Introduction
Salivary duct carcinoma (SDC) is a rare type of
salivary malignancy which accounts for <10% of all salivary
malignancies, and the majority of its histological characteristics
are similar to those of mammary duct carcinoma (1–3). SDC
exhibits characteristic ductal lesions and tumor cells are often
arranged in a ‘Roman bridge’ formation and cribriform architecture,
with comedo necrosis (2). Due to the
rarity of SDC, little data regarding its clinicopathological
characteristics exists. The standard treatment for SDC is surgery
in combination with radiotherapy, however, the prognosis of SDC is
poor (4–7). Effective therapeutic strategies rely on
a sufficient understanding of SDC and its prognostic factors,
therefore, the aim of the present retrospective study was to
summarize the clinicopathological characteristics of SDC and to
evaluate the current treatment modalities currently used at the
Affiliated Hospital of Stomatology of Nanjing University (Nanjing,
Jiangsu, China).
Patients and methods
Patients
Between 2001 and 2011, 11 cases of
histopathologically diagnosed with SDC, according to the 2005 World
Health Organization classification of salivary gland tumors
(2), were identified at the
Affiliated Hospital of Stomatology. Subsequent to excluding any
patients with distant metastasis or a previous history of head-neck
surgery, all 11 patients primarily underwent surgical treatment,
predominantly consisting of local extensive resection with neck
dissection, followed by post-operative radiation therapy. All cases
were followed up from the date of the surgical procedure to the
date of mortality or the date patients were lost to follow-up.
Clinical and histological data were reviewed (Table I).
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
|
| Patients |
|---|
|
|
|
|---|
| Characteristic | n | % |
|---|
| Age, years |
|
|
|
38–60 | 6 | 54.5 |
| ≥61 | 5 | 45.5 |
| Gender |
|
|
| Male | 7 | 63.6 |
|
Female | 4 | 36.4 |
| Mortality due to
disease | 2 | 18.2 |
| Site |
|
|
| Parotid
gland | 7 | 63.6 |
|
Submandibular gland | 3 | 27.3 |
|
Palate | 1 | 9.1 |
| Nerve paralysis |
|
|
| Yes | 4 | 36.4 |
| No | 7 | 63.6 |
| T stage |
|
|
| 1 | 2 | 18.2 |
| 2 | 2 | 18.2 |
| 3 | 2 | 18.2 |
| 4 | 5 | 45.5 |
| N stage |
|
|
| N0 | 5 | 45.5 |
| ≥N1 | 6 | 54.5 |
| AJCC stage |
|
|
| I | 1 | 9.1 |
| II | 1 | 9.1 |
| III | 1 | 9.1 |
| IV | 8 | 72.7 |
| Surgical margin |
|
|
|
Negative | 10 | 90.9 |
|
Positive | 1 | 9.1 |
| Perineural
spread |
|
|
| Yes | 5 | 45.5 |
| No | 6 | 54.5 |
| Intravascular tumor
emboli |
|
|
| Yes | 4 | 36.4 |
| No | 7 | 63.6 |
| Neck dissection |
|
|
| Yes | 8 | 72.7 |
| No | 3 | 27.3 |
| Post-operative
radiation |
|
|
| Yes | 8 | 72.7 |
| No | 3 | 27.3 |
| AR expression |
|
|
|
Negative | 4 | 36.4 |
|
Positive | 7 | 63.6 |
| HER-2/neu
expression |
|
|
|
Negative | 2 | 18.2 |
|
Positive | 9 | 81.8 |
| p16 expression |
|
|
|
Negative | 5 | 45.5 |
|
Positive | 6 | 54.5 |
| p53 expression,
% |
|
|
| 0–25 | 8 | 72.7 |
|
26–50 | 1 | 9.1 |
|
>50 | 2 | 18.2 |
| Ki-67 expression,
% |
|
|
|
0–25 | 6 | 54.5 |
|
26–50 | 4 | 36.4 |
|
>50 | 1 | 9.1 |
Statistical analysis
All data were analyzed using SPSS software version
17.0 for Windows (SPSS, Inc., Chicago, IL, USA). Survival analysis
was conducted and survival curves were constructed using the
Kaplan-Meier method. Furthermore, the log-rank test was used to
analyze the statistical differences and P<0.05 was considered to
indicate a statistically significant difference.
