Introduction
Osteosarcoma (OS) is the most frequently diagnosed
primary malignant bone tumor, accounting for 2.4% of all childhood
malignancies. The average age of onset for OS is 10–20 years old
(1,2).
As the primary lesion can be surgically removed, distant migration
is the major cause of OS-associated mortality. However, the
mechanisms of migration remain to be elucidated. Therefore, the
study of OS migration is important for diagnostic and treatment
strategies.
Focal adhesion kinase (FAK) is a non-receptor
tyrosine kinase with tyrosine kinase activity (3). The autophosphorylation of FAK at
tyrosine 397 can activate downstream target proteins, mediate a
number of signal pathways and has a central role in a number of
cellular events. In addition, FAK is involved in tumor cell
proliferation, survival, apoptosis, spreading, migration and
invasion. A number of previous studies have revealed that FAK is
overexpressed in a variety of malignant tumors, including breast,
lung, prostatic and colorectal cancer, hepatocellular carcinoma and
pancreatic adenocarcinoma (4). The
overexpression of FAK has an important role in tumor metastasis and
confers a poor patient prognosis. However, the role of FAK in the
migration of OS remains unclear.
In the present study, a limiting dilution method was
used to analyze the expression of FAK in different OS cell lines.
In addition, small interfering RNA (siRNA) was used to examine the
role of FAK in OS cell migration.
Materials and methods
Materials
The OS 143B cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). The RPMI 1640
medium was purchased from the Beijing Wensent Co. (Beijing, China),
the characterized fetal bovine serum (FBS) was obtained from
TransGen Biotech Inc., (Beijing, China) and trypsin was purchased
from Amresco LLC (Solon, OH, USA). The FAK polyclonal rabbit
anti-human and mouse anti-human α-tubulin primary antibodies were
purchased from Sigma-Aldrich (St. Louis, MO, USA), and the
anti-mouse immunoglobulin G (IgG) and anti-rabbit IgG secondary
antibodies were purchased from GE Healthcare Life Sciences (Logan,
UT, USA). The FAK siRNA sequence (GGUUCAAGCUGGAUUAUUTT) and the
negative control siRNA sequence (UUCUCCGAACGUGUCACGUTT) were
obtained from the Shanghai GenePharma Co., Ltd. (Shanghai, China).
The phalloidin, DAPI antibodies, transfection reagents,
Lipofectamine 2000, Opti medium and staining secondary antibodies
were purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA).
Cell culture
The OS 143B cell lines were cultured in RPMI 1640
medium with 10% FBS, 50 U/ml penicillin and 50 µg/ml gentamicin at
37°C in a 5% CO2 incubator in a humidified atmosphere.
The medium was changed every three days. Upon reaching 80%
confluence, the cells were digested with 0.25% trypsin and
passaged. All cells were in the logarithmic growth phase.
Monoclonal separation of the OS 143B
cell lines
The OS 143B cell lines were diluted into 96-well
plates at 1 cell/well using the limiting dilution method. The
monoclonal cells in the wells were observed and recorded using an
inverted microscope (Eclipse Ti-U; Nikon Corporation; Tokyo,
Japan). Next, the cells were cultured in conditioned medium with
20% FBS and 50 U/ml penicillin and 50 µg/ml streptomycin, and kept
at 37°C in a 5% CO2 incubator in a humidified atmosphere
for 2–3 weeks. Upon covering a third of the well, the cells were
transferred to 24-well plates to expand in culture, then to 6-wells
plates and finally to a dish.
OS cell migration detection using the
wound healing assay
In total, five small dots were drawn with a marker
pen in parallel to scratches made on the back of a 12-well plate.
An equal number of cells from each 143B subclone cell line were
then inoculated and cultured at 37°C in a 5% CO2
incubator in a humidified atmosphere. When the cell monolayers had
reached 90–100% confluency, single scratches were made using 200-µl
tips along the bottom of the culture plates. The cellular debris
were rinsed three times with phosphate-buffered saline (PBS), added
to the medium and then analyzed with an inverted microscope
(Eclipse Ti-U; Nikon Corporation). The ‘A’ value was taken as the
distance of the scratch area in the vicinity of each dot, and the
‘B’ value was taken after the cells had been cultured for 24 h or
subsequent to scratch healing. Relative migration was calculated
according to the following equation: Relative migration = (A - B) /
A. In the same period, the relative displacement of the subclone
cells indicated the migration ability. The experiment was performed
in triplicate.
