Introduction
V-akt murine thymoma viral oncogene homolog
[SGK3; also known as cytokine-independent survival kinase
(CISK)], as a downstream target of the phosphoinositide
3-kinase (PI3K) cascade and a member of the AGC family of kinases,
has certain similar substrates and shares certain target-signaling
molecules with v-akt murine thymoma viral oncogene homolog (AKT) in
cell proliferation, growth and survival (1–4). SGK3 is a
serine/threonine protein kinase, and its key phosphorylation sites
are Thr256 and Ser422 (5).
The residues of SGK3 (also known as CISK) equivalent to those of
AKT are targeted by 3-phosphoinositide-dependent kinase 1 (PDK1)
and PDK2 respectively (5).
Functionally parallel to AKT, SGK3 is involved in the malignant
transformation of tumors by regulating cell proliferation, cell
growth, cell survival and cell migration (4,6,7). The oncogenic effect of SGK3 in
tumors has been demonstrated by in vitro and in vivo
functional assays (2,3). Xu et al (2) reported that the positive correlation
between SGK3 expression and tumor prognosis varies with tumor grade
and lymph node status. Angiogenesis is a key process in tumor
malignant transformation, which involves blood vessel endothelial
cell proliferation and migration. The signaling pathways of the
cellular processes that SGK3 mediates, particularly in cell
survival, have been well studied. However, angiogenesis, another
tumor malignant transformation process, is seldom reported in
comparison to AKT. Therefore, the present study aims to find
evidence that SGK3 may be involved in angiogenesis.
Hypothesis
An abundance of experiments have been performed to
investigate the function of the PI3K/AKT pathway in regulating
tumor angiogenesis, and so the mechanism of angiogenesis regulated
by the PI3K/AKT pathway is, to a certain extent, clear. However,
the association between angiogenesis and SGK3 remains unclear. As
they are downstream mediators of the PI3K/PDK1 signaling pathway,
AKT and SGK3 have certain similar substrates and share certain
targeting molecules. Thus, we hypothesize that a strong signaling
connection may exist between angiogenesis and SGK3, contributing to
tumor malignant transformation.
Evidence and discussion
The deteriorative progress of tumor growth includes
several alterations that collectively dictate malignant
transformation, including insensitivity to growth-inhibitory
signals, evasion of cell apoptosis, limitless cell proliferation,
sustained angiogenesis, and tissue invasion and metastasis
(8). SGK3, a downstream effector of
PI3K, induces several pro-malignant pathways through the
PI3K/PDK1/SGK3 route. Functionally parallel with AKT (Fig. 1) (3,4), SGK3
participates in cell growth, cell survival and cell migration
(1,2,9,10). In contrast to the AKT pleckstrin
homology domain, SGK3 contains a phox homology domain, through
which SGK3 binds to phosphatidylinositol 3-phosphate-rich endosomal
and vesicular compartments to remain active. Overexpression of SGK3
increases cell cycle progression through G1 by
inactivating glycogen synthase kinase-β (GSK3-β) and stabilizing
CCND1, as previously observed in hepatocellular carcinoma (3,7,9). Similar to AKT, SGK3 is involved in cell
growth signaling by the increase of phosphorylated tuberous
sclerosis factor 2, ribosomal protein S6, proline-rich AKT
substrate of 40 kDa and eIF4E-binding protein 1 in normal cell
physiology and malignant transformation (9,11). Induced
by interleukin (IL)-3 (4), SGK3
increases the level of B-cell lymphoma-extra large (Bcl-xL), and
inhibits the pro-apoptotic proteins Bcl-2-associated death promoter
(BAD) and forkhead family of transcription factors (FKHRs)
(3,4,9,10), thus promoting cell survival.
Consistent with the fact that SGK3 and AKT are functionally
parallel, SGK3 and AKT have synergetic responses to the cell
survival pathways through Bcl-xL, BAD and FKHRs. SGK3 mediates
estrogen receptor-positive cancer cell survival by phosphorylating
its co-activator, flightless-I (2,6).
Observations by Slagsvold et al (12) support the potential role of SGK3 as a
cell survival effector by the downregulation of C-X-C chemokine
receptor type 4 (CXCR4) through the interaction with ubiquitin
ligase atrophin-1-interacting protein 4. CXCR4 is strongly
associated with promoting cell invasion, migration and adhesion
during the process of metastasis in breast cancer and liver tumor
cells (13,14), showing the potentially significant
role of SGK3 in cell migration. Thereby, further studies are
required to more specifically characterize the role of SGK3 in
these processes.
