Introduction
Increasing experimental and clinical data has
identified an association between increased levels of matrix
metalloproteinase (MMP)-9, a type IV collagenase, and shortened
patient survival, cancer progression and metastasis. MMP-9 has a
significant role in tumor cell invasion and metastasis, as it
digests the basement membrane and components of the extracellular
matrix (1–6). In addition to proteolysis, MMP-9 has
been identified to have a significant role in cellular migration
(7,8).
A unique characteristic of MMP-9 is its ability to be secreted in
monomeric and disulfide-bonded dimeric forms. A study by Dufour
et al (7) indicated that
dimerization of MMP-9 through the hemopexin domain is required for
MMP-9 enhanced cellular migration. Our previous study investigated
the secretion patterns of the MMP-9 monomer and dimer in a number
of cancer cell lines, and identified varying degrees of dimer
secretion (8). High MMP-9 and MMP-9
dimer secretion levels were associated with the most aggressive
cancer cell lines (8).
As tumor invasion is dependent upon cellular
migration, and MMP-9 dimer secretion has been demonstrated to be
required for cellular migration (7),
the present study investigated MMP-9 monomer and dimer secretion
patterns in a number of normal human cells from various tissues. It
has been established that signal transduction pathways and
cytokines, including those activated by phorbol 12-myristate
13-acetate (PMA), regulate the expression of MMPs. Therefore, the
aim of the present study was to analyze the expression patterns of
MMP-2, MMP-9 and MMP-9 dimer in normal human cells from a number of
tissues treated with PMA. Muscle, epithelial and connective tissue
tissues were selected for use in the present study, since
adenosarcomas, carcinomas and sarcomas are derived from these
tissue types, respectively.
Materials and methods
Cancer cell lines and reagents
In total, 14 normal human cell types obtained from
three primary tissues, namely epithelial, connective and muscle
tissues, were analyzed in the present study. The cell lines, in
addition to their recommended media, were purchased from Lonza
(Walkersville, MD, USA), with the exception of the kidney
parenchyma, gingival cells and hepatocytes, which were obtained
from the American Type Culture Collection (Manassas, VA, USA) along
with their recommended media, respectively. The human rheumatoid
synovial fibroblasts were isolated from rheumatoid arthritic
membranes following enzymatic dissociation using trypsin-EDTA
(Gibco Life Technologies, Carlsbad, CA, USA). All other high-grade
reagents, including fetal bovine serum (FBS), PMA, streptomycin and
penicillin, were purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell culture
First, the cells were grown in their respective
recommended media containing 10% FBS, 100 mg/ml streptomycin and
100 U/ml penicillin. Next, the cells were plated at a density of
1×105 cells/ml in triplicate in 24-well tissue culture
plates (Coster, Cambridge, MA, USA) and allowed to grow to
confluency at 37°C and 5% CO2 in a humidified
atmosphere. The serum-supplemented medium was removed and the cell
monolayer was washed twice with phosphate-buffered saline and then
once with the recommended serum-free medium. Next, the cells were
cultured for 24 h in an incubator with 0.5 ml serum-free medium.
The parallel sets of cultures were treated with 100 ng/ml PMA for
the induction of MMP-9. The resulting conditioned media were then
collected separately, pooled and centrifuged at 4°C at 704 × g for
10 min in order to remove the cells and cellular debris. Finally,
the supernatant was collected and used for gelatinase zymography, a
highly-sensitive assay for gelatinolytic enzymatic activity, which
is able to detect pro and active forms of MMP-2 and MMP-9.
Gelatinase zymography
Gelatinase zymography was performed under
non-reducing conditions with 10% Novex Pre-Cast SDS polyacrylamide
gel (Invitrogen Life Technologies, Carlsbad, CA, USA) and 0.1%
gelatin. In total, 20 µl of each culture medium was mixed with
sample buffer and loaded onto gels for SDS-PAGE with
Tris-glycine-SDS buffer, according to the manufacturer's
instructions (Thermo Fisher Scientific, Waltham, MA, USA). The
samples were not boiled prior to electrophoresis. Subsequent to
electrophoresis, the gels were washed twice in 2.5% Triton X-100 at
room temperature for 30 min in order to remove the SDS. Next, the
gels were incubated overnight at 37°C in a substrate buffer
containing 50 mM Tris-HCl and 10 mM CaCl2 at pH 8.0,
stained with 0.5% Coomassie Blue R250 in 10% glacial acetic acid
and 50% methanol for 30 min, and then destained. Subsequent to
renaturation of the enzyme, the gelatinases digested the gelatin
contained within the gel, which produced clear bands against an
intensely-stained background. In addition, protein standards were
run and approximate molecular weights were established by plotting
the relative mobilities of known proteins.
