Introduction
Liver cancer is one of the common malignant tumors
worldwide. Currently, surgery is the most effective treatment for
liver cancer. However, some patients may not be suitable for
surgical treatment due to postoperative complications including
liver failure caused by small future liver remnants (FLRs). Portal
vein embolization (PVE) is a procedure that induces regrowth on one
side of the liver prior to planned hepatic resection of the other
side. The procedure greatly reduces the risk of postoperative liver
failure by increasing FLR, and may enable patients whose tumors
were previously deemed inoperable due to small FLR, eligible for
surgical treatment (1). Portal vein
embolization (PVE) is used pre-operatively for patients with
primary or metastatic liver cancer, or as a palliative treatment
combined with chemotherapy for non-surgical cases. Embolic
materials are the key factor determining the success of PVE. At
present, common embolic agents for portal veins include absolute
ethanol, gelatin sponge particles, spring rings and Bletilla
striata. Absolute ethanol mainly affects the secondary
embolization of peripheral vessels and the great arteries (2,3), and is a
type of vascular embolic agent, as well as a tissue necrotic agent.
However, it is likely to cause regurgitation and the dosage is
difficult to control, thereby inducing inadvertent occlusion of the
wrong vessels. Alternative liquid embolic agents are likely to
adhere to the blood vessels, leading to vascular injury, multiple
complications, a high risk of vascular complications, vascular
toxicities and even malignant tumors. The use of Onyx® requires a
specific, expensive catheter. These limitations prevent the
clinical application of the agents.
Fuaile medical adhesive is the third generation of
medical adhesive invented by Chinese researchers. Fuaile is
characterized as being highly adhesive with good diffusion
properties; it is a low polymerization heat adhesive with a mild
bleaching phenomenon and desirable polymer toughness. Moreover,
Fuaile medical adhesive exerts a stable effect without causing
side-effects, toxicity, abnormalities or carcinoma (4,5). When
Fuaile medical adhesive is diluted with lipiodol, an inexpensive
agent, at varying ratios, the price of medical treatment may
decrease. Fuaile possesses the preliminary requirements for use as
a vascular embolic agent, including being stable, non-toxic,
non-teratogenic and non-carcinogenic. In China, experimental
studies have confirmed the successful use of Fuaile medical
adhesive in the embolization of the canine mesentery and branch
vessels of the gastroepiploic veins. However, there have been no
studies to verify that the application in the portal vein causes
embolization.
In the present study, Fuaile medical adhesive mixed
with lipiodol ultra-fluid at various ratios was used as a liquid
embolic agent for the selective embolization of the main trunk or
branch vessels of the portal vein. This experiment aimed to
investigate the feasibility and efficacy of applying Fuaile medical
adhesive for rabbit PVE, providing experimental evidence for its
subsequent clinical application.
Materials and methods
Experimental animals
A total of 22 healthy white rabbits, similar in age
and weight, both male and female, were selected for the present
study (Laboratory Animal Center, Guiyang Medical College, Guiyang,
China). This study, inclusive of all experimental procedures
involving animals, was approved by the Ethical Committee of the
Affiliated Hospital of Guiyang Medical College.
Preparation of experimental
materials
The study equipment consisted of a modified 18-gauge
puncture needle, a 2.7F concentric microcatheter system
(microcatheter and microlead) and 1-, 5- and 10-ml syringes.
Additionally, an Axiom Artis angiography instrument (Axiom Artis
dFC; Siemens Medical Solutions, Erlangen, Germany), GE HiSpeed CT/i
scanner (GE Medical Systems, Milwaukee, WI, USA), Lecia RM 2025
microtome (Lecia Instruments Ltd,. Nussloch, Germany), HB-2 optical
microscopy and imaging system (Olympus, Tokyo, Japan), MPIAS-9000
multimedia true-color pathology image analysis system (Tongji
University, Shanghai, China) were employed.
The agents used were as follows: Fuaile medical
adhesive was evenly daubed at the indicated sites (specification,
0.5 ml/U; batch number, 20070829; Beijing Fuaile Science And
Technology Development Co., Ltd., Beijing, China); lipiodol
ultra-fluid injection (specification, 10 ml; batch number,
06LU006A; Guerbet, Villepinte, France); iopromide injection
(Uhravist 370; specification, 100 ml, 37 g/l; batch number,
1000367; Bayer Healthcare Co., Ltd., Guangzhou, China); ketamine
hydrochloride injection (specification, 2 ml, 0.1 g; batch number,
KH070401; Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China);
and heparin sodium injection (specification 2 ml, 12,500 units;
Tianjin Biochem Pharmaceutical Co., Ltd., Tianjin, China).
Experimental procedure
Prior to embolization, under anesthesia, a blood
sample was collected from the rabbits via the auricular veins in
order to perform hepatic and renal function tests. These tests were
performed again at 1, 7 and 14 days post-operatively.
First, preliminary experiment
A total of 14 white rabbits were randomly divided
into 7 groups, with 2 rabbits in each group. Medical adhesive and
lipiodol ultra-fluid were mixed at a ratio of 1:0, 1:1, 1:2, 1:3,
1:4, 1:5 (adhesive groups) and 0:1 (lipiodol group). The
microcatheter head was placed at the portal trunk and the injection
was gradually delivered at 0.01 ml/s for a total volume of 0.2 ml.
