Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare
soft-tissue neoplasms that have become increasingly prevalent in
recent years. The tumors are a type of inflammatory pseudotumor
(IPT); a mass that contains a mix of myofibroblastic and
fibroblastic spindle cells with infiltration of inflammatory cells.
IMTs have been referred to as plasma cell granulomas, inflammatory
fibrosarcomas and inflammatory myofibrohistiocytic proliferations,
reflecting the variable pathological manifestations, and
controversial nature and origin (1).
An IMT begins as a benign reactive process, and progresses to an
intermediate neoplasm with local destruction and recurrence
(2).
IMTs can affect people of any age, with a
predilection for young adults and children. The tumors occur most
commonly in the lung, with the most common extrapulmonary sites as
the omentum and mesentery (3). IMTs
of the head and neck region are considered to be rare, and can
occur in the orbit, maxillary sinus, nasopharynx, parapharyngeal
space, larynx, skull base, temporal bone and neck (4–6). IMTs
demonstrate various clinical manifestations and pathobiological
behaviors, depending on the site affected (3). IMTs in the orbit usually cause only
inflammation, which is easily treated by corticosteroids. By
contrast, IMTs of the maxillary sinus can recur and can become
sarcomatous following incomplete resection. Computed tomography
(CT) scans and magnetic resonance imaging (MRI) of IMT lesions
differ for each location, and the lesions can be mistaken for other
diseases (7). The variable imaging
findings of these lesion may be due to the preponderance of spindle
cells or inflammatory cells.
In the present study, a rare case of IMT of the neck
is described and the associated literature is reviewed. The
clinical manifestations, radiographic characteristics and