Results
Diagnosis and staging
The occurrence of only 11 cases of SDC in the head
and neck during a 10-year period in a busy institution confirms the
rarity of the cancer. In the current cohort, the male:female gender
ratio was 7:4 and the mean age of the patients was 58.8 years. The
parotid gland was the most commonly affected location (seven cases;
63.6%), followed by the submandibular gland (three cases; 27.3%)
and the palate (one case; 9.1%). Furthermore, the majority of cases
presented with a painless mass in the early period of the disease,
however, in the advanced stage, the majority of patients suffered
from pain, and nerve paralysis was identified in four cases. All
cases in the cohort were staged according to the American Joint
Committee on Cancer (AJCC) staging system (8) and the majority (72.7%) of cases were
determined to be stage IV. Six patients (54.5%) exhibited regional
lymph node metastasis during routine neck dissection and only one
patient exhibited a positive resection margin. In addition, 45.5
and 36.4% of patients presented with perineural spread and
intravascular tumor emboli, respectively.
Treatment strategies
All cases were treated with local extensive
resection, 72.7% of which simultaneously underwent neck dissection.
Six patients (54.5%) exhibited an N stage of ≥N1, and all seven
patients with tumors located in the parotid gland underwent
parotidectomy, among which three cases were accompanied by
resection of the facial nerve. Additionally, eight patients
underwent post-operative radiation therapy of a moderate dose
ranging between 50 and 60 Gy.
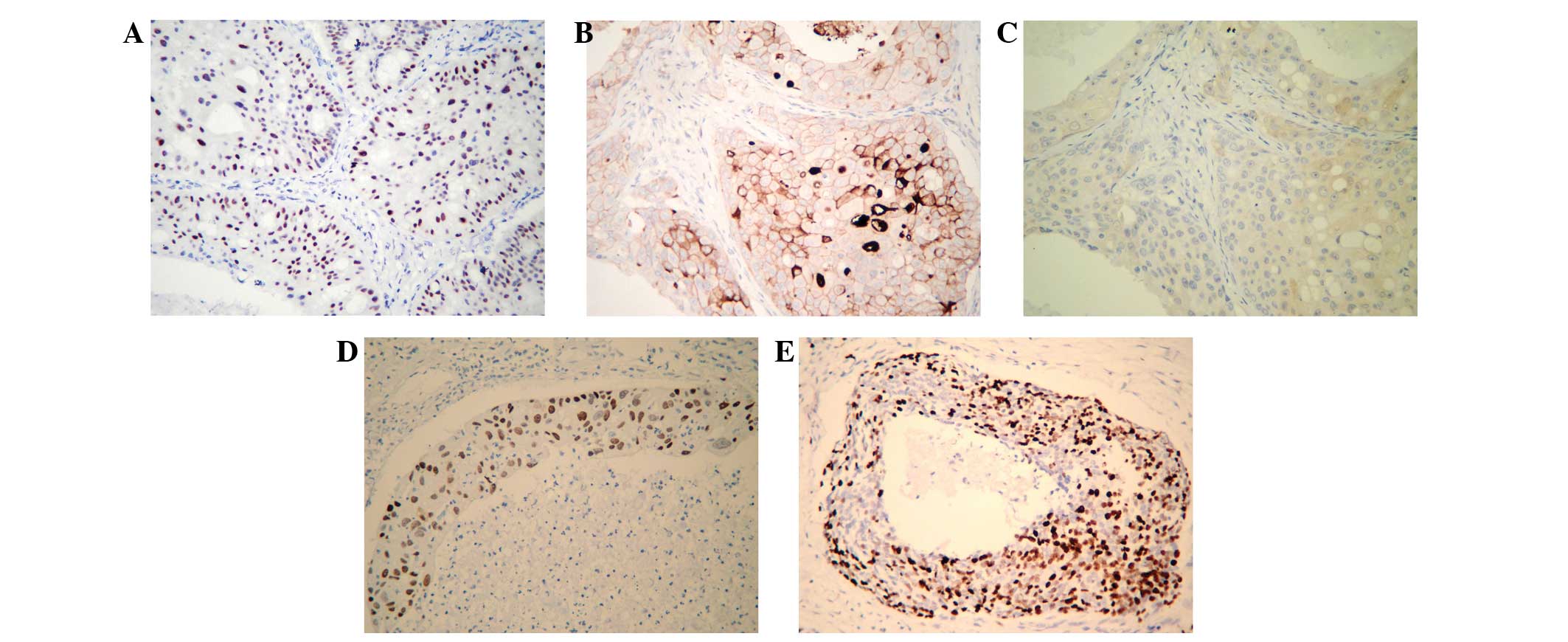
Immunohistochemistry
The results of the immunohistochemical analysis of
the 11 samples are indicated in Table
I. Examination of HER-2/neu protein expression revealed a high
positivity rate of 81.8% (9/11 cases) in the examined tumor