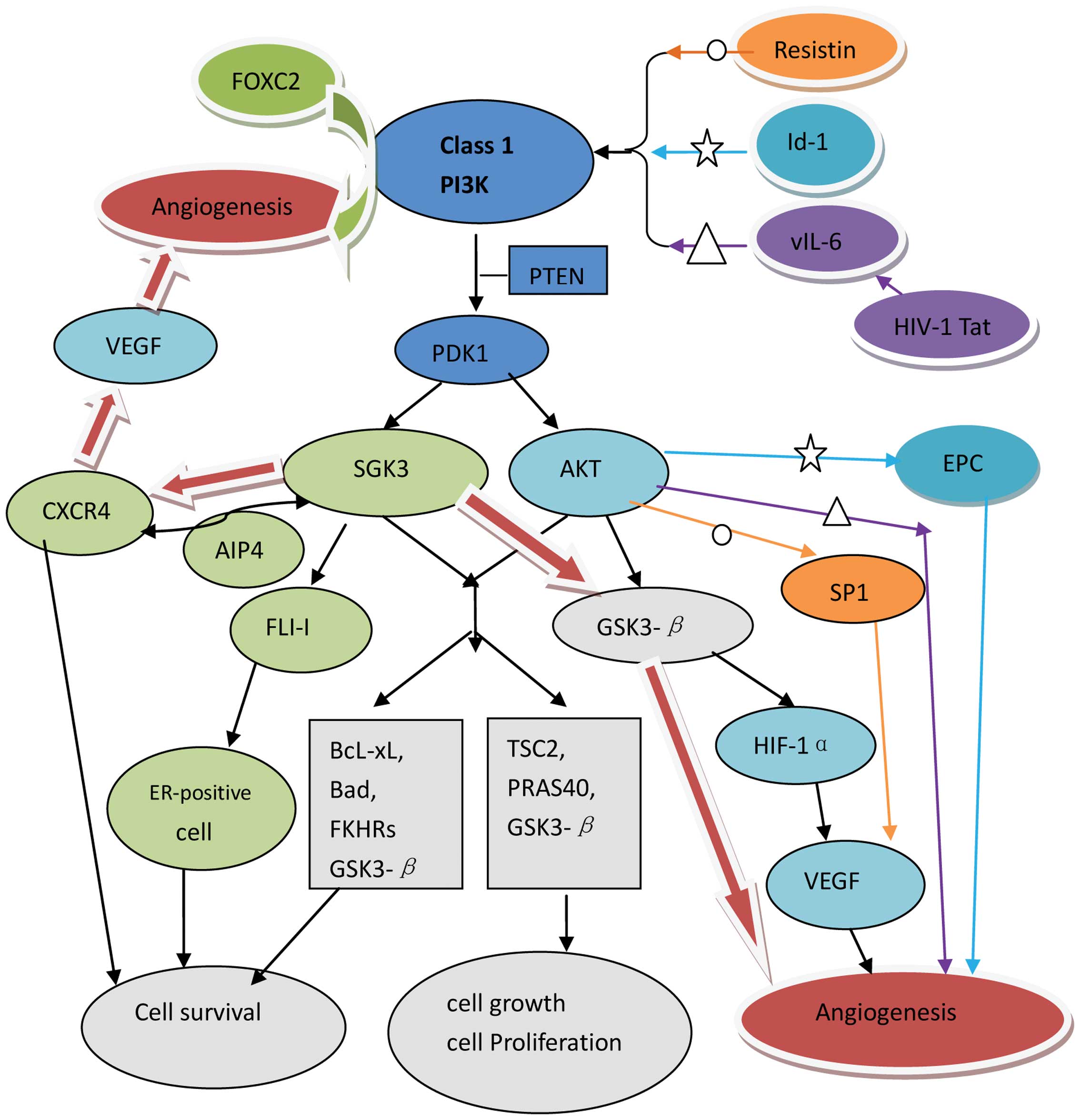 | Figure 1.Signaling of SGK3 and AKT. Activation
of PI3K leads to phosphorylation of PDK1, subsequently leading to
phosphorylation and activation of AKT and SGK3. Cell survival and
cell growth/proliferation: By regulating the downstream targets,
SGK3 and AKT show a parallel function in cell survival (Bcl-xL,
BAD, FKHRs, GSK3-β) and cell growth/proliferation (GSK3-β, TSC2,
PRAS40). SGK3 also induces cell survival by downregulating CXCR4
through the interaction with ubiquitin ligase AIP4. SGK3 mediates
ER-positive cancer cells survival by phosphorylating its
co-activator, FLI-I. Angiogenesis: PI3K signaling pathways involve
in the Foxc2-mediated angiogenesis process. Inhibition of GSK3-β by
PI3K/AKT up-regulates the expression of HIF-1α, which induces VEGF
transcriptional activation to promote angiogenesis.