management of these tumors are discussed, with a focus on imaging
modalities.
Case report
A 43-year-old female patient presented to The First
Affiliated Hospital (College of Medicine, Zhejiang University,
Hangzhou, Zhejiang, China) in April 2012 with a two-month history
of a firm, painless, asymptomatic mass in the right side of the
neck. No history of trauma, surgery or infection was recorded. Upon
physical examination, the patient appeared to be in good health,
with no evidence of lymphadenopathy, hepatomegaly or splenomegaly.
Results of blood tests and a C-reactive protein test were within
the normal ranges.
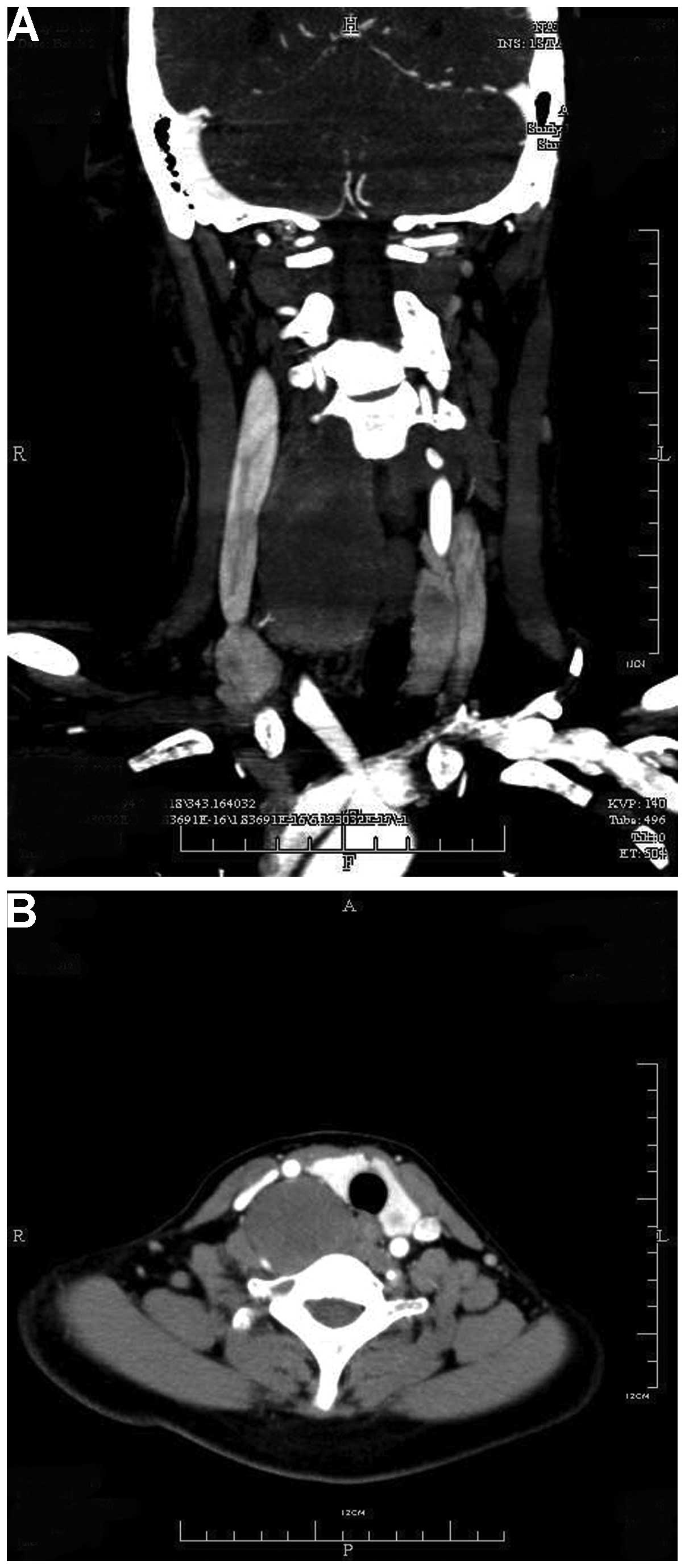
Ultrasonography (USG) examination showed a 3.76-cm
mass in the right side of the neck. CT scans revealed a
well-defined, non-homogeneous, 3.0×4.2×11.0-cm mass of the right
deep neck, with slight enhancement compared with the surrounding
tissues. The tumor was close to the cervical vertebrae (from C3 to
T2), and had pushed the carotid sheath and thyroid laterally. Bony
erosion was not observed in the adjacent osseous structures and the
tumor itself was not calcified (Fig.
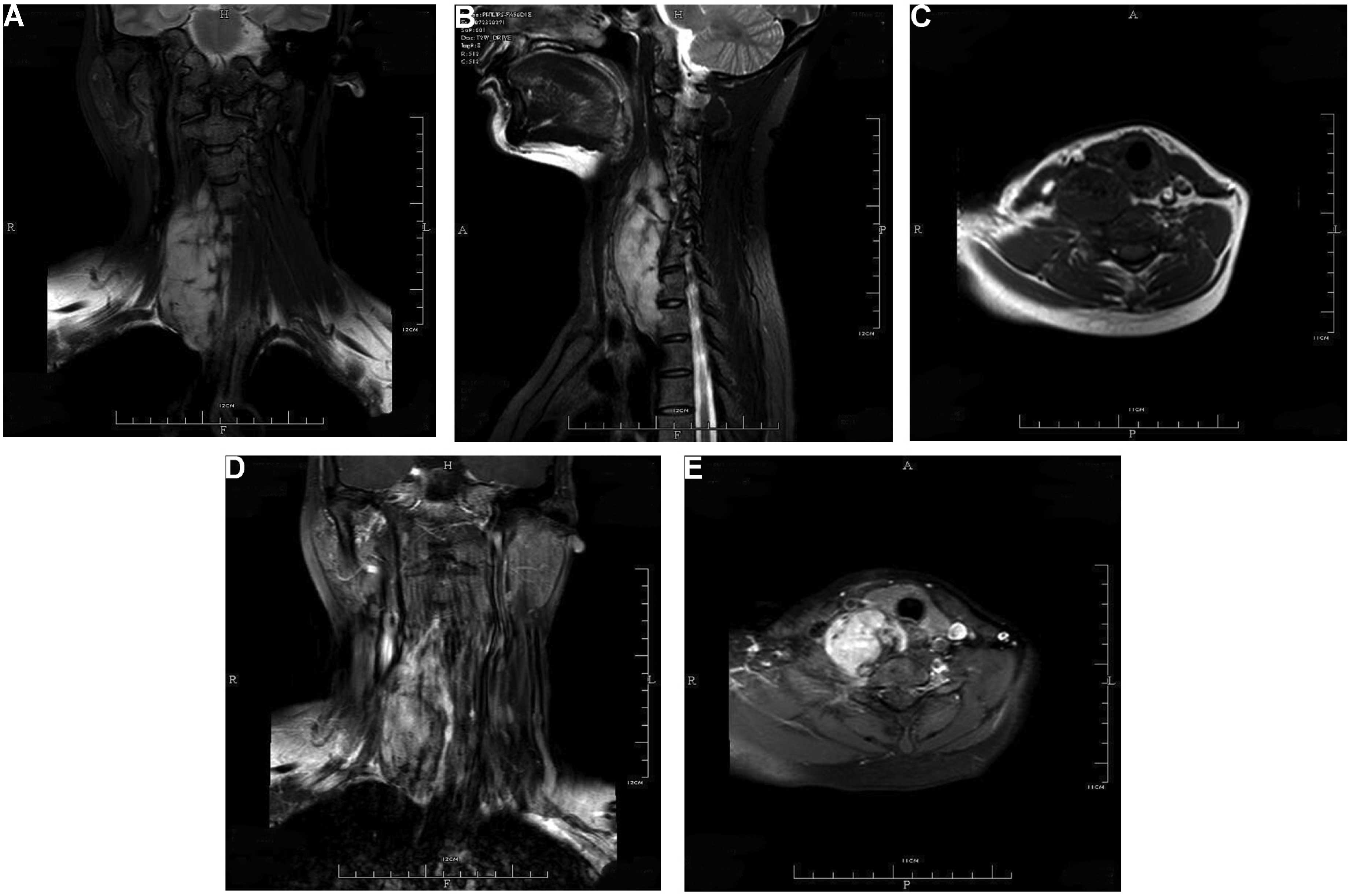
1). MR images were obtained with T1-weighted, T2-weighted and
gadolinium-enhanced T1-weighted sequences. The fusiform,
well-defined and non-homogeneous mass displayed a hypointense to
isointense signal on T1-weighted sequences and a hyperintense
signal on T2-weighted sequences compared with the surrounding
tissues. The tumor appeared non-homogeneously enhanced on
gadolinium-enhanced T1-weighted images (Fig. 2). The radiographic appearance
initially suggested a nerve sheath tumor and the marked enhancement
on MRI indicated a possible malignancy.
A USG-guided aspiration biopsy was performed under
local anesthesia, and histology showed numerous spindle and
inflammatory cells, but the result was non-diagnostic. For surgical