samples. Furthermore, androgen receptor (AR) expression was
detected in seven of the tumor specimens (63.6%) and five cases
(45.5%) were p16-negative. However, Ki-67 and p53 demonstrated
>50% positive expression in only one and two cases, respectively
(Fig. 1).
Follow-up
In the present study, the range for the follow-up
period was 10–89 months and the mean overall survival time was 72.8
months. At the termination of the follow-up period, only two of the
11 cases had succumbed to the disease, while distant metastasis
occurred in two patients, with the lung identified as the
metastatic site.
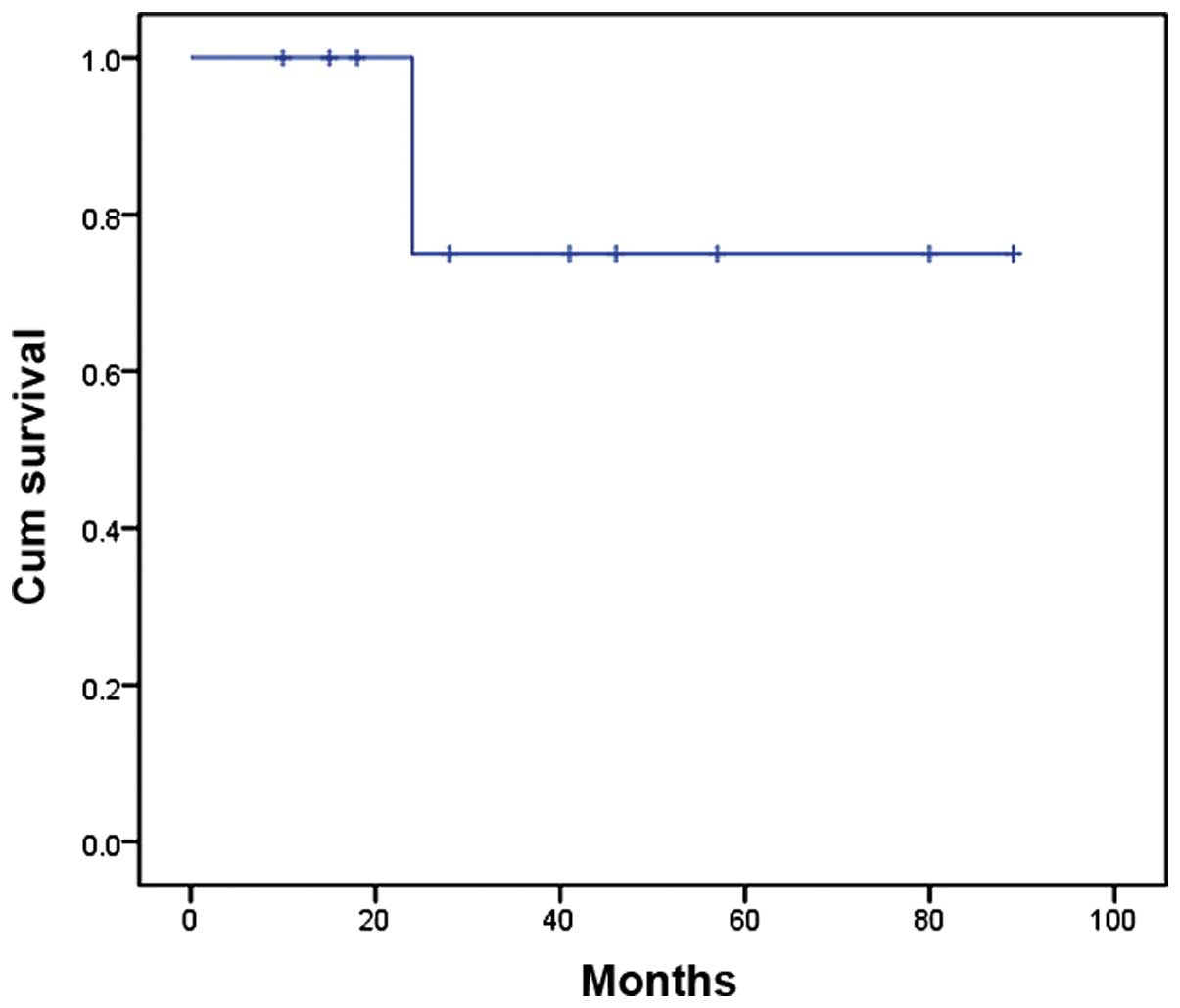
Furthermore, the two-year overall survival rate was
75% according to Kaplan-Meier analysis (Fig. 2). The site of the tumor (P=0.049)
appeared to be significantly associated with a poor prognosis,
whereas age, gender, nerve paralysis, post-operative radiation, T
stage, N stage, AJCC stage, neck dissection, and expression of AR,
Ki-67 and HER-2/neu did not appear to significantly affect the
survival rate.
Discussion
SDC in the salivary gland region is a type of
carcinoma that is histologically indistinguishable from mammary
duct carcinoma, exhibiting intraductal and invasive components
(2–5).
SDC is rare and thus clinicians have relatively limited experience
to aid in guiding the development of novel treatment strategies for
the cancer. SDC has been reported to occur with a male predominance
and an average age of onset of ≥50 years (2,6,7). The present study revealed similar
results, with a preponderance of males and an average age of 58.8
years. SDC typically presents with a painful or painless rapidly
growing, firm tumor, and the symptom of nerve palsy is also common
(1,2,9); in the
present study, nerve palsy occurred in >36% of patients.
Furthermore, the parotid gland was the most frequently involved
site of SDC, followed by the submandibular gland, while only one