The approximate adhesion time, degree of adhesion and range of
distribution were observed.
Second, formal experiment
A total of 12 white rabbits were collected and
randomly assigned into 2 groups, with 6 rabbits in each group. The
first and second blood vessel branches were used as the target
vessels. Based upon the preliminary experiment data, medical
adhesive was mixed with lipiodol ultra-fluid at a ratio of 1:4 and
used at the same speed. Blood vessel embolization was performed in
each group.
Experimental methods
Preoperative preparation
All animals were subject to fasting pre-operatively,
fixed on the operative bench in a supine position and underwent
anesthesia using 1 ml ketamine plus 5 ml normal saline via the
auricular veins. The animals also received persistent intravenous
administration of 2 ml ketamine plus 150 ml 5% glucose in water
(GS) (6,7). The hairs on the abdomen were shaved and
conventional sterilization was performed.
Arterial intubation
Following repeated disinfection, an incision was
made in the skin of the abdomen, exposing the portal trunk, and a
puncture needle was used for portal trunk puncture. A microcatheter
was introduced into the portal trunk for CT portography and then
intubation was performed into the portal vein branches, guided by
the microcatheter lead.
Embolization
The mixture of Fuaile medical adhesive and lipiodol
ultra-fluid was injected at a constant speed following proper
intubation, to avoid the incidence of embolic agent regurgitation.
Following embolization, 5% dextrose solution was used for
irrigating the catheter, the microcatheter was withdrawn to the
portal trunk and portal vein angiography was repeatedly performed
to confirm the effect of embolization.
Observations
In each group, two animals were sacrificed at 1, 7
and 14 days post-angiography, and CT scan results and the liver
tissues were collected for subsequent observation. The tissues were
fixed in 10% formalin, and stained with hematoxylin and eosin to
observe the liver cell necrosis and PVE. Prior to embolization,
blood samples were collected via the auricular veins at 1, 7 and 14
days post-operatively to measure the liver and renal functions.
Statistical analysis
SPSS 11.5 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for the data analysis. All data are presented as
the mean ± standard deviation. A paired t-test was conducted and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Preliminary experimental results
A total dose of 0.01 ml of embolic agent was infused
into the portal trunk at varying ratios (Table I). The high doses of embolic agents
(1:1 and 1:2) were much more likely to adhere to the catheter
compared with the low doses (1:4 and 1:5). While high doses of
embolic agents were distributed in the main trunk and first branch,
the low doses were localized to the first-second and second
branches. Furthermore, rabbits in the high-dose groups had a
stronger systemic reaction to the embolic agents compared with the
low-dose groups.
 | Table I.The reactions of the portal trunk
following infusion with embolic agent containing varying ratios of
medical adhesive and lipiodol. |
Table I.
The reactions of the portal trunk
following infusion with embolic agent containing varying ratios of
medical adhesive and lipiodol.
| Group | Degree of adhesion to
microcatheter | Distribution
area | Vascular
recanalization | Rabbit systemic
reactions |
|---|
| Pure medical
adhesive | Tightly adheres to
catheter | Main trunk | None | Death |
| 1:1 | Prone to tightly
adhere to catheter | Main trunk | None | Serious, prone to
die |
| 1:2 | Adheres to catheter,
but not tightly | Main trunk, first
branch | None | Moderately
serious |
| 1:3 | Partially adheres to
catheter | First-second
branches | None | Moderate to
significant |
| 1:4 | Not prone to adhesion
to catheter | First-second
branches | None | Mild to moderate |
| 1:5 | Not prone to adhesion
to catheter | Second branch and
below | None | Relatively mild |
| Pure lipiodol | Does not adhere to
catheter | Second branch and
below | None | Extremely slight |
Dose of embolic agents
The injected doses of embolic agents were determined
according to rabbit size and the speed of blood flow. The doses
were ~0.15 ml for the portal trunk, and 0.02 ml for the first
branch and 0.01 ml for the second branch of the portal veins.
Speed of embolization
The overall speed was extremely slow and maintained
under strict supervision. The high doses (1:1 and 1:2) more
commonly induced regurgitation and embolized the blood vessels in
error due to heavy resistance, while the low doses of embolic
agents (1:4 and 1:5) less commonly caused the incidence of
regurgitation, and was easy to manage.
Formal experimental results
Based on the preliminary experimental outcomes,
embolization was found to be optimal at a 1:4 ratio. In
consideration of the severe reactions, including death, following
portal trunk embolization, the first or second branches of the
portal vein were also selected for embolization. The dose of
embolic agents for the first branch of the portal vein was 0.02 ml
and the dose for the second branch was 0.01 ml. A total of 6
rabbits were subjected to embolization of the first branch of the
portal vein and 6 were subjected to embolization of the second
branch, with a success rate of 100%.