excision, a lateral neck incision was made under general
anesthesia. A large, firm, reddish-yellow tumor was resected en
bloc. Part of the tumor was close to the cervical vertebrae, but
bony erosion was not observed, as indicated on CT.
Intraoperatively, frozen sections revealed a benign spindle cell
tumor. Histologically, the tumor was composed mainly of spindle
myofibroblasts with a few infiltrating inflammatory cells.
Immunochemistry showed that the tumor cells were positive for
smooth muscle actin (SMA), muscle-specific actin (MSA), vimentin,
desmin and ALK, but negative for S-100. These results confirmed the
diagnosis of a neck IMT. The post-operative recovery was uneventful
and 23 months of follow-up revealed no signs of recurrence.
Discussion
IPT has been used to describe a wide range of
reactive and neoplastic lesions, including IMT and certain
infectious processes (8). These
lesions show similar pathological characteristics, but differ
biologically. IMT, a distinctive neoplasm with a few reactive
inflammatory cells that occurs primarily in viscera and soft
tissues, predominantly affects children and young adults (9). As IMT of the neck is a rare disease, to
the best of our knowledge, the present study is the first case
report of the lesion. For the present literature review, PubMed was
searched regarding IMT of the neck between 1990 and 2013 using the
following keywords: ‘Inflammatory myofibroblastic tumor’ and
‘neck’; or ‘inflammatory pseudotumor’ and neck’; or ‘plasma cell
granuloma’ and ‘neck’. Seven patients were found in seven
English-language studies, including the present case, and one
patient in one Chinese-language study, which described clinical and
imaging details (Table I) (7,10–15).
 | Table I.Review of the clinical and imaging
details of inflammatory myofibroblastic tumors of the neck. |
Table I.
