Introduction
Dendritic cells (DCs) were first identified in the
early 1970s (1). Since then, it has
been established that DCs are the primary antigen-presenting cells
(APCs) to T-lymphocytes, which initiate and also regulate cellular
immune responses (2). Immunotherapy
using DCs has been investigated in a number of cancers during the
last two decades. The results of these studies have revealed the
induction of tumor-specific immune responses. DCs can be isolated
ex vivo from blood mononuclear cells using granulocyte
macrophage colony-stimulating factor (GM-CSF) and interleukin-4
(IL-4) (3). However, DCs are usually
present in extremely small numbers in the circulation, and exhibit
marked diversity. There are a number of previous studies that have
described methods for culturing DCs in vitro (4–6). The cells
can be generated from various cellular sources, including bone
marrow, umbilical cord blood and peripheral blood. CD34+
hematopoietic progenitor cells are often used as an alternative
source of DCs, cord blood is rich in these cells. Although the
cellular sources and culture conditions are diverse, the majority
of protocols generate DCs using GM-CSF, IL-4 and tumor necrosis
factor (TNF)-α (7,8).
The present study compares two different mononuclear
cell isolation methods to obtain DCs from cord blood, namely
plastic adherence and magnetic activated cell sorting (MACS). The
results revealed that either method is able to produce DCs, but
that the cells differ in their differentiation pathways, phenotypes
and functions. The differentiated DCs obtained by the adherent
method were more mature than those isolated by MACS.
Materials and methods
Cord blood collection
Subsequent to obtaining written informed consent, 50
ml samples of human umbilical cord blood were collected from
healthy, full-term deliveries. All mothers and infants were healthy
and demonstrated no abnormal laboratory results. This study was
approved by the ethics committee of Beijing Chao-Yang Hospital,
Capital Medical University (Beijing, China).
Mononuclear cell isolation
The umbilical cord blood mononuclear cells (UBMCs)
were isolated from fresh cord blood with 200 IU/ml heparin using
1.077 g/ml Ficoll-Hypaque (Gibco-Invitrogen, Paisley, UK) density
gradient centrifugation, and then centrifuged for 30 min at 700 × g
at room temperature.
MACS
The UBMCs were isolated by the positive selection of
CD34+ cells using an immunomagnetic separation kit
(MiniMACS CD34 Isolation kit; Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany), according to the manufacturer's instructions.
The cells isolated by the MACS method were washed twice with
phosphate-buffered saline (PBS; GE Healthcare Life Sciences, Logan,
UT, USA) and then seeded into six-well culture plates (Costar,
Cambridge, MA, USA) at a density of 1×106/2 ml per well.
The cells were cultured in RPMI-1640 medium containing 10% fetal
bovine serum (FBS; Gibco Life Technologies, Carlsbad, CA, USA) and
supplemented with 1000 U/ml recombinant human GM-CSF (PeproTech,
Inc., Rocky Hill, NJ, USA) and 1000 U/ml IL-4 (PeproTech, Inc.) at
37°C in an incubator with a humidified 5% CO2
atmosphere.
Adherence method
The UBMCs were seeded into six-well culture plates
containing RPMI-1640 medium and 10% FBS at a density of
1×106/2 ml per well. After 2 h incubation at 37°C in a
humidified incubator with a 5% CO2 atmosphere, the
non-adherent cells were removed. The adherent cells were then
further cultured in RPMI-1640 medium containing 10% FBS, 1000 U/ml
recombinant human GM-CSF and 1000 U/ml IL-4 at 37°C in an incubator
with a humidified 5% CO2 atmosphere.
Generation of dendritic cells
Every three days, 50% of the spent medium was
replaced with fresh medium containing GM-CSF and IL-4 in order to
yield final concentrations of 1000 U/ml. On the 5th day, the cells
were cultured in TNF-α (PeproTech, Inc.) for a further two days.
Following 7 days of culture, the DCs were harvested.