Western blot analysis
An equal number of cells with different migration
abilities from each OS subclone cell line were plated into 6-well
plates at 37°C in a 5% CO2 incubator with a humidified
atmosphere. When the cells had reached 80% confluency, the medium
was removed. Next, the cells were washed twice with PBS and added
to SDS. The cells were then scraped and boiled for 5 min. Following
centrifugation at 10,000 × g for 5 min, the supernatant was stored
for subsequent use. Equal quantities of the samples were separated
by SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes. Subsequent to blocking with 5% skimmed milk, the
membranes were incubated overnight at 4°C with primary antibodies
against FAK (dilution, 1:400) and α-tubulin (dilution, 1:800).
Next, the membranes were washed three times with Tris-buffered
saline and Tween 20 (TBST) and incubated with the secondary
antibodies for 2 h at room temperature. The membranes were then
washed a further three times with TBST, for 10 min each time.
Subsequent to development and fixing, the blots were exposed to
X-ray film in order to observe the protein expression level.
Subclone cell lines with strong
migration ability transfected with FAK-siRNA
The subclone cell lines with strong migration
abilities were separated and total of 2×105 cells were
plated into 6-well plates at 37°C in a humidified atmosphere of 5%
CO2 for 24 h. The cells grew to 40–60% confluency within
the 24 h. The control cells were transfected with siRNA, and the
experimental cells with siRNA specific to FAK at concentrations of
30, 50 and 60 nM/l. The control cells were transfected with
Lipofectamine 2000 according to the manufacturer's instructions.
The FAK-siRNA and control siRNA were mixed with Lipofectamine 2000
and left to sit for 20 min prior to being added to 6-well plates at
37°C in 5% CO2 incubator in a humidified atmosphere. The
culture medium was changed after 6 h. The transfection level,
migration ability and pseudopodia formation were examined after 48
h.
Lamellipodia were counted using an
immunofluorescence staining method
The siRNA and FAK-siRNA at a concentration of 50
nM/l were added to the cell lines with strong migration abilities
for 48 h. Next, the cells were fixed with 2% paraformaldehyde at
room temperature for 8 h and then washed with PBS three times for 5
min each. Staining with fluorescein isothiocyanate
(FITC)-phalloidin (dilution, 1:500) and DAPI (dilution, 1:500) was
then performed at room temperature for 60 min. The cells were then
washed three times with PBS for 5 min each. Next, anti-fluorescence
quenching liquid was added and the cells were examined under a
fluorescence microscope (Delta Vision microscope, Applied Precision
Inc., Issaquah, WA, USA). A total of 200 cells were used to
calculated the number of lamellipodia and proportion of the total
number. The experiment was performed in triplicate.
Statistical analysis
All data were analyzed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). The results are presented as
the mean ± standard deviation. The data was analyzed by a one-way
analysis of variance and χ2 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Separation of the OS 143B subclone
cell lines
Single cells were successfully isolated from ~20 in
a 96-well plate. In total, 14 clones continued to grow successfully
following transmission to the dish. The rest had undergone
apoptosis or were unable to be passaged. Under the same culture
conditions, two subclone cell lines underwent spontaneous cell
death at passages 8–12, and so could not continue through
passaging.