Review of the clinical and imaging
details of inflammatory myofibroblastic tumors of the neck.
|
|
|
|
| CT | MRI |
|
|
|---|
|
|
|---|
| First author,
year | Gender/age | Etiology |
Symptoms/duration | Plain | Enhanced | Plain | T1 | T2 | Enhanced | Treatment | Outcome |
|---|
| Browne et al,
2004 | F/12 | Unknown | Neck mass, LS/6
months | WD, IHG Soft-tissue
density | – | – | – | – | – | Resect en bloc | No recurrence |
| Babar-Craig et al,
2005 | M/31 | Unknown | Painful neck mass,
RS/5 months | – | – | WD, IHG | – | – | – | Steroids | Improvement |
| Ceruse et al,
2005 | M/26 | Unknown | Neck mass, LS/several
months | – | – | – | – | – | – | Conservative
surgery | Recurrence at 6
months |
| Chen et al, 2008 | M/39 | Unknown | Painful neck mass,
RS/7 years | – | – | – | – | – | – | Resect en bloc | No recurrence |
| Park et al, 2009 | F/17 | Unknown | Neck mass, LS | ID, HG Soft-tissue
density | Yes, HG | – | – | – | – | – | – |
| Chen et al, 2011 | M/39 | Unknown | – | – | – | – | – | – | – | Resect en bloc | No recurrence |
| Marraoui et al,
2012 | F/51 | Unknown | Painless neck mass,
LS/5 days | ID, IHG Soft-tissue
density | Yes, IHG | ID, IHG | OS | OS | Yes, IHG | Steroids | No recurrence |
| Present study,
2014 | F/43 | Unknown | Painless neck mass,
RS/2 months | WD, IHG Soft-tissue
density | Yes, WD, IHG | WD, IHG | OS, IS | HS | Yes, IHG | Resect en bloc | No recurrence |
The patients consisted of four males and four
females who ranged in age between 12 and 51 years old at initial
presentation, with a mean age of 32.25 years old. In the present
review, two patients (25%) were younger than 18 years, and six
patients (75%) were younger than 40 years. This indicates that IMT
of the neck is overrepresented in young people. The most common
symptom was a painful or painless neck mass, with four cases
affecting the left side, three affecting the right side and one
unknown. The etiology of this disease is unknown, as it does not
appear to be associated with a history of surgery, infection or
trauma. The disease duration ranged between 5 days and 7 years,
with the majority recorded as several months. The tumors ranged in
size between 30 and 110 mm.
The diagnosis of IMT is based on the histological
and immunohistochemical criteria. Microscopically, these lesions
are composed of spindle cells and inflammatory cells in a stroma
that can be myxoid, fibrotic or hyalinized (16). Mitotic rates are 0–2 per 10 high-power
fields, but abnormal mitotic figures and necrosis are absent. Upon
immunohistochemical examination, IMTs usually express antigens
indicating myoid differentiation, including SMA, MSA, desmin and
vimentin, but are negative for S-100 proteins and epithelial
markers. Additionally, 50% of IMT lesions display ALK protein
overexpression (17). In the present
review, three cases included the immunohistochemical findings, with
one case positive for SMA, one positive for calponin, and one
positive for SMA, MSA, vimentin, desmin and ALK.
The therapy for IMT includes conservative or
aggressive surgical resection and steroid treatment. The prognosis
for neck IMT is good, although the lesion can recur; however, it
rarely metastasizes. In the present review, four lesions were
resected en bloc, while two patients opted for steroid
treatment; none of these patients experienced recurrence or
metastasis. The one lesion that was conservatively resected
recurred 6 months later. In our opinion, the gold standard
treatment of IMT of the neck is aggressive surgical resection.
However, in certain cases of short duration or evident infection,
steroids may be preferable.