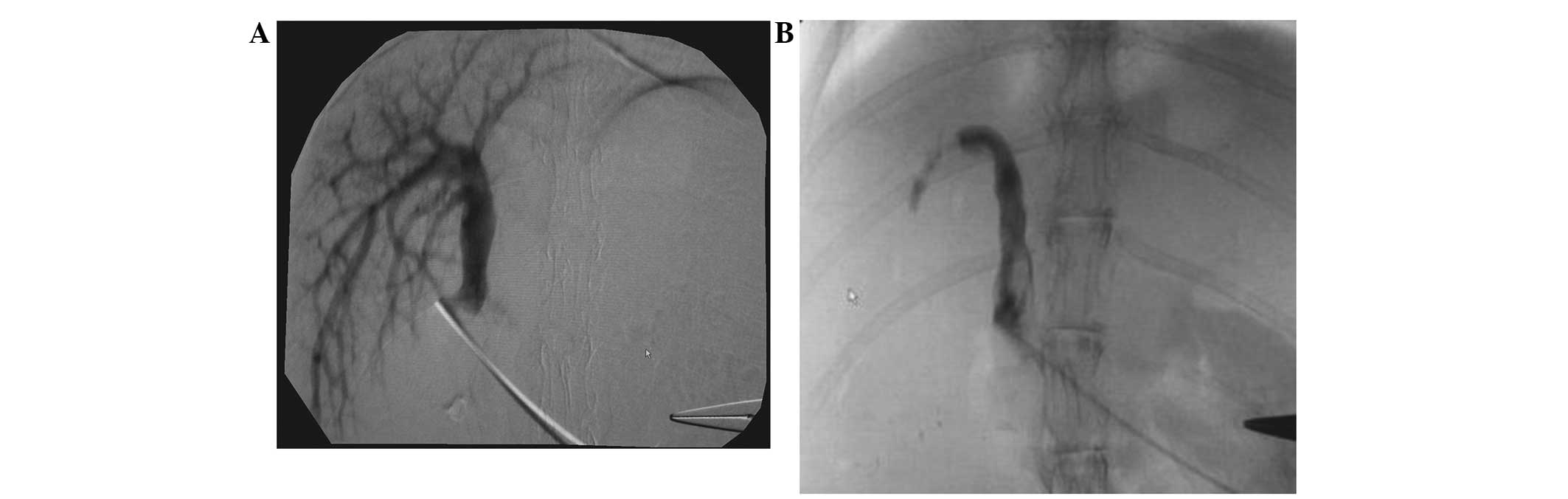
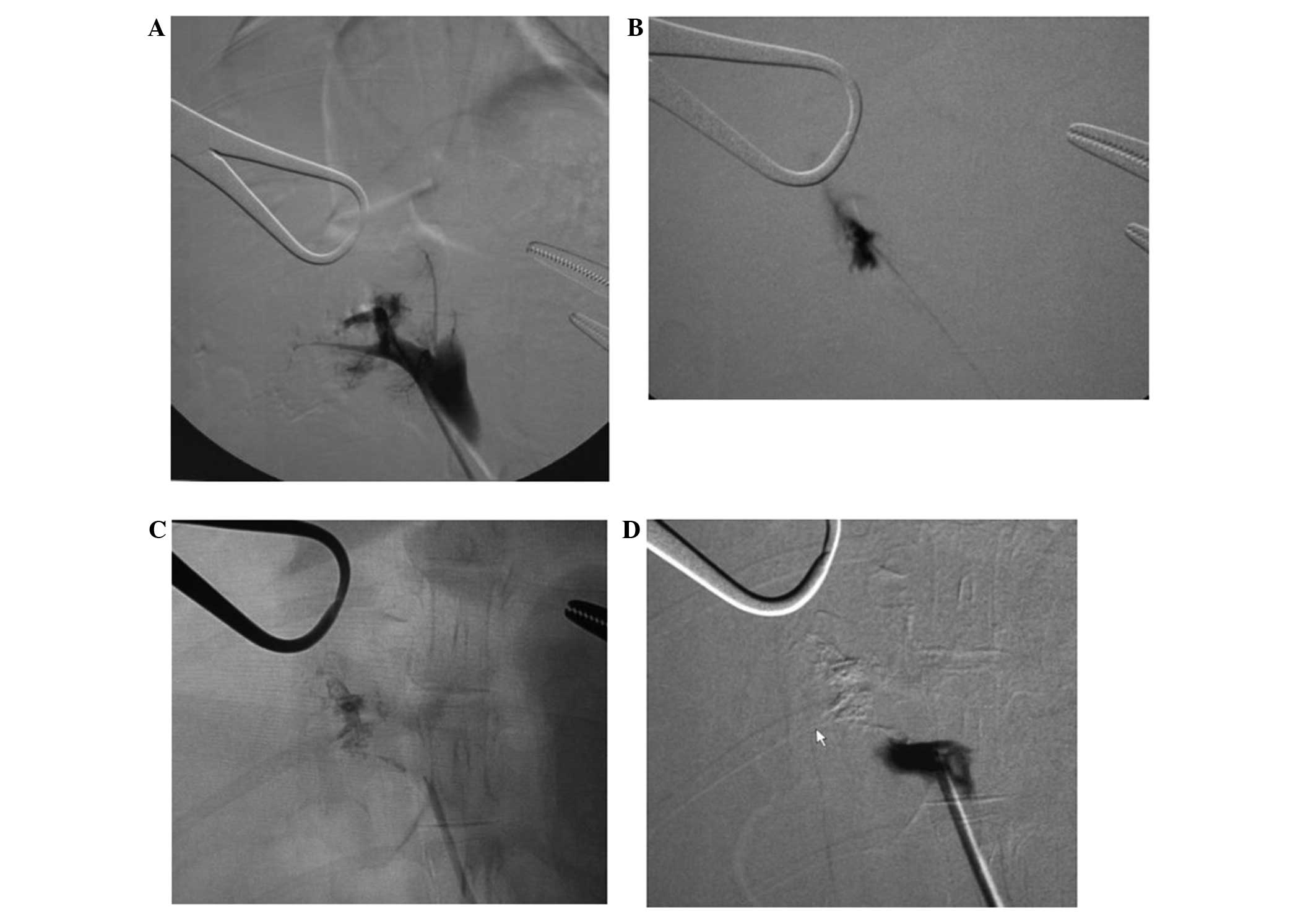
CT portography
All cases received an acute and complete
embolization. The embolization rate achieved was 100%. With the
exception of the pure lipiodol group, no other groups experienced
significant recanalization at 7 and 14 days post-embolization
(Figs. 1–4).