Separation of the OS 143B subclone
cell lines according to different migration abilities
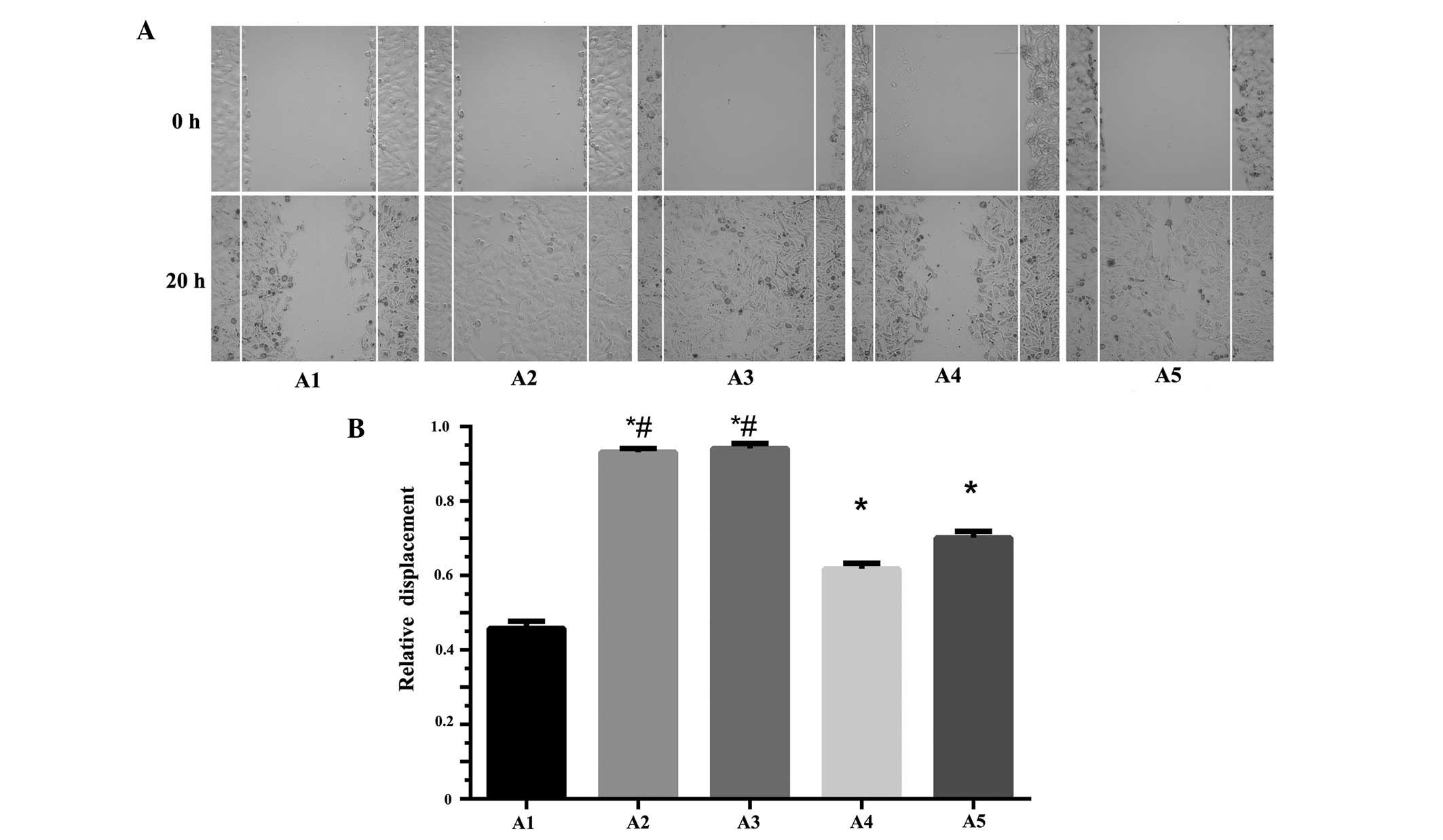
In total, five subclone cell lines (A1, A2, A3, A4
and A5) under the same generation and with similar growth rates
were selected for the scratch test. The results indicated that the
migration abilities of A2 (0.931±0.010) and A3 (0.941±0.014) were
significantly higher than A1 (0.458±0.019), A4 (0.618±0.015) and
the other cell lines (P<0.05). Of the cell lines analyzed, A1
exhibited the slowest migration. No significant difference in
migration was observed between A2 and A3 (P>0.05) (Fig. 1).
