Introduction
Employing percutaneous nephrolithotomy (PCNL)
surgery for the management of large renal stones has been proven to
be effective and is an accepted treatment strategy worldwide
(1–3).
The good functional results and associated decrease in surgical
morbidity have encouraged the use of PCNL, as opposed to open
pyelolithotomy or nephrolithotomy, even in complicated cases, such
as complete staghorn stones associated with chronic infection and
previous procedures (4,5).
During the treatment of such challenging cases, the
possibility of an underlying renal cell carcinoma (RCC) or
urothelial carcinoma requires consideration (6,7).
Pre-operative diagnosis of such lesions may be challenging due to
the presence of a stone and the associated inflammatory process.
PCNL enables direct investigation of the renal pelvis. However, it
is difficult for PCNL to identify RCC during surgery. The current
study reports the case of a 39-year-old male patient exhibiting a
recurrent waist tumor subsequent to PCNL that was determined to be
metastatic adenocarcinoma. To the best of our knowledge, there are
no previous reports of recurrent metastatic adenocarcinoma of the
waist following PCNL in the literature. Notably, no tumor was
detected in the right kidney using B-ultrasound, computed
tomography (CT) or positron emission tomography (PET)-CT.
Therefore, the current study is unique.
Case report
In April 2014, a 39-year-old man presented to the