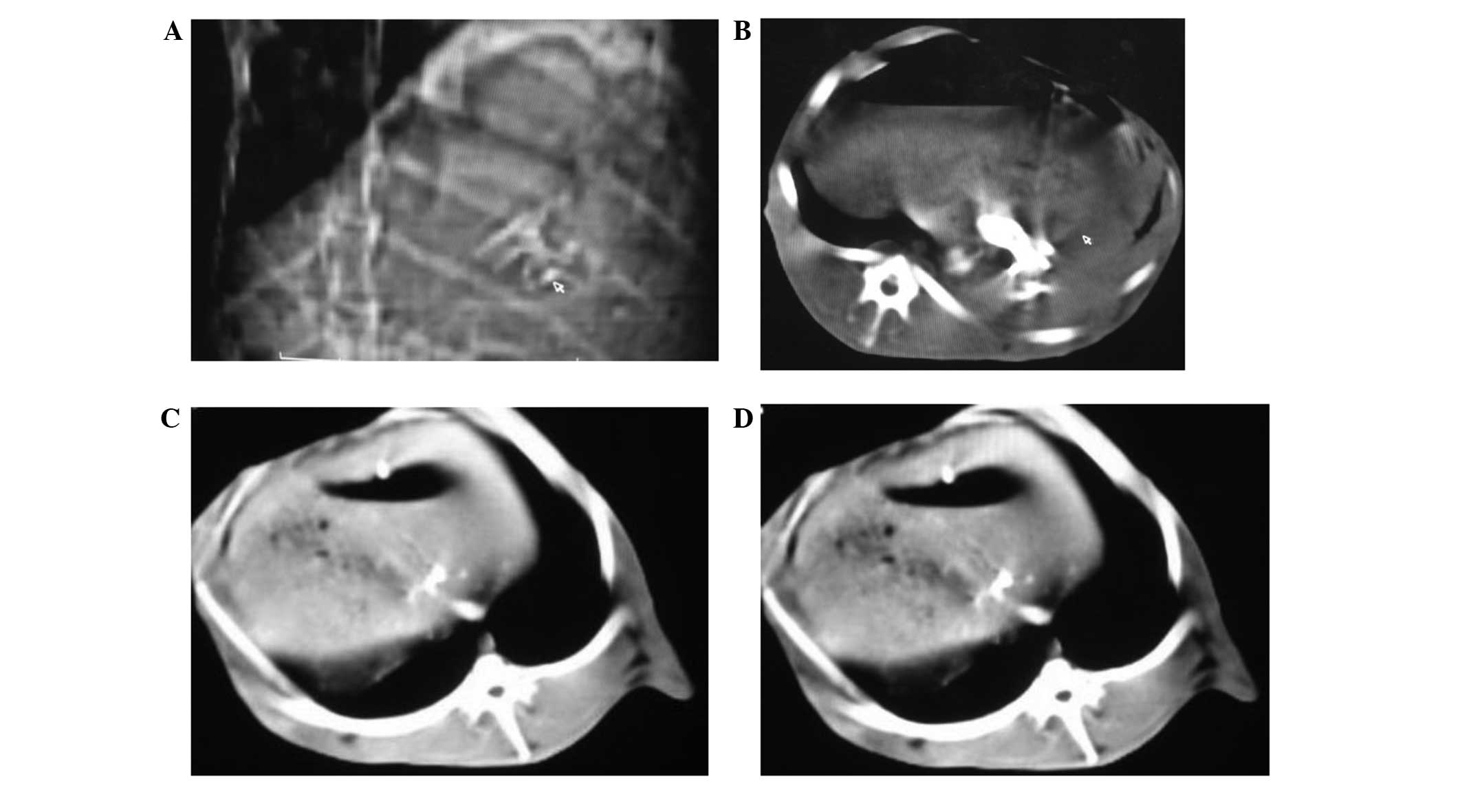
CT scanning
Conventional CT scanning revealed high-density
shadows in the targeted blood vessels, but the density of the liver
tissues did not decrease significantly. Post-embolization
reexamination identified the presence of high-density shadows.