○Resistin activates PI3K/AKT to increase the interaction
with Sp1, resulting in upregulation of VEGF expression to promote
angiogenesis. ★PI3K/AKT is mediated by Id1 to enhance
EPC angiogenesis in ovarian cancer. ∆By regulating
PI3K/PTEN/AKT/GSK-3β pathway, HIV-1 Tat in KSHV vIL-6-induced
angiogenesis. SDF-1/CXCR4 receptor ligand system up-regulates VEGF
expression to promote angiogenesis. Hypothesis: The red arrow
pathways are our hypothesis: SGK3 may involve in tumor angiogenesis
by regulating CXCR4 or/and GSK-3β. PI3K, phosphoinositide 3-kinase;
PDK1, 3-phosphoinositide-dependent kinase 1; AKT, v-akt murine
thymoma viral oncogene homolog; SGK3, serum- and
glucocorticoid-inducible protein kinase 3; Bcl-xL, B-cell
lymphoma-extra large; FKHRs, forkhead family of transcription
factors; BAD, Bcl-2-associated death promoter; FLI-I, flightless-I;
GSK3-β, glycogen synthase kinase-β; TSC2, tuberous sclerosis factor
2; PTEN, phosphatase and tensin homolog; PRAS40, proline-rich AKT
substrate of 40 kDa; SDF-1, stromal cell-derived factor 1; CXCR4,
C-X-C chemokine receptor type 4; HIF1α, hypoxia-inducible
factor-1α; VEGF, vascular endothelial growth factor; Id1, inhibitor
of DNA binding/differentiation 1; EPC, endothelial progenitor cell;
KSHV vIL-6, Kaposi's sarcoma-associated herpesvirus viral
interleukin-6; HIV-1, human immunodeficiency virus type 1; Tat,
transactivator of transcription; ER, estrogen receptor; AIP4,
atrophin-1-interacting protein 4; FOXC2, forkhead box protein C2;
Sp1, specificity protein 1. |
Tumor angiogenesis is recognized as an essential
step for tumor growth, invasion and metastasis, and has become an
intriguing target of tumor investigators worldwide for the
development of anticancer drugs. A number of studies have shown
that vascular endothelial growth factor (VEGF)/VEGF receptor are
essential in the tumor angiogenesis process (15,16).
Study has also been focused on the role of the
PI3K/AKT pathway in angiogenesis (Fig.
1) (17). Inhibition of GSK3-β by
PI3K/AKT upregulates the expression of hypoxia-inducible factor-1α,
inducing VEGF transcriptional activation to promote angiogenesis
(17). In early 2013, Su et al
(18) reported that PI3K/AKT is
mediated by inhibitor of DNA binding/differentiation 1 to enhance
endothelial progenitor cell angiogenesis in ovarian cancer. In a
study by You et al (19),
extracellular signal-regulated kinases and PI3K signaling pathways
showed strong involvement in the forkhead box protein C2-mediated
angiogenesis process. Zhou et al (20) showed the promotion of human
immunodeficiency virus type 1 transactivator of transcription in
Kaposi's sarcoma-associated herpesvirus viral IL-6-induced
angiogenesis by regulating the PI3K/phosphatase and tensin
homolog/AKT/GSK-3β pathway in vivo. A recent study revealed
that PI3K/AKT is activated by resistin and increases the
interaction with specificity protein 1, resulting in the
upregulation of VEGF expression to promote angiogenesis (21).