Affiliated Cancer Hospital of Xiangya Medical School with a
one-month history of a recurrent right waist tumor. Examination of
the patient's medical history revealed that PCNL had been performed
for right calculus of the kidney at Xiangya Hospital, Central South
University (Changsha, China) in June 2008. Following PCNL, the
patient did not receive post-operative therapy and was in a good
general condition. However, seven months prior to admission to our
clinic, a soft tissue mass was noted at the previous PCNL tract
site. The patient underwent local mass resection for the right
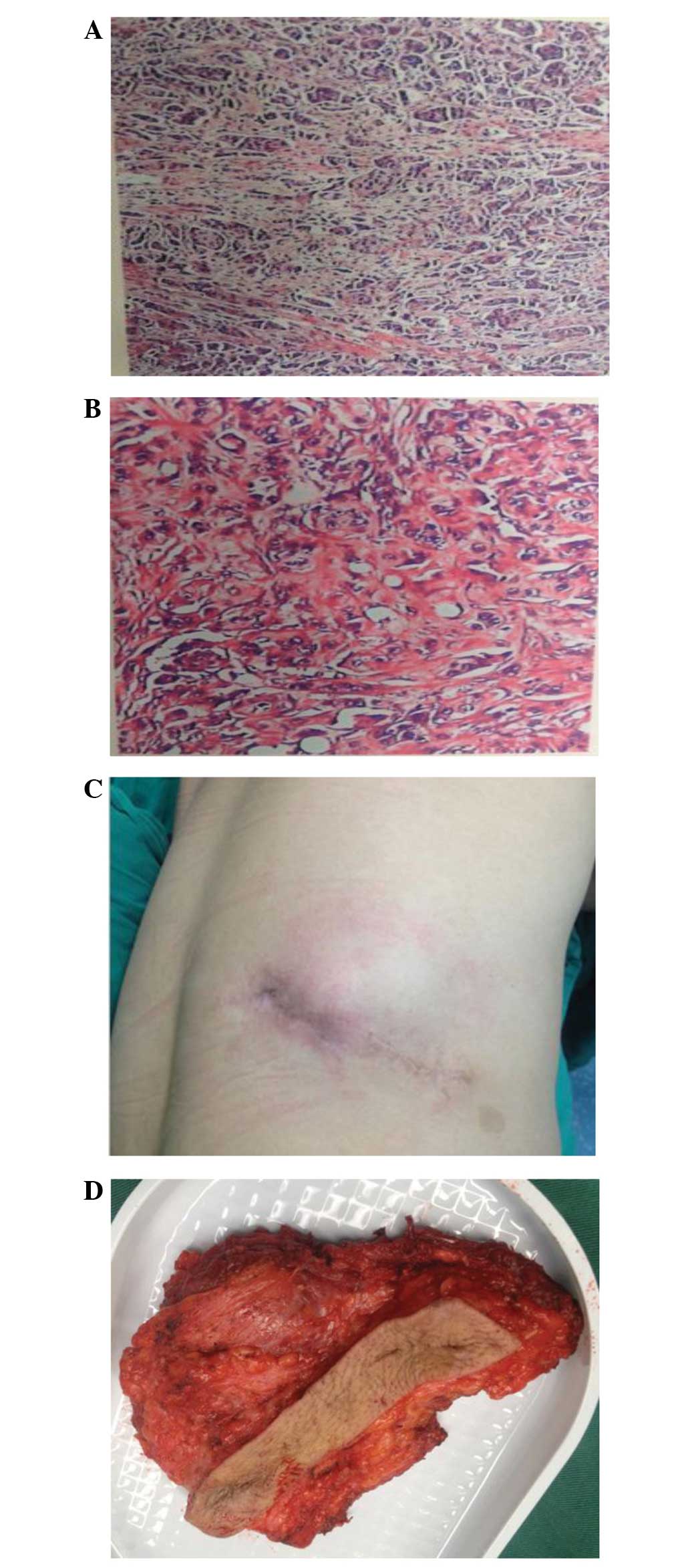
waist tumor and immunohistochemical examination of the resected
specimen revealed poorly differentiated metastatic adenocarcinoma
(Fig. 1A and B); Mitotic figures in
high-power fields were observed, as well as intercellular bridges
and focal keratinization. Furthermore, tumor cells exhibited strong
positive staining for Vim and epithelial membrane antigen.
Considering that the patient had previously undergone PCNL, it was
proposed that the metastatic tumor had originated from RCC. The
patient did not receive any post-operative adjuvant therapy and was
in a good general condition. Upon presentation at our clinic, the
patient underwent a physical examination. The examination revealed
a well-healed right flank scar and a 4×7-cm tumor under the scar
exhibiting a painless, indurated, firm and immobile nodule
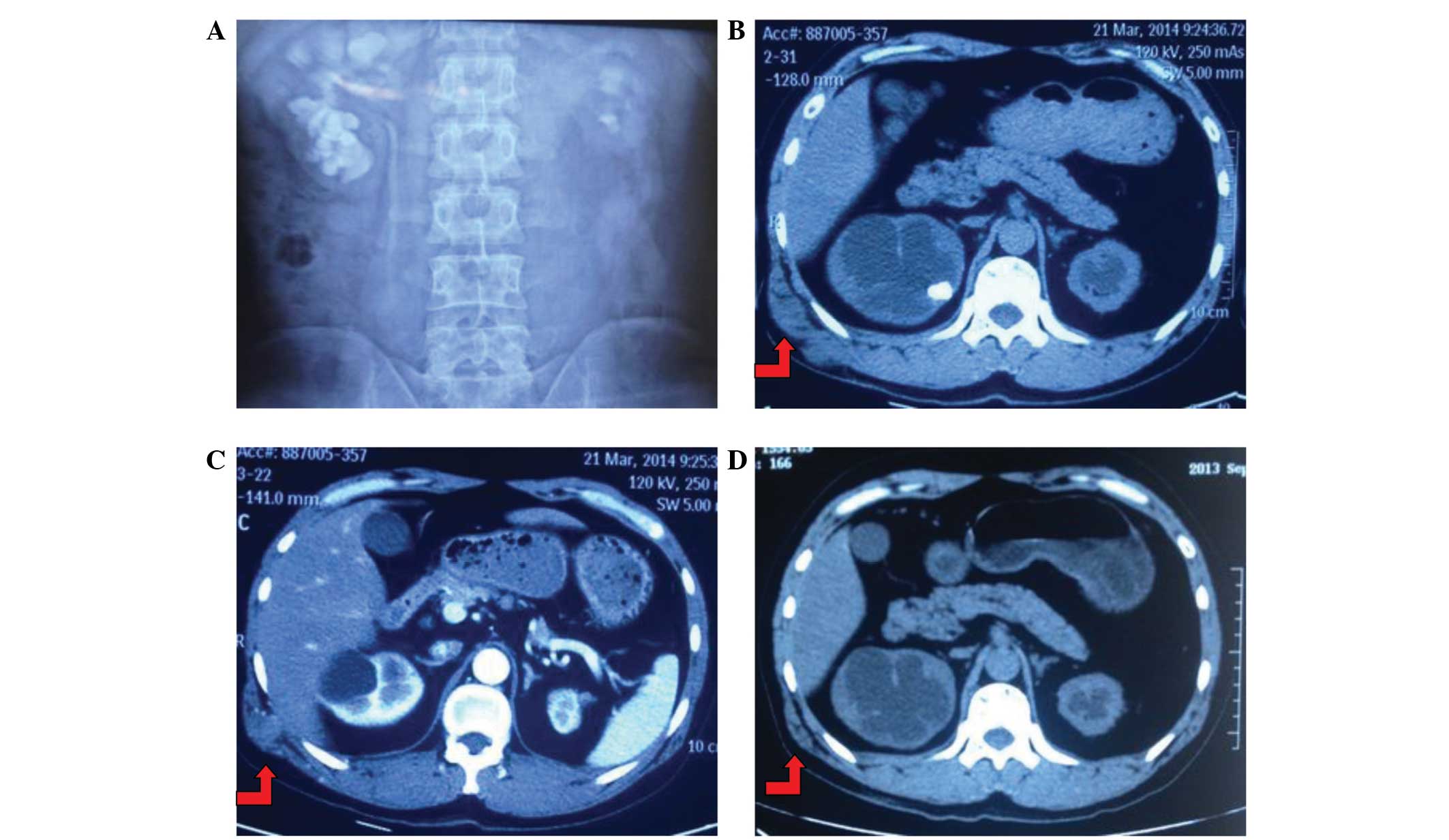