Results
Normal human cell lines express MMP-2,
but not MMP-9
The human dermal fibroblasts, gingival fibroblasts,
lung fibroblasts, prostate stromal cells, smooth muscle cells,
synovial fibroblasts and human vein endothelium cells secreted
MMP-2 only, even when treated with 100 ng/ml PMA, as shown in
Table I. Zymograms of MMP secretion
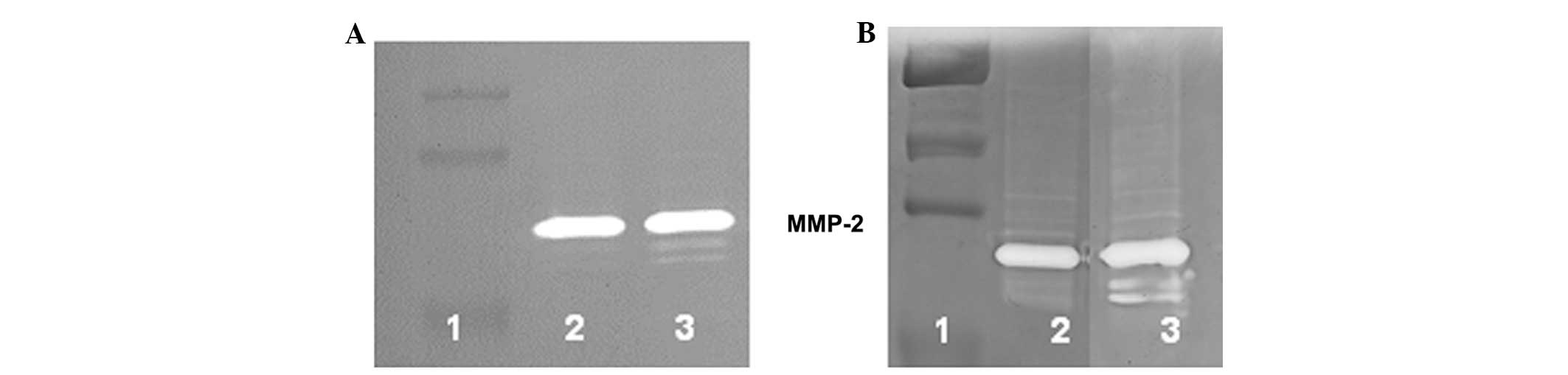
by representative cell lines, human gingival fibroblasts and human
prostrate stromal cells, are shown in Fig. 1.
 | Table I.MMP-2, MMP-9 and MMP-9 dimer secretion
patterns of normal human cells treated with 100 ng/ml phorbol
12-myristate 13-acetate. |
Table I.
MMP-2, MMP-9 and MMP-9 dimer secretion
patterns of normal human cells treated with 100 ng/ml phorbol
12-myristate 13-acetate.
| Cell line | Tissue | MMP-2 | MMP-9 | MMP-9 dimer |
|---|
| Dermal fibroblast
(NHDF) | Stromal connective
tissue | + | – | – |
| Lung fibroblast
(NHLF) | Stromal connective
tissue | + | – | – |
| Gingival fibroblast
(HGF) | Stromal connective
tissue | + | – | – |
| Synovial
fibroblast | Stromal connective
tissue | + | – | – |
| Prostate stromal
(PrSC) | Stromal connective
tissue | + | – | – |
| Vein endothelium
(HUVEC) | Endothelial
epithelial tissue | + | – | – |
| Aortic smooth muscle
cells (Ao-SMC) | Smooth muscle
tissue | + | – | – |
| Chondrocytes
(NHAC-Kn) | Supportive connective
tissue | + | + | – |
| Osteoblasts
(NHOst) | Supportive connective
tissue | + | + | – |
| Keratinocytes
(NHEK-Ad) | Glandular epithelial
tissue | + | + | – |
| Skeletal muscle
(SK-SMC) | Striated muscle
tissue | + | + | – |
| Hepatocytes | Glandular epithelial
tissue | + | + | – |
| Bronchial smooth
muscle | Smooth muscle
tissue | + | + | – |
| Uterine smooth
muscle | Smooth muscle
tissue | + | + | – |
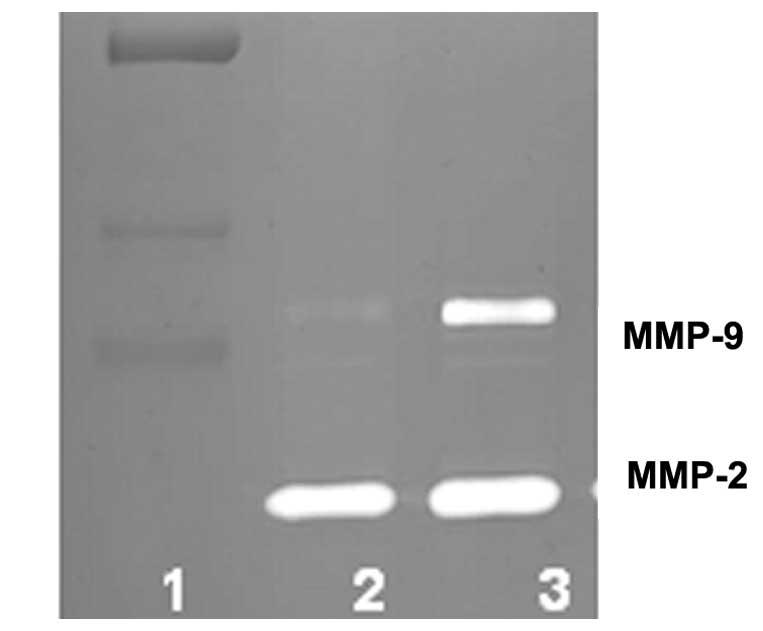
Normal human cells secrete MMP-2 and
MMP-9 upon stimulation by PMA, but fail to form MMP-9 dimers
The human chondrocytes, osteoblasts, skeletal muscle
cells, hepatocytes, bronchial muscle cells, uterine muscle cells
and keratinocytes secreted MMP-2 and MMP-9 following treatment with
PMA, but failed to secrete MMP-9 dimers, as shown in Table I. Zymograms of MMP secretion by human
osteoblasts, are shown in Fig. 2.
Discussion
Normal human stromal connective tissue cells (dermal
fibroblasts, lung fibroblasts, gingival fibroblasts, synovial
fibroblasts and prostate stromal cells), endothelial epithelial
tissue cells (vein endothelium cells) and aortic smooth cells
treated with PMA were found to secrete MMP-2, but not MMP-9 monomer
or dimer in the present study. By contrast, normal human supportive
connective tissue cells (chondrocytes and osteoblasts), striated
muscle cells from skeletal muscle), epithelial glandular tissue
cells (hepatocytes and keratinocytes) and smooth muscle cells from