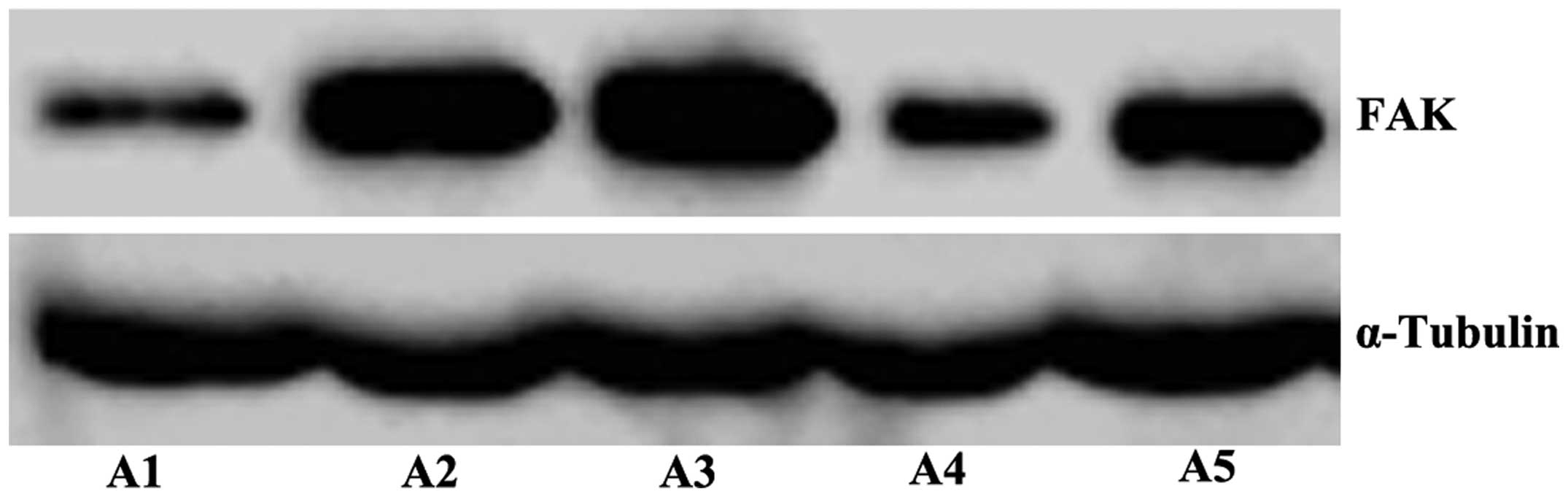
Expression of FAK in the OS 143B
subclone cell lines with different migration abilities
The results of the western blot analysis revealed
that the expression of FAK in the A2 and A3 cell lines was markedly
higher than that in the A1 cell line (Fig. 2).
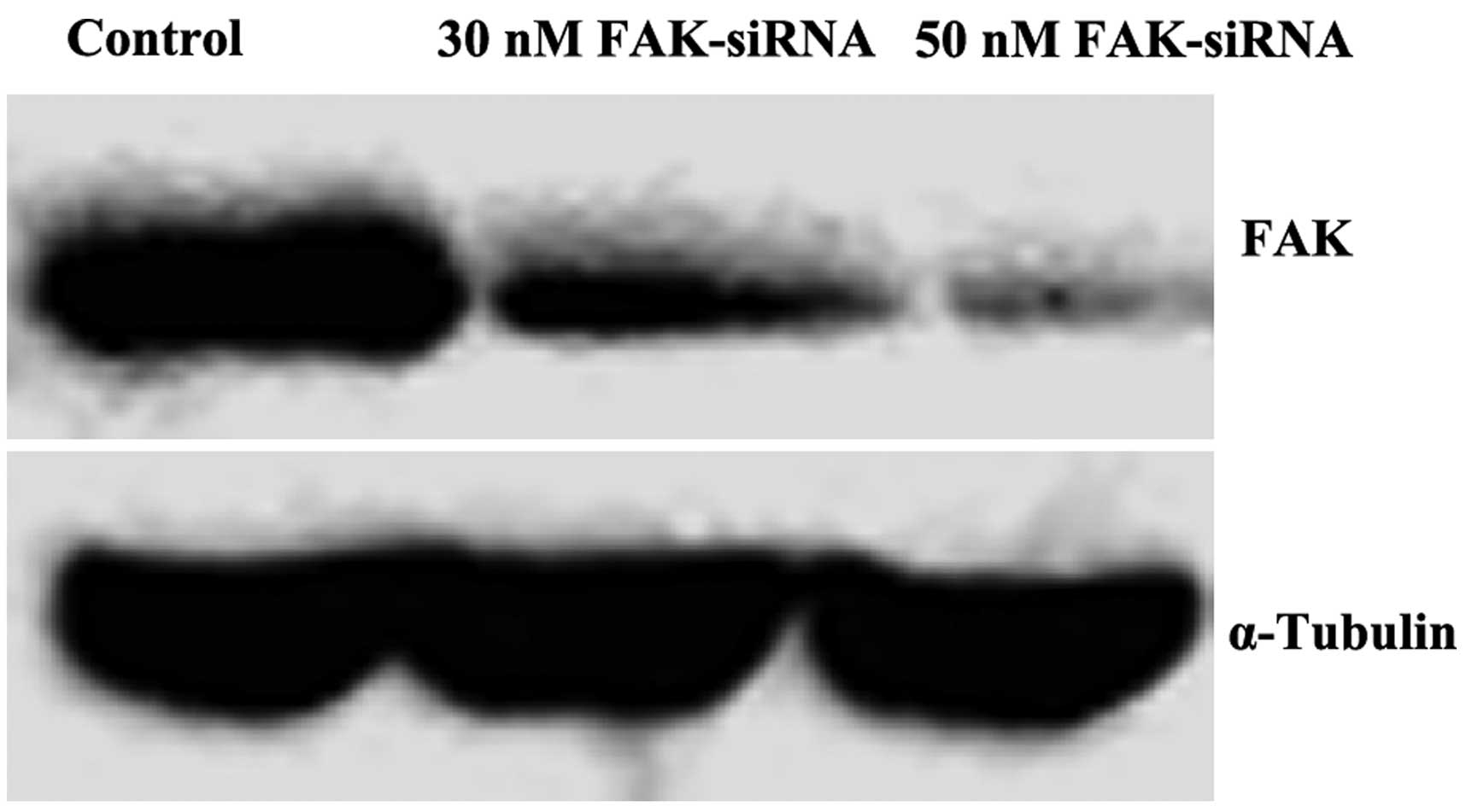
Differential expression of FAK in OS
cells transfected with FAK-siRNA
The results of the western blot analysis revealed
that the expression of FAK in the experimental group was lower than
that in the control group. The OS cells transfected with 50 nM/l
FAK-siRNA scarcely expressed FAK, which indicated that the
experiment had succeeded in knocking down the protein. FAK-siRNA,
at a concentration of >50 nM/l, induced significant
cytotoxicity. Therefore, FAK-siRNA at a concentration of 50 nM/l
was selected for use in the experiments (Fig. 3).
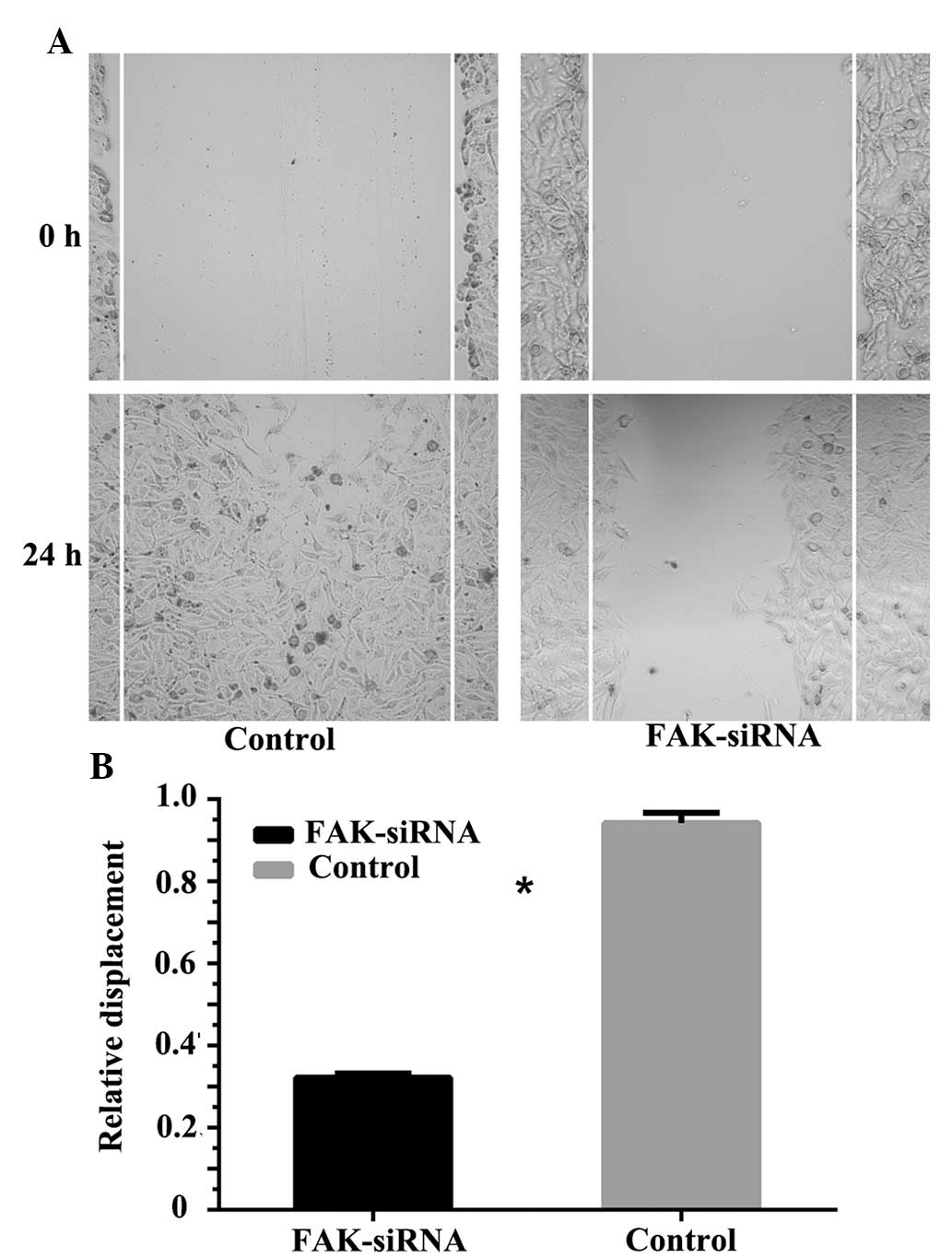
Effect of FAK-knockdown on OS cell
migration
The wound scratch assay demonstrated that following
transfection with FAK-siRNA, the migration ability of the A3 cell
line was significantly lower than that of the negative control
group. After 24 h, the relative displacement of the negative