The stromal cell-derived factor 1/CXCR4 receptor
ligand system has also been reported to play a potential role in
cancer metastases via the upregulation of VEGF expression to
promote angiogenesis (22–24).
Bevacizumab, a monoclonal antibody targeted against
VEGF, and a number of other anti-angiogenesis molecules have been
used in numerous anti-angiogenesis strategies (25). The use of anti-angiogenesis strategies
may present a new epoch in tumor research, however, the exact
pathway of the angiogenesis mechanism remains unknown.
Since PI3K/AKT plays an important role in tumor
angiogenesis, we hypothesize that SGK3, as a downstream target of
PI3K and functionally parallel to AKT, may also be involved in the
malignant transformation of tumors by promoting angiogenesis. It
has also been reported that CXCR4 and GSK3-β, both downstream of
SGK3, may also have potential capacity in angiogenesis (3,17). These
facts demonstrated the potential role of SGK3 in promoting
angiogenesis and is evidence that confirms our hypothesis (Fig. 1).
SGK3 has been studied in depth with regard to tumor
malignant transformation, but the exact association between
angiogenesis and SGK3 is rarely reported. Possible reasons for this
include the fact that SGK3 does not significantly correlate with
angiogenesis, or that the amplification and overexpression of SGK3
may be an early stage event in tumor growth (3).
In conclusion, the mechanism of SGK3 in oncogenesis
is, to a certain extent, clear. However, its role in malignant
transformation, particularly in angiogenesis, remains to be
elucidated. Based on the present datum, detailed characterization
of any role of SGK3 in the promotion of angiogenesis via CXCR4 and
GSK3-β, the association between SGK3 and VEGF, and the exact
mechanisms behind this are required.
Clinical implications
These data generates an overall impression of SGK3
as an important oncogenic signaling mediator, and stresses the
vital nature of further research on the elucidation of the
signaling mechanisms associated with SGK3 in tumor angiogenesis.
Determining the role of SGK3 in tumor angiogenesis will surely
present a novel perspective on tumor malignant transformation, as
well as a target for tumor therapy.
Acknowledgements
This study was financially supported by grants from
the National Natural Science Foundation of China (nos.
30900650/H1615, 81372501/H1615, 81172232/H1615 and 81172564/H1625),
the Guangdong Natural Science Foundation (nos. S2012010008378,
S2013010015327 and S2012010008270) and the Science and Technology
Plan of Guangdong Province (no. 2011B031800025).
Abbreviations:
|
PI3K
|
phosphoinositide 3-kinase
|
|
PDK1
|
3-phosphoinositide-dependent kinase
1
|
|
AKT
|
v-akt murine thymoma viral oncogene
homolog
|
|
SGK3
|
serum- and glucocorticoid-inducible
protein kinase 3
|
|
CISK
|
cytokine-independent survival
kinase
|
|
Bcl-xL
|
B-cell lymphoma-extra large
|
|
FKHRs
|
forkhead family of transcription
factors
|
|
BAD
|
Bcl-2-associated death promoter
|
|
GSK3-β
|
glycogen synthase kinase-β
|
|
CXCR4
|
C-X-C chemokine receptor type 4
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Xu J, Liu D, Gill G and Songyang Z:
Regulation of cytokine-independent survival kinase (CISK) by the
Phox homology domain and phosphoinositides. J Cell Biol.
154:699–705. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu J, Wan M, He Q, et al: SGK3 is
associated with estrogen receptor expression in breast cancer.
Breast Cancer Res Treat. 134:531–541. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu M, Chen L, Chan TH, et al: Serum and
glucocorticoid kinase 3 at 8q13.1 promotes cell proliferation and
survival in hepatocellular carcinoma. Hepatology. 55:1754–1765.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu D, Yang X and Songyang Z:
Identification of CISK, a new member of the SGK kinase family that
promotes IL-3-dependent survival. Curr Biol. 10:1233–1236. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi T and Cohen P: Activation of
serum- and glucocorticoid-regulated protein kinase by agonists that
activate phosphatidylinositide 3-kinase is mediated by
3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2.