Flow-based analysis of labeled
cells
The DCs were washed twice with PBS and then
incubated in a 10% Fc receptor (FcR) blocking solution (Miltenyi
Biotec GmbH) for 30 min at 4°C in order to block non-specific
binding to the FcR. The cells were then stained with phycoerythrin
(PE)-conjugated monoclonal mouse anti-human-cluster of
differentiation (CD)80 (1:100; cat. no. ab-155374; Abcam,
Cambridge, MA, USA) and monoclonal mouse anti-human human leukocyte
antigen (HLA)-DR (1:100; cat. no. ab95830; Abcam) antibodies or
APC-conjugated monoclonal mouse anti-human CD11c antibodies (1:100;
cat. no. 657713; BD Biosciences, Franklin Lakes, NJ, USA), whilst
control staining was performed using isotype-matched, irrelevant
antibodies that were also directly conjugated to PE or APC. The
fluorescence intensity of the cells was analyzed using a FACS
Calibur flow cytometry system (BD Biosciences).
Statistical analysis
The data in each figure corresponds to one
representative experiment of at least three independent
experiments. A t-test was used to determine the significance
of data comparison. A one-way analysis of variance was used for the
statistical analysis of differences among the experimental groups.
All statistical analyses were performed using SPSS 19.0 statistical
software (SPSS, Inc., Chicago, IL, USA). A value of P<0.05 was
used to indicate a statistically significant difference.
Results
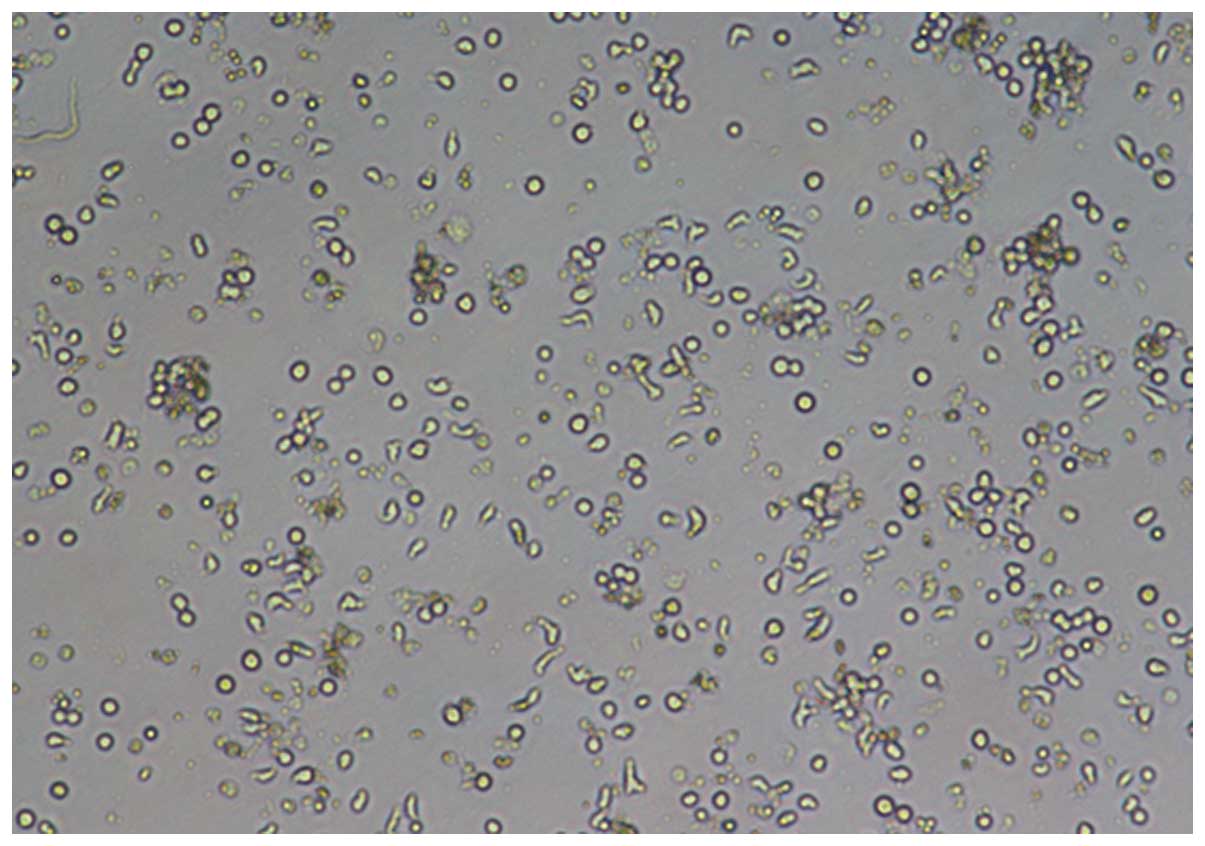
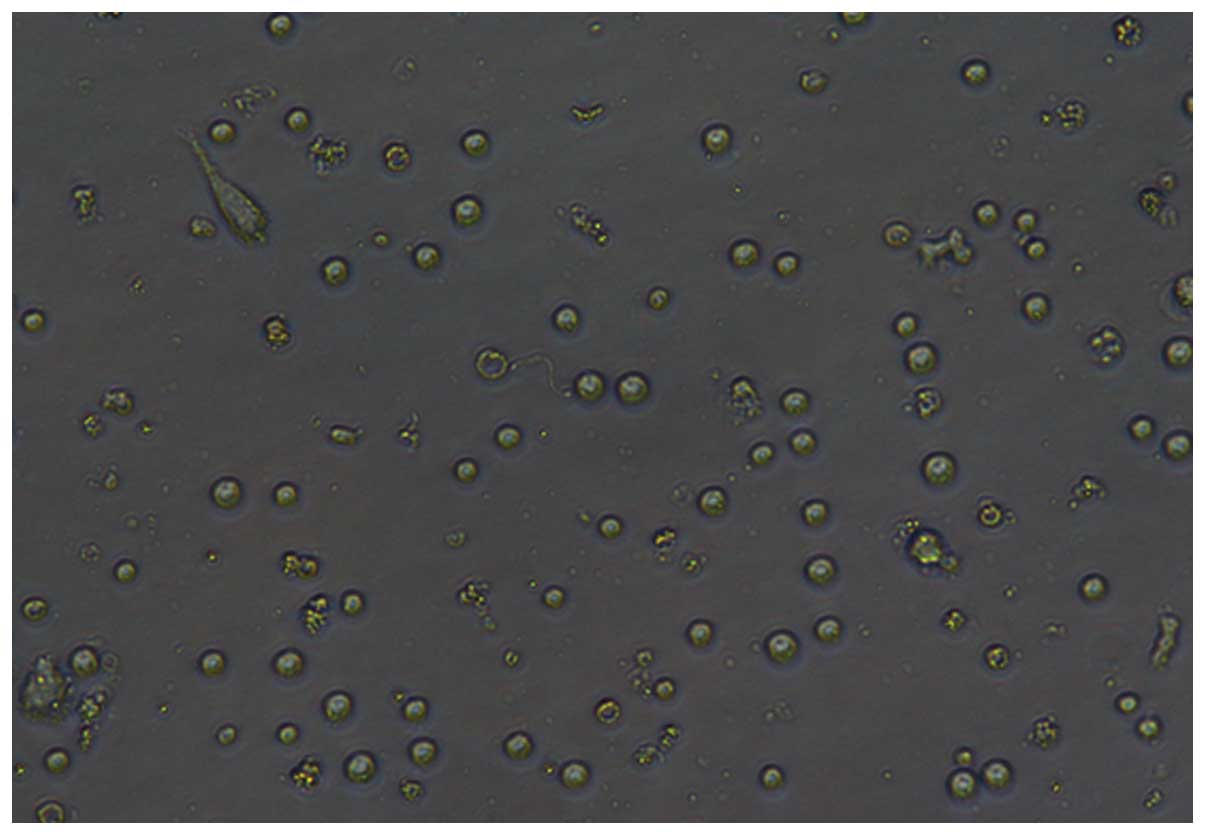
Following 7 days of incubation, microscopic images
of the DCs were captured with an inverted microscope (IX51; Olympus
Corporation, Tokyo, Japan) using the x10 or x20 objective (Figs. 1–4).