However, the density of the hepatic tissues was significantly
decreased and a gas density shadow was visible (Fig. 5).
Histological examinations
To the naked eye, no significant changes occurred
during the early stages post-embolization, and then topical swollen
tissues gradually became darker. Upon light microscopy, during the
early stage, the blended embolus of Fuaile medical adhesive and
lipiodol was observed in the portal vein and partial central vein,
followed by secondary formation of a thrombus, liver tissue
necrosis within the embolization area, liver cell edema and
cytoplasmic relaxation. The inflammatory response surrounding the
embolization area was not significant during the early stage;
inflammatory cell infiltration of varying degrees was evident and
apparent inflammatory fibroplasia occurred 14 days later. A
substantial amount of lipiodol and medical adhesive mixture was
observed followed by secondary formation of a thrombus. No
significant vascular recanalization was recorded (Fig. 6).
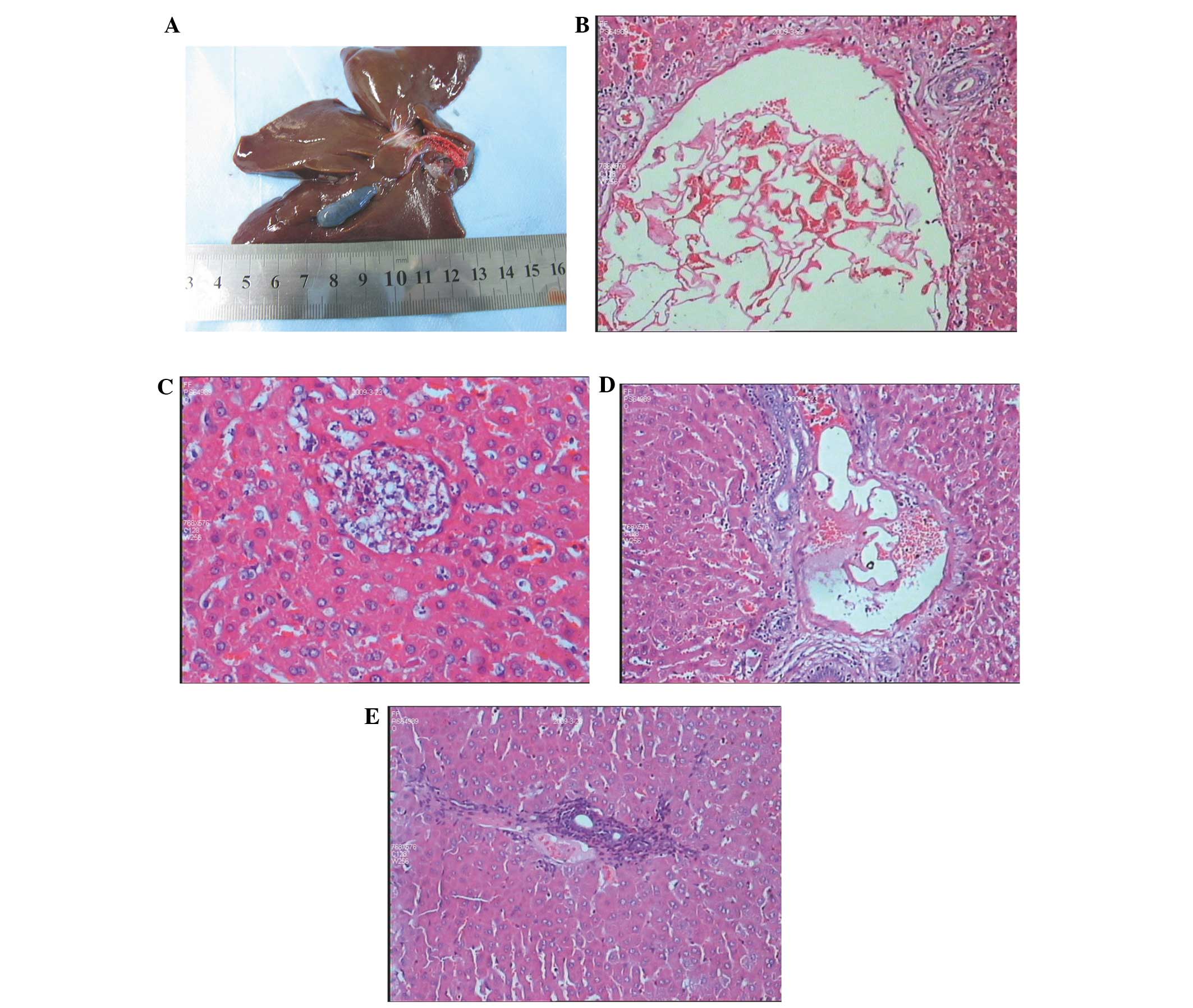 | Figure 6.(A) Following portal trunk
embolization, red embolic agents and secondary formation of a
thrombus were noted in the portal trunk. (B) Embolization of the
largest branch of the portal vein (magnification, x100). A large
amount of blended lipiodol and Fuaile medical adhesive was observed
as an embolus, followed by secondary formation of a thrombus. (C)
At 1 day post-embolization, liver cell injuries were observed,
typical necrotic lesions formed and inflammatory cell infiltration
was insignificant (magnication, x200). (D) At 7 days
post-embolization of the portal vein branches, an embolus and
secondary formation of a thrombus were noted. The inflammatory cell
infiltration was more serious surrounding the embolized blood
vessels (HE staining; magnification, x100). (E) At 14 days
post-embolization, inflammatory hyperplasia was apparent in the
embolized area, and adjacent liver cell necrosis was also noted (HE
staining, magnification, x100). HE, hematoxylin and eosin. |
Changes in hepatic and renal functions
before and after embolization (Table
II)
As shown in Table II,
the liver function damage occurred at 1 day post-embolization,
prior to the severity peaking at 7 days and subsequently being
almost restored to normal status at 14 days, as indicated by levels
of alanine aminotransferase (ALT) and aspartate aminotransferase
(AST). Renal function damage was identified at 1 day
post-embolization, and normal function resumed at 7 days, as
indicated by levels of blood urea nitrogen (BUN) and ceruloplasmin
(CR).