Biochem J. 339:319–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J, Liao L, Qin J, et al: Identification
of Flightless-I as a substrate of the cytokine-independent survival
kinase CISK. J Biol Chem. 284:14377–14385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Buse P, Tran SH, Luther E, et al: Cell
cycle and hormonal control of nuclear-cytoplasmic localization of
the serum- and glucocorticoid-inducible protein kinase, Sgk, in
mammary tumor cells. A novel convergence point of
anti-proliferative and proliferative cell signaling pathways. J
Biol Chem. 274:7253–7263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caers J, van Valckenborgh E, Menu E, et
al: Unraveling the biology of multiple myeloma disease: Cancer stem
cells, acquired intracellular changes and interactions with the
surrounding micro-environment. Bull Cancer. 95:301–313.
2008.PubMed/NCBI
|
|
9
|
Bruhn MA, Pearson RB, Hannan RD and
Sheppard KE: AKT-independent PI3-K signaling in cancer - emerging
role for SGK3. Cancer Manag Res. 5:281–292. 2013.PubMed/NCBI
|
|
10
|
Ellson CD, Andrews S, Stephens LR and
Hawkins PT: The PX domain: a new phosphoinositide-binding module. J
Cell Sci. 115:1099–1105. 2002.PubMed/NCBI
|
|
11
|
Bruhn MA, Pearson RB, Hannan RD and
Sheppard KE: Second AKTthe rise of SGK in cancer signalling. Growth
Factors. 28:394–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Slagsvold T, Marchese A, Brech A and
Stenmark H: CISK attenuates degradation of the chemokine receptor
CXCR4 via the ubiquitin ligase AIP4. EMBO J. 25:3738–3749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gassmann P, Haier J, Schlüter K, et al:
CXCR4 regulates the early extravasation of metastatic tumor cells
in vivo. Neoplasia. 11:651–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Müller A, Homey B, Soto H, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai W and Chen X: Multimodality molecular
imaging of tumor angiogenesis. J Nucl Med. 49(Suppl 2): 113S–128S.
2008.PubMed/NCBI
|
|
16
|
Ferrara N: VEGF and the quest for tumour
angiogenesis factors. Nat Rev Cancer. 2:795–803. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okumura N, Yoshida H, Kitagishi Y, et al:
PI3K/AKT/PTEN signaling as a molecular target in leukemia
angiogenesis. Adv Hematol. 2012:8430852012.PubMed/NCBI
|
|
18
|
Su Y, Gao L, Teng L, et al: Id1 enhances
human ovarian cancer endothelial progenitor cell angiogenesis via
PI3K/Akt and NF-κB/MMP-2 signaling pathways. J Transl Med.
11:1322013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You W, Gao H, Fan L, et al: Foxc2
regulates osteogenesis and angiogenesis of bone marrow mesenchymal
stem cells. BMC Musculoskelet Disord. 14:1992013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou F, Xue M, Qin D, et al: HIV-1 Tat
promotes Kaposi's sarcoma-associated herpesvirus (KSHV)
vIL-6-induced angiogenesis and tumorigenesis by regulating
PI3K/PTEN/AKT/GSK-3β signaling pathway. PLoS One. 8:e531452013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang L, Zhang Y, Yu Y and Zhang S:
Resistin promotes the expression of vascular endothelial growth
factor in ovary carcinoma cells. Int J Mol Sci. 14:9751–9766. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koshiba T, Hosotani R, Miyamoto Y, et al:
Expression of stromal cell-derived factor 1 and CXCR4 ligand
receptor system in pancreatic cancer: a possible role for tumor
progression. Clin Cancer Res. 6:3530–3535. 2000.PubMed/NCBI
|
|
23
|
Darash-Yahana M, Pikarsky E, Abramovitch
R, et al: Role of high expression levels of CXCR4 in tumor growth,
vascularization, and metastasis. FASEB J. 18:1240–1242.
2004.PubMed/NCBI
|
|
24
|
Wang J, Wang J, Sun Y, et al: Diverse
signaling pathways through the SDF-1/CXCR4 chemokine axis in
prostate cancer cell lines leads to altered patterns of cytokine
secretion and angiogenesis. Cell Signal. 17:1578–1592. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dandamudi UB, Ghebremichael M, Sosman JA,
et al: A phase II study of bevacizumab and high-dose interleukin-2
in patients with metastatic renal cell carcinoma: a Cytokine
Working Group (CWG) study. J Immunother. 36:490–495. 2013.
View Article : Google Scholar : PubMed/NCBI
|