(Fig. 2C and D). Upon excretory
urography, the left kidney was determined to be poorly functioning,
demonstrating atrophy, and right moderate hydronephrosis with large
staghorn stones was diagnosed (Fig.
2A). Additionally, a CT scan identified the soft tissue mass
(Fig. 2B and C) and a PET-CT scan
revealed an increased radioactivity concentration at the tumor site
in the right waist (Fig. 2D). There
was no evidence of additional tumor spread or lymph node
involvement. Notably, there were also no abnormal findings on the
right kidney, excluding recurrent calculi. In consideration of the
aforementioned findings, a local mass resection of the waist tumor
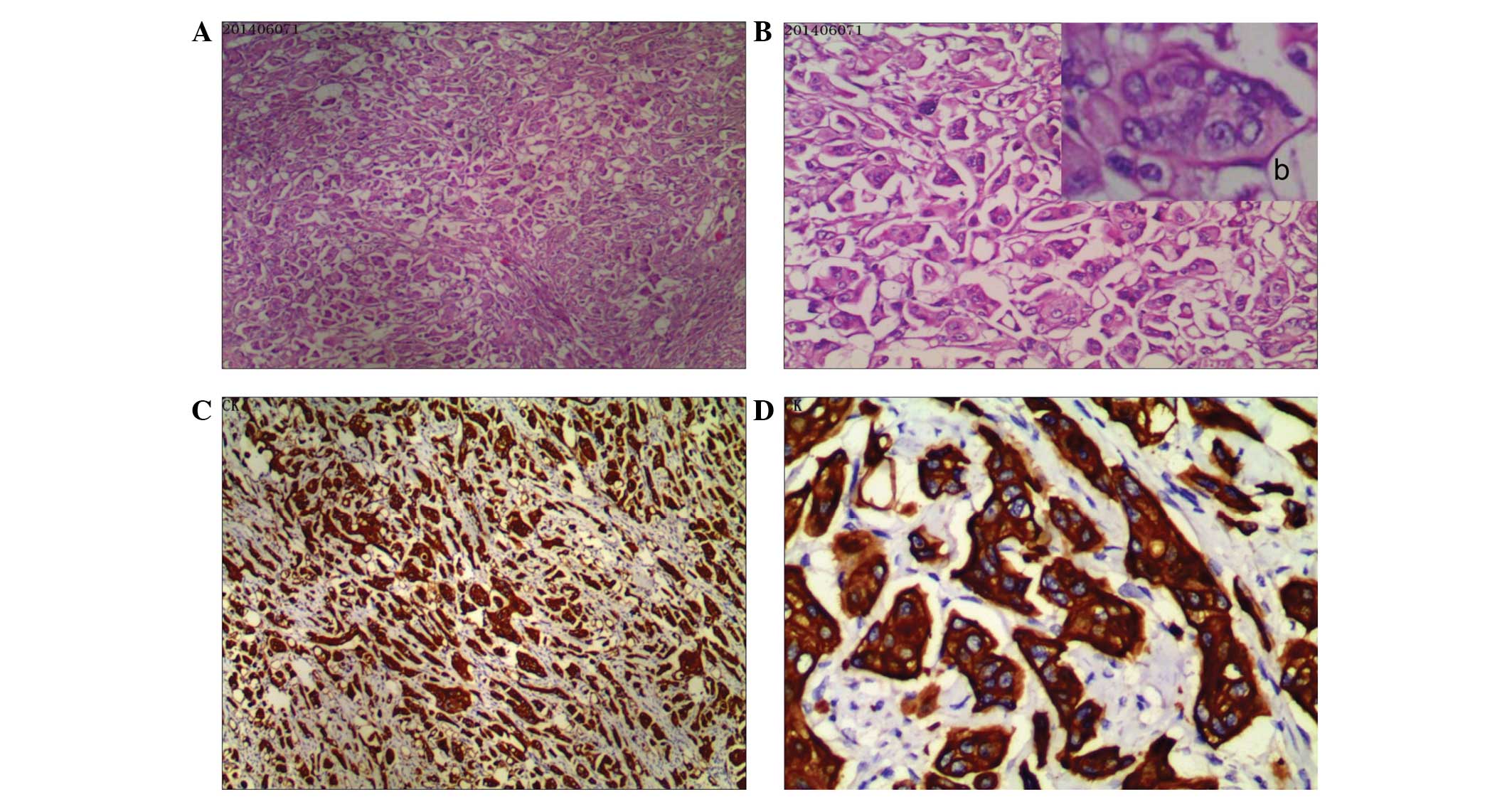
was performed. Subsequent histopathological examination revealed a
poorly differentiated adenocarcinoma that conformed to the
characteristics of neoplasm recurrence (Fig. 3). Post-operatively, the patient
received systemic chemotherapy, consisting of the administration of
gemcitabine (1000 mg/m2; days 1 and 8; Q3W) plus
carboplatin (area under curve, 5; day 1, Q3W), for the treatment of
the metastatic tumor, and was disease-free at the most recent
three-month follow-up. However, notably, the primary tumor was not
identified by imaging and clinical analysis. Considering that the
left kidney was poorly functioning and demonstrated atrophy, right
moderate hydronephrosis with large staghorn stones was
identified.
In accordance with the regulations of the Human
Investigation Committee of the Central South University (Changsha,
China), written informed consent was obtained from the patient for
publication of the current report and any accompanying images.
Discussion
Following its introduction in 1976, PCNL has become
the preferred surgical procedure for the treatment of patients with
large and complex calculi of the kidneys (6). For example, according to the 2005
American Urological Association clinical guidelines, PCNL is the
recommended first-line treatment strategy for calculi with surface
area >500 mm2 (7).
Although PCNL can result in a good stone-free rate of 78–95%
(8), it has been associated with
significant complications, such as loss of a kidney (due to the
necessity for complete resection), urinary leakage, uncontrolled
hemorrhage, sepsis, tumor seeding, injury to the collecting system
and surrounding viscera, or mortality (8,9).
Therefore, undergoing PCNL surgery poses a significant risk for
patients, particularly those exhibiting occult RCC.
To the best of our knowledge, no cases of RCC
extension along a PCNL tract have been reported in the literature
in recent years. The current study reports the case of a recurrent
right waist tumor extending along a PCNL tract that was diagnosed
as RCC of suspicious origin upon pathological analysis of the tumor
specimen. However, prior to intervention, intravenous pyelography,
CT and PET-CT scans were performed without presenting evidence of
the suspected RCC.