control cells was 0.942±0.025, whereas the relative displacement of
the cells transfected with FAK-siRNA was 0.322±0.01 (P<0.05)
(Fig. 4).
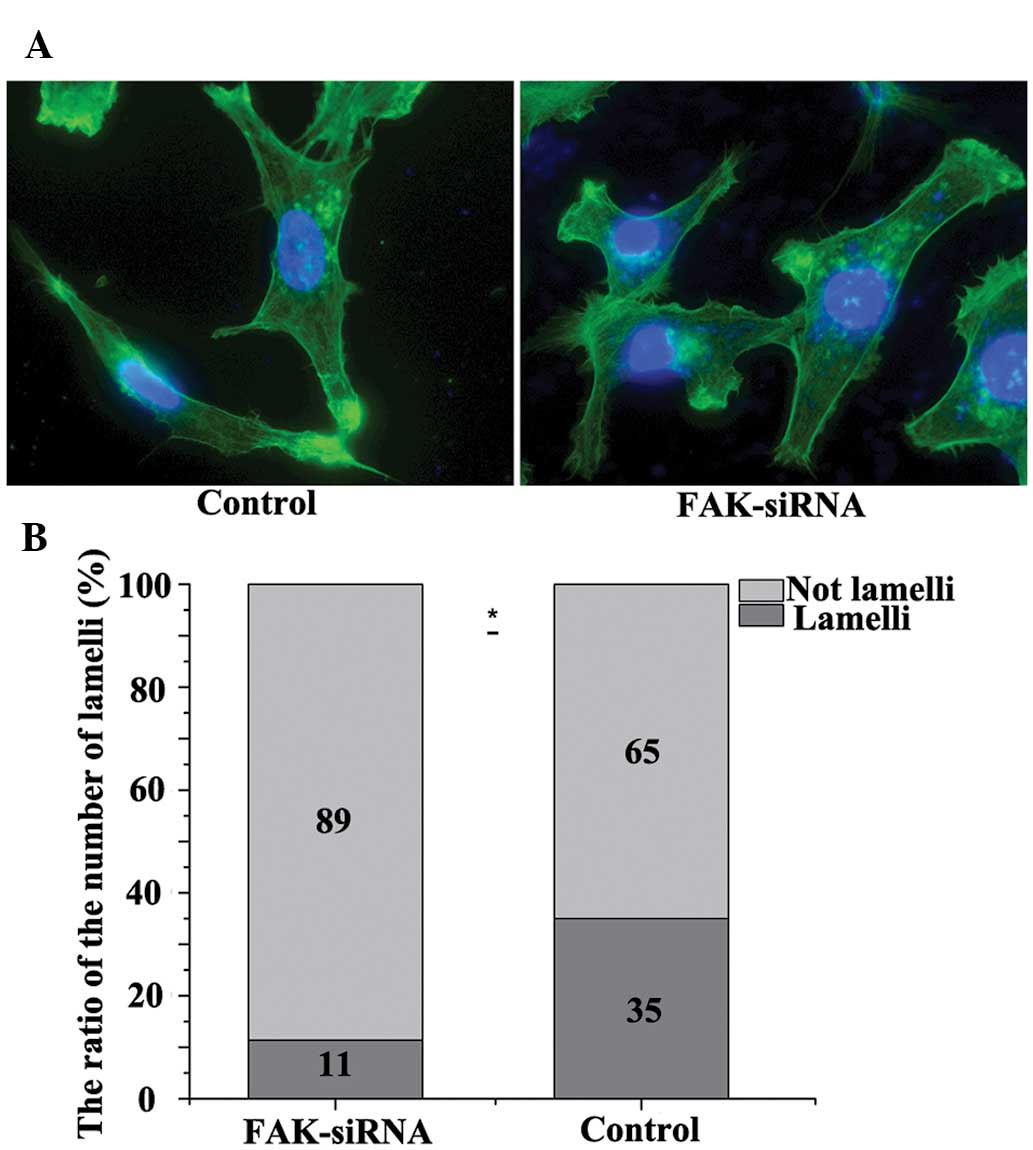
Effect of FAK-knockdown on the
lamellipodia formation of the OS cells
In contrast to the negative control group, silencing
of FAK in the FAK-siRNA group lead to a sparse appearance of
filaments surrounding the cells and a low fluorescence intensity.
The ratio of the number of filopodia dropped from 33.3 to 12.8% in
the FAK-siRNA group (P<0.05) (Fig.
5).
Discussion
OS is a disease with rapid progression, a poor
prognosis, and a high morbidity and mortality rate (5,6). Despite
significant improvements in imaging techniques and the use of
neoadjuvant chemotherapies, the five-year survival rate for OS
patients remains at 65% (7,8). Furthermore, there have been no
significant improvements in the rate of mortality compared with
several years ago. Lung metastases are the main cause of mortality
in patients with OS. Therefore, studying the metastatic mechanisms
allows for effective assessment of the clinical prognosis and
treatment of OS. In 1977, Fidler and Kripke (9) put forward the concept of heterogeneity