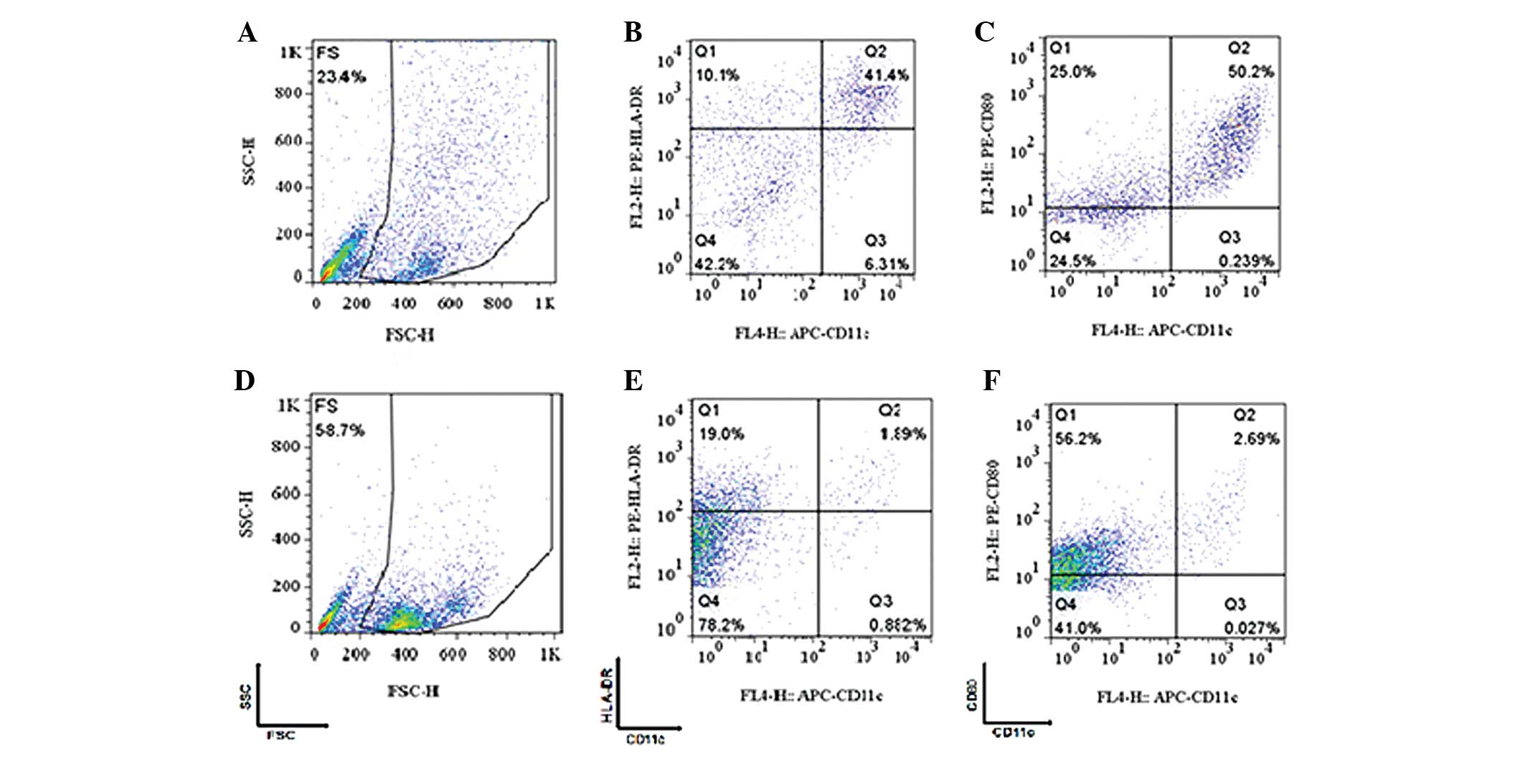
The results of the flow cytometry analysis are shown
in Fig. 5. Fig. 5A–C refer to the cells that were
cultured by the adherent method and Fig.
5D–F refers to the cells that were cultured by the MACS method.
The cells in the gated population were further analyzed and
identified to be CD11C+/HLA-DR+ (Fig. 5B and E) and
CD11C+/CD80+ (Fig.
5C and F).