case was located in the palate. These data are similar to those
determined by previously conducted studies (2,3,5,6).
According to previous studies, SDC is typically
characterized by aggressive behavior and a poor prognosis (2,3,5). Thus, the cases investigated in the
present study were representative of typical SDC, as they
demonstrated aggressive biological behavior. Furthermore, cervical
lymph node involvement occurred in 54.5% of cases and nerve
paralysis in 36.4%, and the majority of patients (72.7%) presented
with AJCC stage IV disease. In addition, the incidence of
intravascular tumor emboli and perineural spread were relatively
high, at 36.4 and 45.5%, respectively.
For the majority of salivary malignancies at our
institution, local tumor resection with a free surgical margin is a
suitable treatment strategy, however, a more aggressive method is
required for the treatment of SDC. For example, in the current
cases from the Affiliated Hospital of Stomatology, the most
commonly administered therapeutic strategy was local extensive
resection with neck dissection. The present study evaluated the
prognostic parameters for SDC and identified that the tumor site
was a significantly predictive factor of SDC survival, whereas age,
gender, nerve paralysis, post-operative radiation, T stage, N
stage, AJCC stage, neck dissection, and the expression of
HER-2/neu, AR and Ki-67 did not appear to significantly affect
survival. Furthermore, SDC in the parotid glands was associated
with an improved prognosis compared with that of the palate and
submandibular gland. In the current series of patients, distant
metastasis occurred in two patients, with the lung identified as
the metastatic site. Distant metastasis is one of major clinical
problems in the management of SDC and requires the development of a
novel alternative strategy to the current treatment methods.