as the basis of tumor migration. From then on, it was realized that
primary malignant tumor cells exhibit heterogeneity (10). Within a tumor, the biological
characteristics and metastatic behaviors of various subgroups are
not identical. Furthermore, the invasion and migration of the
subsets are inconsistent during the progression of the tumors.
However, not all tumor cells have the ability of invasion and
migration, and only certain subgroups have the potential to
establish a stable, subline tumor metastatic phenotype. The present
study analyzed OS cell lines (A1, A2, A3, A4 and A5) with different
migration abilities using the limiting dilution method.
Tumor metastasis is a complex process. The
three-step, molecular-level hypothesis for malignant cells was
proposed by Liotta (11) and includes
the following stages: i) Adhesion, ii) degradation and iii)
migration and invasion. The migration of malignant cells largely
determines their ability to transfer to the others. FAK is a
widely-expressed, non-receptor protein tyrosine kinase located in
the cytoplasm. Phosphorylation of FAK mediates signaling events, is
widely involved in various biological processes within cells, and
contributes to tumor progression, including invasion, metastasis
and angiogenesis (8–11). FAK is closely associated with the
migration of malignant cells. In the present study, the expression
of FAK in the OS subclone A2 and A3 cell lines with high migration
abilities was significantly higher than that in the A1 cell line
with a low migration ability. Similarly, Chen et al
(12) identified that the expression
of FAK in hepatoma cells with a stronger migration and invasion
ability was significantly higher than that in the hepatoma cells
with weaker migration and invasion abilities. In the same study,
the knockdown of FAK expression by siRNA affected the cellular
migration of the hepatoma cells, a result also observed in human
neuroblastoma (13) and melanoma
(14) cells. The results of the
present study also indicated that in the early migration process,
reduced expression of FAK significantly decreased the number of
lamellipodia. This result is consistent with the results of a study
by Kwiatkowska et al (15),
which found that the decreased expression of phosphorylated FAK
(phospho-FAK) suppressed the migration of malignant glioma cells.