Discussion
Transhepatic portal catheterization for PVE is
effective in pre-operative embolization for patients with primary
or metastatic liver cancer, or as a palliative treatment combined
with chemotherapy for non-surgical cases (8–12). For
patients receiving pre-operative embolization, embolization of the
branch of the partial portal vein can cause the atrophy of liver
tissue lesions, reduced tumor size and lead to hepatic tissue
hyperplasia within the non-embolization area, thereby widening the
extent of the surgical excision and surgical indications.
Furthermore, PVE also avoids the acute liver cell
damage induced by a decline in portal venous pressure
intraoperatively or abnormal metabolism resulting from excessive
hyperplasia post-operatively, which enhances the tolerability of
surgery and decreases the incidence of post-operative complications
(13,14). For non-surgical patients, large
peripheral lesions of primary liver cancer supply blood for the
portal vein, while metastatic liver cancer depends on the portal
vein for its blood supply. Liver artery chemotherapy combined with
embolization yields poor clinical effects, with a high incidence of
metastasis and recurrence post-operatively (12,13).
Consequently, performing combined chemotherapy and embolization
therapy in the liver artery and portal vein of patients with liver
cancer not only thoroughly treats intrahepatic malignant masses,
but also reduces the incidence of tumor recurrence and metastasis.
In addition, it is also capable of treating portal venous tumor
thrombosis.
Fuaile medical adhesive was initially invented by
using N-octyl-α-cyanoacrylate (NOCA) to alter the property
of N-butyl cyanoacrylate (NBCA). In total, >99% of its
ingredients are α-cyano methoxyethyl acrylate, NOCA and NBCA. Xia
et al (4) adapted the formula
of Fuaile in 2003 to create a spray-adehesive. Fuaile medical
adhesive is categorized as a 6865-Ⅲ medical device, which is widely
applied in intraoperative hemostasis, adhesion, sealing and
embolization. Thus far, 17 biological detections and >100
pathological tests, including asepsis, pyrogen, acute systemic
toxicity, sub-acute poisoning, skin sensitization, intracutaneous
irritation, hemolysis, cell toxicity and the Ames test, have been
completed and yielded negative results. The experimental outcomes
for mutagenic, teratogenic and carcinogenic effects, and
propagation were also negative. Antibacterial tests revealed that
the adhesive exerts an inhibitory effect on 11 species of bacteria,
including Staphylococcus aureus. No adverse reactions have
been reported in the 29-year follow-up of >10 million cases in
clinical settings. Pathological studies conducted in the Second
Affiliated Hospital of Xi'an Medical University in order to utilize
Fuaile medical adhesive to embolize the canine mesentery and branch
vessels of the gastroepiploic vein, noted aseptic inflammatory
reactions at days 1–4, which were alleviated at 7 days and
significantly eased at 14 days. Intravenous membrane hyperplasia
invaded the vascular lumen and surrounded the medical adhesive,
splitting it into different partitions. At 14 days, fibrosis
appeared in the vascular wall and fibrous embolization was observed
in the venular lumen. Residual medical adhesive embraced by fiber
tissues remained in the large venous lumen for up to one year, and
was classified as permanent embolization.
NOCA and NBCA are useful as blood vessel embolic
agents in a clinical setting. However, they exhibit significant
adhesion and there is a risk of the microcatheter adhering to the
blood vessels. Additionally, aggregation effects may give off heat.
Fuaile medical adhesive precludes a variety of additives and is
safe for human use. Compared with NOCA AND NBCA, Fuaile medical
adhesive has an appropriate polymerization speed, low
polymerization heat and desirable diffusion. The preliminary
experiments of the present study found that if Fuaile medical
adhesive was mixed with lipiodol ultra-fluid at a proper ratio and
infused at proper speed, the incidence of adherence to the blood
vessels was prevented and the goal of being able to perform repeat
injections in the embolized vessels was met.
Compared with non-adhesive liquid embolic agents,
such as Onyx, Fuaile medical adhesive has a lower price, yields no
vascular toxicity and can be easily injected via common catheters.
