in myelodysplastic syndromes. Blood.
116:1124–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thanaketpaisarn O, Waiwut P, Sakurai H and
Saiki I: Artesunate enhances TRAIL-induced apoptosis in human
cervical carcinoma cells through inhibition of the NF-ĸB and
PI3K/Akt signaling pathways. Int J Oncol. 39:279–285.
2011.PubMed/NCBI
|
|
20
|
Chou TC and Talalay P: Analysis of
combined drug effects: a new look at a very old problem. Trends
Phamacol Sci. 4:450–454. 1983. View Article : Google Scholar
|
|
21
|
Chen H, Shi L, Yang X, Li S, Guo X and Pan
L: Artesunate inhibiting angiogenesis induced by human myeloma
RPMI8226 cells. Int J Hematol. 92:587–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Han Y, Yang Y, et al: Effect of
interaction of magnetic nanoparticles of
Fe3O4 and artesunate on apoptosis of K562
cells. Int J Nanomedicine. 6:1185–1192. 2011.PubMed/NCBI
|