bronchial and uterine tissues were found to secrete MMP-2 and MMP-9
following treatment with PMA, but failed to secrete MMP-9
dimer.
Our previous study investigated the relative
secretion patterns of MMP-9 monomer and dimer by a variety of
carcinomas, sarcomas, adenosarcomas and leukemia cell lines with
and without PMA induction (8). It was
identified that cancer cells derived from the same connective
tissues as those analyzed in the present study, namely fibrous
tissue (fibrosarcoma HT-1080), cartilage tissue (chondrosarcoma
SW-1353) and bone tissue (osteosarcoma U-2OS), exhibited MMP-9
dimer secretion subsequent to treatment with PMA. Among the tissue
cancer cell lines, sarcomas exhibited the highest levels of MMP-9
monomer and dimer secretion. In particular, osteosarcoma cells
demonstrated the highest MMP-9 to MMP-9 dimer ratio, which
indicated an extremely aggressive form of cancer. Muscle
tissue-derived sarcomas, including leiomyosarcoma SK-UT-1 (smooth
muscle) and rhabdomyosarcoma (striated muscle) also secreted MMP-9
dimers, but with a slightly lower level of MMP-9. Similarly,
epithelial tissue cancers, including adenocarcinomas
(hepatocellular carcinoma SK-Hep-1 and renal cell carcinoma RCC
786-0) secreted MMP-9 dimers, but at a lower level of MMP-9.
The aim of the present study was to analyze the
expression patterns of MMP-2, MMP-9 and MMP-9 dimer in normal human
cells from a number of tissues treated with PMA. As expected, none
of the normal human cells that were analyzed secreted MMP-9 dimer,
which indicated that cellular migration was not supported by these
cells. Therefore, it can be concluded that the MMP-9 dimer appears
to be associated with cancer cells.
Acknowledgements
The present study was funded by a grant from the Dr
Rath Health Foundation (Santa Clara, CA, USA), which is a
non-profit organization.
References
|
1
|
Kleiner DE and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43:(Suppl). S42–S51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yurchenko PD and Schitny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
3
|
Barsky SH, Siegel GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Invest. 49:140–147.
1983.PubMed/NCBI
|
|
4
|
Liotta LA, Tryggvason K, Garbisa S, et al:
Metastatic potential correlates with enzymatic degradation of
basement membrane collagen. Nature. 284:67–68. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
6
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dufour A, Zucker S, Sampson NS, et al:
Role of matrix metalloproteinase-9 dimers in cell migration: Design
of inhibitory peptides. J Biol Chem. 285:35944–35956. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: Effect of a nutrient mixture on matrix
metalloproteinase-9 dimers in various human cancer cell lines. Int
J Oncol. 44:986–992. 2014.PubMed/NCBI
|