Moreover, Fuaile medical adhesive can be prepared as an embolic
agent for different branches of target blood vessels, whereas
absolute ethanol is limited to application in peripheral vessel
embolization (4,14). Therefore, Fuaile medical adhesive is
feasible in clinical practice as a liquid embolic agent.
With regard to the safety of PVE, the hepatic and
renal function tests performed in the present study indicated that
liver function damage emerged at 1 day post-embolization, prior to
the severity peaking at 7 days and then being almost restored to
normal status at 14 days. Renal function damage appeared at 1 day
post-embolization, and normal function resumed at 7 days. These
results are consistent with previous findings. Dong et al
conducted PVE in rat models and noted alanine aminotransferase
(ALT) elevation at 1 day post-embolization, which started to
decline at 7 days and was finally restored to a normal level at 14
days (15). Wan et al
(16) utilized Bletilla
microspheres and absolute ethanol to perform PVE in rabbits, and
subsequent liver function examination indicated that liver function
damage occurred at 1 day post-embolization, and that the severity
of the injuries peaked at approximately 3 days and were generally
restored to a normal level 14 days later. Wu et al (17) applied a type of medical adhesive, DTH
for selective PVE in a rat model and found that liver function
presented with transient changes characterized as ALT and AST
elevation 1 day post-operatively, which started to decline at 3
days and eventually returned to normal levels 14 days
post-operatively. Dong et al (15) used NBCA to perform PVE in rats. This
caused an increase in the level of ALT at 1 day post-embolization.
A decreasing trend was evident at 7 days. At 14 days, liver and
renal functions appeared to be at normal levels. In this study,
renal function injuries were relatively mild and rapidly recovered
(15), however, the possibility of
contrast agent-induced renal toxicity must always be
considered.
Wan et al (16)
suggested that PVE is generally safe if the extent of embolization
does not exceed three liver segments. In this investigation, the
animals showed a slightly poor performance with regard to diet,
physical strength and activity at 1 day post-embolization, but all
were subsequently restored to normal status (16).
The rabbits died during the preliminary experiment,
which may be explained by the following reasons: i) Excessive
anesthesia-related mortality; ii) intraperitoneal
hemorrhage-related mortality due to the improper management of
hemostasis; and iii) acute complete portal trunk
embolization-related mortality due to an excessive dose of embolic
agents. Therefore, overall, it is safe to perform PVE in rabbit
models if the procedures, including anesthesia, hemostasis and
extent of embolization, are controlled.
Since Fuaile medical adhesive experiences a
transient transition from a hydrophilic to hydrophobic status in
water at 37°C, attention is required upon the injection of embolic
agents. The presence of sediment from the embolic agents must be
avoided in the microcatheter, as it could possibly block the
microcatheter and affects the surgery. Based on the findings from
the in vitro embolization experiment and preliminary study,
the following procedures are necessary: i) Prior to injection, the
microcatheter should be washed using a moderate amount of
phosphate-buffered saline to lower the temperature of the wall and
cavity of the microcatheter, thereby extending the time of phase
transition of the polymer in the microcatheter.
ii) Prior to and following injection, 5% GS
irrigation should be used to wash the microcatheter cavity to
prevent the formation of sediment from the embolic agents within
the microcatheter or around the mouth, which may lead to catheter
obstruction or make the microcatheter adhere to the blood vessel
wall.
iii) The total dose of embolic agents should be
under control, with ~0.15 ml for the portal trunk, 0.02 ml for the
first branch of portal vein and ~0.01 ml for the second branch of
the portal vein in rabbits. An increase in dosage of embolic agents
is necessary for multiple injections. The dose for each injection
should not be excessively high to avoid the incidence of
embolization of the surrounding blood vessels or adhesion to the
microcatheter.
iv) The speed of injection for different doses of
embolic agents should vary accordingly. It is critical that the
embolic agents be injected extremely slowly using a 1-ml syringe.
Repeated injections will prevent the incidence of regurgitation and
embolization of other blood vessels in error. Special attention is
necessary when injecting a high dose of embolic agents.
v) The entire process of embolization should be
monitored to avoid errors.
vi) Angiography is to follow immediately after
embolization to prevent the incidence of incomplete
embolization.
vii) Injury to the animals must be avoided as much
as possible. An incision of 3–4 cm in size on the abdominal wall at
the site of the portal vein can be enlarged as necessary.
Pre-operative fasting reduces the volume of the stomach to minimize
the risk of anesthesia and to expose the portal vein easily.
viii) The frequency of intraoperative portal vein
puncture should be reduced as much as possible. The puncture, the
microcatheter and the needle should be in the correct position to
prevent damage to the portal vein blood vessel wall and to avoid
repeated punctures, which aggravate the severity of the trauma and
enhance the difficulty of hemostasis. Hemostasis is achievable by
suppressing the puncture site for several minutes after pulling out
the needle. A suitable quantity of Fuaile medical adhesive should
be daubed on the puncture point as necessary.
ix) The surgery must be performed in aseptic
conditions. In the abdominal wall, incision sutures are applied
layer by layer, then wrapped by bandages to minimize the risk of
infection. The use of antibiotics in the diet is acceptable as
necessary.