The differential diagnosis of IMT usually requires
immunohistochemical examinations, which require several days to be
performed post-operatively. Hence, pre-operative imaging is
important to make the correct diagnosis and select options for
therapy. IMT can be divided into three main microscopic subtypes:
Myxoid-vascular, hypocellular fibrous and compact spindle cell
subtypes (18). One previous study
concluded that IMTs arising from different locations demonstrate
different histological subtypes, which are evident on imaging
(19). By contrast, another study
found that CT scans of IMTs arising from the lung were not
indicative of the pathological characteristics (20).
Kim et al (21)
studied the CT features of 10 pulmonary IMTs and found that the
tumors all showed mild enhancement, with eight homogeneous and two
heterogeneous cases. Takayama et al (22) reported that IMTs of the lung were
homogeneous and hypointense on T1-weighted images and hyperintense
on T2-weighted images, with delayed enhancement. Yuan et al
(23) found that seven IMTs of the
maxillary sinus showed heterogeneous enhancement on
contrast-enhanced CT and MRI, an isointense signal on T1-weighted
images and an isointense to hyperintense signal on T2-weighted
images. The MRI findings of IMTs of the liver in further studies
were heterogeneous, hypointense or hyperintense on T1-weighted
images and isointense or hyperintense on T2-weighted images, with
delayed enhancement (24,25). IMT of the limbs exhibited low signal
intensity on T1-weighted sequences and intermediate-low signal
intensity on T2-weighted sequences on MRI, with enhancement on
contrast-enhanced CT and MRI (26).
Due to the rarity of neck IMTs, no review of the
imaging characteristics of this disease has been previously
reported. In the present review, two cases reported CT and MRI
results, two reported CT results only and one reported MRI results
only. On the CT scans, all tumors appeared as soft-tissue
densities. On MRI, all tumors displayed a heterogeneous
hypointense-isointense signal on T1-weighted sequences and an
isointense-hyperintense signal on T2-weighted sequences. All tumors
showed enhancement on enhanced CT and MR images.
The imaging features of neck IMT can be summarized
as follows: i) A soft-tissue density, rarely exhibiting
calcification or necrosis on CT scans; ii) when enhanced, the mass
displays enhancement on CT and MR images; iii) MRI is superior to
CT scans in the differential diagnosis of this disease; iv) as this
tumor often has multiple components, it usually presents with
heterogeneous signals; v) in general, the lesion displays a
hypointense-isointense signal on T1-weighted sequences and an
isointense-hyperintense signal on T2-weighted sequences; vi) due to
the fibrous tissue in the tumor, delayed enhancement may be
observed on gadolinium-enhanced MR images; and vii) due to its
benign or intermediate features, the tumor is usually a
well-defined mass.
Acknowledgements
The present study was supported by the Zhejiang
Province Health Department of Scientific Research Funds (grant no.
2013KYB112).
References
|
1
|
Ma L, Wang K, Liu WK and Zhang YK: Is
radical surgery necessary to head and neck inflammatory
myofibroblastic tumor (IMT) in children? Childs Nerv Syst.
25:285–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ong HS, Ji T, Zhang CP, Li J, Wang LZ, Li
RR, et al: Head and neck inflammatory myofibroblastic tumor (IMT):
evaluation of clinicopathologic and prognostic features. Oral
Oncol. 48:141–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Magill JC, Ferguson MS, Butler CR,
Sandison A and Grant WE: Inflammatory myofibroblastic tumour of the
tonsil: case report and literature review. J Laryngol Otol.
124:1123–1125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee DH, Shin OR, Cho KJ and Kim JH:
Inflammatory pseudotumor in the middle ear cavity. Int J Pediatr
Otorhinolaryngol. 72:1569–1572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curry JM, King N, O'Reilly RC and Corao D:
Inflammatory pseudotumor of the inner ear: are computed tomography
changes pathognomonic? Laryngoscope. 120:1252–1255. 2010.PubMed/NCBI
|
|
6
|
Biron VL, Waghray R, Medlicott SA and
Bosch JD: Inflammatory pseudotumours of the larynx: Three cases and
a review of the literature. J Otolaryngol Head Neck Surg.
37:E32–E38. 2008.PubMed/NCBI
|
|
7
|
Chen YF, Zhang WD, Wu MW, Ou-Yang D and
Zhang Q: Inflammatory myofibroblastic tumor of the head and neck.