The study also revealed that lamellipodia formation was
significantly prolonged in the cells with low levels of
phospho-FAK. These findings, in addition to those of the present
study, suggest that the silencing of FAK affects the formation of
lamellipodia.
In summary, the results of the present study
suggested that FAK has an important role in the migration of OS
cells. FAK decreased the formation of lamellipodia, which in turn
affected the migration ability of the cells. These findings provide
a breakthrough in the study of the migration mechanisms involved in
OS, and present a novel route for the clinical assessment,
prognosis and treatment of the disease.
Acknowledgements
The study was supported by the Jiangsu Province
Natural Science Foundation from the Science and Technology
Department of Jiangsu Province of China (grant no. BK2012775), the
Key Scientific and Technological Project of ChangzhouHealth Bureau
(grant no. ZD201404), the Jiangsu Province Science and Technology
Support Program of Social Development Projects (grant no.
BE2013712).
References
|
1
|
Whelan JS: Osteosarcoma. Eur J Cancer.
33:1611–1619. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murphey MD, Robbin MR, McRae GA, et al:
The many faces of osteosarcoma. Radiographics. 17:1205–1231. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai IR, Chu PY, Lin HS, Liou JY, Jan YJ,
Lee JC and Shen TL: Phosphorylation of focal adhesion kinase at
Tyr397 in gastric carcinomas and its clinical significance. Am J
Pathol. 177:1629–1637. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Golubovskaya VM: Focal adhesion kinase as
a cancer therapy targy. Anticancer Agents Med Chem. 10:735–741.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Link MP, Goorin AM, Miser AW, et al: The
effect of adjuvant chemotherapy on relapse-free survival in
patients with osteosarcoma of the extremity. N Engl J Med.
314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wunder JS, Nielsen TO, Maki RG, et al:
Opportunities for improving the therapeutic ratio for patients with
sarcoma. Lancet Oncol. 8:513–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, et al: Osteosarcoma. J Am Acad Orthop Surg. 17:515–27.
2009.PubMed/NCBI
|
|
8
|
Parkes SE, Parke S, Mangham DC, et al:
Fifty years of paediatric malignant bone tumours in the West
Midlands, UK, 1957–2006: incidence, treatment and outcome. Paediatr
Perinat Epidemiol. 24:470–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fidler IJ and Kripke ML: Metastasis
results from preexisting variant cells within a malignant tumor.
Science. 197:893–895. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mark M, Reinhat-King CA and Erickson D:
Microfabricated physical spatial gradients for investigating cell
migration and invasion dynamic. PLoS One. 6:e208252011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liotta LA: Cancer cell invasion and
metastasis. Sci Am. 266:54–59, 62–63. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JS, Huang XH, Wang Q, et al: FAK is
involved in invasion and metastasis of hepatocellular carcinoma.
Clin Exp Metastasis. 27:71–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones ML, Shawe-Taylor AJ, Williams CM and
Poole AW: Characterization of a novel focal adhesion kinase
inhibitor in human platelets. Biochem Biophys Res Commun.
389:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tavora B, Batista S, Reynolds LE, et al:
Endothelial FAK is required for tumour angiogenesis. EMBO Mol Med.
2:516–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kwiatkowska A, Kijewska M, Lipko M, et al:
Downregulation of Akt and FAK phosphorylation reduces invasion of
glioblastoma cells by impairment of MT1-MMP shuttling to
lamellipodia and downregulates MMPs expression. Biochim Biophys
Acta. 1813:655–667. 2011. View Article : Google Scholar : PubMed/NCBI
|