Insights gained from the present study included the
fact that lipiodol ultra-fluid not only dilutes the concentration
of Fuaile medical adhesive, but also allows for close monitoring
throughout the process of embolization. By comparing CT portography
images prior to and following embolization, it was found that the
higher the dose of Fuaile medical adhesive injected, the larger the
size of the branches of the portal vein that could be embolized.
However, a higher dosage enhances the incidence of adhesion to the
microcatheter and regurgitation. The present experimental results
confirmed that the effect of embolization in the blood vessels was
favorable and no significant recanalization was noted. The ratio of
lipiodol ultra-fluid and lipiodol ultra-fluid should be <1:3,
and a ratio ranging from 1:4 to 1:5 is highly recommended. Further
dilution is likely to prolong the time of polymerization and
provide more time for the surgery, however, an excessively low
concentration may negatively affect the effect of embolization.
In summary, Fuaile medical adhesive is a safe and
efficacious liquid embolic agent that may be used for PVE in white
rabbit models. The total dose differs depending upon the
embolization site (0.15 ml for the portal trunk, 0.02 ml for the
first branch of the portal vein and 0.01 ml for the second branch
of the portal vein). In addition, the adhesive is useful in the
embolization of different branches of the portal vein using
selective catheterization and by combination with lipiodol
ultra-fluid at varying ratios (recommended ratio, 1:3–1:5). Fuaile
medical adhesive is cheap and easy to prepare, and therefore
warrants widespread application in the clinical setting.
References
|
1
|
Abulkhir A, Limongelli P, Healey AJ, et
al: Preoperative portal vein embolization for major liver
resection: a meta-analysis□J□. Ann Surg. 247:49–57. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jungreis CA: Skuff-base tumors: ethanol
embolization of the cavernous carotid artery. Radiology.
181:741–743. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadato A, Numaguchi Y, Taki W, et al:
Nonadhesive liquid embolic agent: role of its components in
histologic changes in embolized arteries. Acad Radiol. 5:198–206.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia HS, Tian X and Lu YS: A new generation
of spray-type Fuaile medical adhesive (fundamental research). J
Clin Surg. 11:2003.(In Chinese).
|
|
5
|
Xia HS, Tian X and Lu YS: A new generation
of spray-type Fuaile medical adhesive (clinical research). J Clin
Surg. 11:2003.(In Chinese).
|
|
6
|
Zheng ZL, Pan JS, Ma LJ, et al: The
comparison of different anesthesia approaches for vascular surgery
operation in rabbits. Journal of Hebei Medical University.
29:384–387. 2008.(In Chinese).
|
|
7
|
Li YQ, Yang XL, Qin JQ, et al: Analysis of
application of ketamine in anesthesia of experimental animals.
Shanghai Laboratory Animal Science. 21:169–170. 2001.(In
Chinese).
|
|
8
|
Mao G, Yu Z, Zhang Y, et al: Combined
transcatheter arterial chemoembolization and beta-ultrasound guided
portal vein embolization in the treatment of hepatocellular
carcinoma. Zhonghua Zhong Liu Za Zhi. 24:391–393. 2002.(In
Chinese). PubMed/NCBI
|
|
9
|
Bartolozzi C, Lencioni R, Caramella D, et
al: Treatment of large HCC: transcatheter arterial
chemoembolization combined with percutaneous ethanol injection
versus repeated transcatheter arterial chemoembolization.
Radiology. 197:812–818. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamada K, Kitamoto M, Aikata H, et al:
Combination of transcatheter arterial chemoembolization using
cisplatin-lipiodol suspension and percutaneous ethanol injection
for treatment of advanced small hepatocellular carcinoma. Am J
Surg. 184:284–290. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tanaka H, Hirohashi K, Kubo S, et al:
Preoperative portal vein embolization improves prognosis after
right hepatectomy for hepatocellular carcinoma in patients with
impaired hepatic function. Br J Surg. 87:879–882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inaba S, Takada T, Amano H, et al:
Combination of preoperative embolization of the right portal vein
and hepatic artery prior to major hepatectomy in high-risk
patients: a preliminary report. Hepatogastroenterology.
47:1077–1081. 2000.PubMed/NCBI
|
|
13
|
Liu H, Peng YH, Yang ZH, et al:
preoperative portal vein embolization increases the excision rate
of liver cancer surgery. Chinese Journal of General Surgery.
9:5832004.(In Chinese).
|
|
14
|
Ji W, Ma KS, Dong JH, et al: Role of
preoperative selective portal vein embolization in two-stage
hepatectomy for primary hepatocellular carcinoma. Chinese Journal
of Hepatobiliary Surgery. 348–350. 2003.(In Chinese).
|
|
15
|
Dong BW, Liang P, Luo YK, et al:
Experimental studies of the portal vein puncturing embolization
with NBCA in rats. Chinese Journal of Ultrasonography. 10:494–497.
2001.
|
|
16
|
Wan ZY, Feng GS, Liang HM, et al:
Experimental portal vein embolization in rabbits: a comparison of
Bletilla microsphere with absolute ethanol. J Clin Radiol.
23:908–912. 2004.(In Chinese).
|
|
17
|
Wu XF, Fan J, Lin ZY, et al: Experimental
studies on selective portal vein embolization. Journal of Chinese
Microcirculation. 4:218–220. 2000.
|