Notably, the present analysis determined a good
short-term outcome for the patients with primary SDC, which was in
disagreement with the findings reported in previous studies
(2,3,6,7,10–13). Mortality in late-stage patients was
22.2% compared with 0% in early-stage patients with similar
prognoses; however, N status did not appear to have a significant
impact on the patient prognosis. The present findings differ from
those of previous studies, in which an association was identified
between tumor size/lymph node involvement and outcome (2,3,5,6,14). In the current study, it was identified
that the two-year overall survival rate was 75% according to
Kaplan-Meier analysis; only two patients succumbed to the disease
within 24 months and no mortalities occurred during the two-year
treatment period. The good prognosis in the present study may be
attributed to the high number of patients administered with
post-operative radiation (72.7%) and exhibiting negative surgical
margins (10 cases). In validation of this proposal, a recent study
demonstrated that post-operative radiotherapy was effective for SDC
locoregional control (14);
therefore, we hypothesize that complete resection combined with
post-operative radiotherapy may be an effective treatment for
SDC.
A number of previous studies demonstrated that
HER-2/neu and p53 expression are statistically associated with SDC
survival rates (3,15–20).
However, in the present study, the protein expression levels of
HER-2/neu, AR, Ki-67, p16 and p53 did not correlate with prognosis,
although HER-2/neu, AR and p16 demonstrated a positive expression
rate of >50%, which may contribute to the diagnosis for SDC.
The role of additional adjuvant therapy for SDC
remains unclear. Due to the limited efficacy and severe
complications of surgery and radiotherapy, a more systematic
therapeutic approach should be analyzed in order to improve the
prognosis of SDC. The present study demonstrated a high positivity
rate for HER-2/neu and AR expression in SDC, indicating that SDC
carcinogenesis may resemble that of breast ductal carcinoma or
prostate cancer (4,7,11,13,15,18,21,22).
As HER-2/neu blockers (trastuzumab) are effective in treating
HER-2-overexpressing breast cancer, this agent may be useful for
the treatment of SDC. Similarly, androgen deprivation therapy may
be applied to the treatment of SDC. These two therapeutic
strategies have achieved positive results in a small number of
cases of head and neck SDC (11,15,22).
Clinicians may expect monoclonal antibody treatments to be a
promising adjuvant therapy for SDC, however, this field requires
additional research prior to the application of such therapies.
In conclusion, the present study determined that SDC
is a rare salivary malignancy with a peak incidence in the fifth
and sixth decades of life, and a clear male preponderance. The
parotid gland was the most commonly affected site and the majority
of cases presented with a painless mass in the early stage of the
disease. In the advanced stage, pain and nerve paralysis were
common. All patients were treated with surgery and the majority
underwent adjuvant post-operative radiotherapy. The range for the
follow-up period was 10–89 months and the mean overall survival
period was 72.8 months. At the completion of follow-up, only two of
the 11 cases had succumbed to the disease, resulting in a two-year
overall survival rate of 75% according to Kaplan-Meier analysis.
Furthermore, the current data demonstrated that SDC in the parotid
glands is associated with a more positive prognosis compared with
SDC in the palate and submandibular gland. However, the limitations
of this study, which include its retrospective nature and the small
sample size used, should be considered. The present study proposes
that the development of a biological treatment strategy for SDC,
targeting HER-2/neu or AR, may provide a more positive outcome for
such patients.
Acknowledgements
The present study was supported by the National Key
Disciplines Constructional Project of China, the Jiangsu Province
Department of Health (grant no. H201344) and the Nanjing Medical
Science and Technique Development Foundation (grant no.
QRX11260).
References
|
1
|
Kikuchi Y, Hirota M, Iwai T, et al:
Salivary duct carcinoma in the mandible: a case report. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 103:e41–e46. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: World Health Organization Classification of Tumors.
Pathology and Genetics of Head and Neck Tumors. IARC Press; Lyon:
2005
|
|
3
|
Jaehne M, Roeser K, Jaekel T, Schepers JD,
Albert N and Löning T: Clinical and immunohistologic typing of
salivary duct carcinoma: a report of 50 cases. Cancer.