Med Oncol. 28:(Suppl 1). S349–S353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: Where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Erickson-Johnson M, Wang X,
Bahrami A, Medeiros F, Lonzo ML, et al: Malignant high-grade
histological transformation of inflammatory myofibroblastic tumour
associated with amplification of TPM3-ALK. J Clin Pathol.
63:1040–1041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SB, Lee JH and Weon YC: Imaging
findings of head and neck inflammatory pseudotumor. AJR Am J
Roentgenol. 193:1180–1186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ceruse P, Ramade A, Vautrin R, Crozes C,
Dubreuil C and Disant F: Inflammatory pseudotumor of the neck: a
long-term result without surgical approach. Otolaryngol Head Neck
Surg. 132:812–813. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Babar-Craig H, Gill H, Almeyda R, Wong WL
and Farrell R: Inflammatory pseudotumour of the neck with
multifocal sites on positron emission tomography scan imaging. J
Laryngol Otol. 119:219–221. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Browne M, Abramson LP, Chou PM, Acton R,
Holinger LD and Reynolds M: Inflammatory myofibroblastic tumor
(inflammatory pseudotumor) of the neck infiltrating the trachea. J
Pediatr Surg. 39:e1–e4. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marraoui W, Jean B, Muheish M, Trouillier
S, Kemeny JL and Dorcier F: Imaging of inflammatory myofibroblastic
cervical tumours: a case report. Diagn Interv Imaging. 93:617–620.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Chen H and Qian Z: Inflammatory
myofibroblastic tumor of neck: A case report and literature review.
Chin J Misdiagn. 28:5551–5553. 2008.(In Chinese).
|
|
16
|
Nonaka D, Birbe R and Rosai J: So-called
inflammatory myofibroblastic tumour: a proliferative lesion of
fibroblastic reticulum cells? Histopathology. 46:604–613. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kelleher FC and McDermott R: The emerging
pathogenic and therapeutic importance of the anaplastic lymphoma
kinase gene. Eur J Cancer. 46:2357–2368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horger M, Pfannenberg C, Bitzer M,
Wehrmann M and Claussen CD: Synchronous gastrointestinal and
musculoskeletal manifestations of different subtypes of
inflammatory myofibroblastic tumor: CT, MRI and pathological
features. Eur Radiol. 15:1713–1716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakitsubata Y, Theodorou SJ, Theodorou DJ,
Nabeshima K, Kakitsubata S and Friedman PJ: Myofibroblastic
inflammatory tumor of the lung: CT findings with pathologic
correlation. Comput Med Imaging Graph. 31:607–613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim TS, Han J, Kim GY, Lee KS, Kim H and
Kim J: Pulmonary inflammatory pseudotumor (inflammatory
myofibroblastic tumor): CT features with pathologic correlation. J
Comput Assist Tomogr. 29:633–639. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takayama Y, Yabuuchi H, Matsuo Y, Soeda H,
Okafuji T, Kamitani T, et al: Computed tomographic and magnetic
resonance features of inflammatory myofibroblastic tumor of the
lung in children. Radiat Med. 26:613–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan XP, Li CX, Cao Y, Singh S and Zhong
R: Inflammatory myofibroblastic tumour of the maxillary sinus: CT
and MRI findings. Clin Radiol. 67:e53–e57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi BY, Kim WS, Cheon JE, Kim IO, Kim CJ
and Yeon KM: Inflammatory myofibroblastic tumour of the liver in a
child: CT and MR findings. Pediatr Radiol. 33:30–33. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu JS, Park C, Kim JH, Chung JJ and Kim
KW: Inflammatory myofibroblastic tumors in the liver: MRI of two
immunohistochemically-verified cases. J Magn Reson Imaging.
26:418–421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masciocchi C, Lanni G, Conti L, Conchiglia
A, Fascetti E, et al: Soft-tissue inflammatory myofibroblastic
tumors (IMTs) of the limbs: potential and limits of diagnostic
imaging. Skeletal Radiol. 41:643–649. 2012. View Article : Google Scholar : PubMed/NCBI
|