In 2011, it was estimated that 60,920 novel cases of
RCC were diagnosed in the United States (10). A large number of RCC masses are
diagnosed in asymptomatic patients in the early stages of the
disease due to the increased application of cross-sectional imaging
(11). However, this cross-sectional
imaging cannot increase the diagnosis rates to manage small or
occult renal masses. Previously, Mullins and Rodriguez (9) demonstrated that RCC may seed at a
percutaneous biopsy tract. The risk of RCC seeding at a PCNL tract
is low; however, upper tract transitional cell carcinoma (TCC)
seeding is not uncommon. For example, various studies have reported
TCC seeding in patients following percutaneous management of upper
tract TCC, nephrostomy tube placement for obstructive uropathy and
renal mass biopsy (12). Clinicians
deciding whether to perform PCNL should consider that TCC tumors
are typically considered to have a greater implantation rate
following percutaneous manipulation compared with RCC tumors. In
the current case, the patient did not present any abnormalities in
the right kidney or change in micturition, excluding the recurrence
of kidney stones. Furthermore, clinical and radiological
observations did not identify any other primary site of disease or
a direct extension of the tumor from the right kidney.
The patient in the present study was aged 39 years.
The left kidney was poorly functioning with atrophy and right
moderate hydronephrosis with large staghorn stones was observed.
Although the risk of PCNL tract seeding with RCC is low, it was
theorized with a high degree of probability that the origin of the
tumor was RCC. However, RCC could not be identified in the right
kidney. Considering the despondent state of the patient,
chemotherapy was recommended following surgery. It remains unclear
how a diagnosis of the origin of the tumor can be definitely
determined and if such patients should be recommended for targeted
therapies.
In conclusion, the present study is, to the best of
our knowledge, the first contemporary report of PCNL tract seeding
with RCC. The current study raises awareness of this rare
complication. Measures such as careful examination of the renal
tissue during PCNL surgery must be taken to minimize the risk of
developing RCC at the time of PCNL, to prevent clinicians from
being discouraged from performing PCNL for the treatment of renal
stones when required.
Acknowledgements
This study was supported by the Medjaden Academy
& Research Foundation for Young Scientists (grant no.
MJR20150025) and supported by the China Medical Foundation (grant
no. 313.2238).
References
|
1
|
Sivalingam S, Cannon ST and Nakada SY:
Current practices in percutaneous nephrolithotomy among
endourologists. J Endourol. 28:524–527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong KA, Sahai A, Patel A, Thomas K,
Bultitude M and Glass J: Is percutaneous nephrolithotomy in
solitary kidneys safe? Urology. 82:1013–1016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Opondo D, Gravas S, Joyce A, et al:
Standardization of patient outcomes reporting in percutaneous
nephrolithotomy. J Endourol. 28:767–774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pérez-Fentes DA, Gude F, Blanco B and
Freire CG: Percutaneous nephrolithotomy: short and long term
effects on health related quality of life. J Endourol. 29:13–17.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kreydin EI and Eisner BH: Risk factors for
sepsis after percutaneous renal stone surgery. Nat Rev Urol.
10:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SS, Ho HC, Su CK, Chen WM, Cheng CL
and Yang CR: Seeding of malignant renal tumor through a nephrostomy
tract. J Chin Med Assoc. 67:308–310. 2004.PubMed/NCBI
|
|
7
|
Huang A, Low RK and de Vere White R:
Nephrostomy tract tumor seeding following percutaneous manipulation
of a ureteral carcinoma. J Urol. 153:1041–1042. 1995.
Fernström I and Johansson B: Percutaneous
pyelolithotomy. A new extraction technique. Scand J Urol Nephrol.
10:257–259. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Preminger GM, Assimos DG, Lingeman JE,
Nakada SY, Pearle MS and Wolf JS Jr: AUA Nephrolithiasis Guideline
Panel: Chapter 1: AUA guideline on management of staghorn calculi:
diagnosis and treatment recommendations. J Urol. 173:1991–2000.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michel MS, Trojan L and Rassweiler JJ:
Complications in percutaneous nephrolithotomy. Eur Urol.
51:899–906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mullins JK and Rodriguez R: Renal cell
carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J.
7:E176–E179. 2013.PubMed/NCBI
|
|
11
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kane CJ, Mallin K, Ritchey J, Cooperberg
MR and Carroll PR: Renal cell cancer stage migration: analysis of
the National Cancer Data Base. Cancer. 113:78–83. 2008. View Article : Google Scholar : PubMed/NCBI
|