103:2526–2533. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cornolti G, Ungari M, Morassi ML, et al:
Amplification and overexpression of HER2/neu gene and HER2/neu
protein in salivary duct carcinoma of the parotid gland. Arch
Otolaryngol Head Neck Surg. 133:1031–1036. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salovaara E, Hakala O, Bäck L, et al:
Management and outcome of salivary duct carcinoma in major salivary
glands. Eur Arch Otorhinolaryngol. 270:281–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guzzo M, Di Palma S, Grandi C and Molinari
R: Salivary duct carcinoma: clinical characteristics and treatment
strategies. Head Neck. 19:126–133. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thompson LD: Salivary duct carcinoma. Ear
Nose Throat J. 91:356–359. 2012.PubMed/NCBI
|
|
8
|
Greene FL, Page DL, Fleming ID, et al:
AJCC Cancer Staging Manual. 6th. Springer; New York, NY: 2002
|
|
9
|
Lewis JE, McKinney BC, Weil LH, Ferreiro
JA and Olsen KD: Salivary duct carcinoma Clinicopathologic and
immunohistochemical review of 26 cases. Cancer. 77:223–230. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes L, Rao U, Krause J, Contis L,
Schwartz A and Scalamogna P: Salivary duct carcinoma. Part I. A
clinicopathologic evaluation and DNA image analysis of 13 cases
with review of the literature. Oral Surg Oral Med Oral Pathol.
78:64–73. 1994.PubMed/NCBI
|
|
11
|
Jaspers HC, Verbist BM, Schoffelen R, et
al: Androgen receptor-positive salivary duct carcinoma: a disease
entity with promising new treatment options. J Clin Oncol.
29:e473–e476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laurie SA and Licitra L: Systemic therapy
in the palliative management of advanced salivary gland cancers. J
Clin Oncol. 24:2673–2678. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moriki T, Ueta S, Takahashi T, Mitani M
and Ichien M: Salivary duct carcinoma: cytologic characteristics
and application of androgen receptor immunostaining for diagnosis.
Cancer. 93:344–350. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JY, Lee S, Cho KJ, et al: Treatment
results of post-operative radiotherapy in patients with salivary
duct carcinoma of the major salivary glands. Br J Radiol.
85:e947–e952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nashed M and Casasola RJ: Biological
therapy of salivary duct carcinoma. J Laryngol Otol. 123:250–252.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Potter CR: The neu-oncogene: more than
a prognostic indicator? Hum Pathol. 25:1264–1268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skálová A, Stárek I, Vanecek T, et al:
Expression of HER-2/neu gene and protein in salivary duct
carcinomas of parotid gland as revealed by fluorescence in-situ
hybridization and immunohistochemistry. Histopathology. 42:348–356.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wee DT, Thomas AA and Bradley PJ: Salivary
duct carcinoma: what is already known, and can we improve survival?
J Laryngol Otol. 126(Suppl 2): S2–S7. 2012.PubMed/NCBI
|
|
19
|
Nagao T, Gaffey TA, Visscher DW, et al:
Invasive micropapillary salivary duct carcinoma: a distinct
histologic variant with biologic significance. Am J Surg Pathol.
28:319–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto H, Uryu H, Segawa Y and
Tsuneyoshi M: Aggressive invasive micropapillary salivary duct
carcinoma of the parotid gland. Pathol Int. 58:322–326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson CJ, Barry MB, Vasef MA and Deyoung
BR: Her-2/neu expression in salivary duct carcinoma: an
immunohistochemical and chromogenic in situ hybridization study.
Appl Immunohistochem Mol Morphol. 16:54–58. 2008.PubMed/NCBI
|
|
22
|
Nabili V, Tan JW, Bhuta S, Sercarz JA and
Head CS: Salivary duct carcinoma: a clinical and histologic review
with implications for trastuzumab therapy. Head Neck. 29:907–912.
2007. View Article : Google Scholar : PubMed/NCBI
|