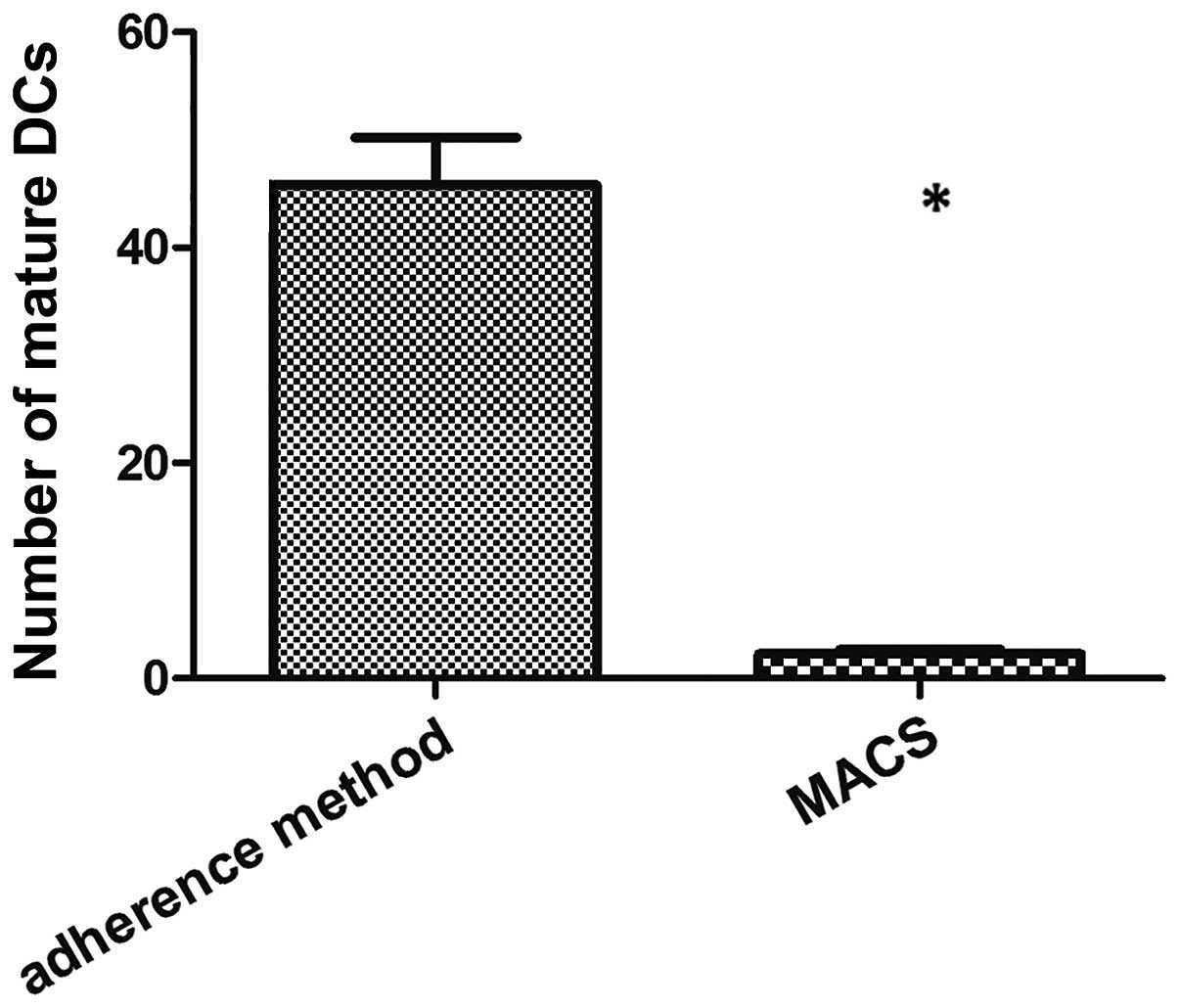
Either method was found to be able to produce DCs.
However, subsequent to 7 days of incubation, the DCs differentiated
by the adherent method were more mature than those isolated using
the MACS method (P=0.0102; Fig.
6).
In addition, it was identified that the DCs isolated
by the adherent method were unable to be maintained in culture for
>9–10 days. By contrast, the immature DCs differentiated from
CD34+ cells by MACS were phenotypically stable and could
be maintained for up to 3 weeks in culture.
Discussion
DCs have an important role in the induction of
immunity (2). In peripheral tissues,
DCs exist as immature cells that must undergo a process of
maturation upon exposure to cytokines and antigens, exemplified by
the upregulation of major histocompatibility complex (MHC) and
costimulatory molecules (CD80/86), activation markers and cytokine
production in order to activate T cells. DCs present acquired
antigens to naive T cells via MHC molecules, and also promote the
differentiation and maturation of antibody-producing B cells. DCs
exhibit certain common characteristics, including an irregular
shape, a distinct cell-surface phenotype, such as extremely high
levels of MHC class II proteins, active motility, and potent
stimulatory activity for T cell-dependent responses (9). However, they also demonstrate marked
heterogeneity (10). The subsets of
DCs include cells in lymphoid organs, blood DCs, Langerhans cells,
and cells in non-lymphoid organs, such as the lung, gut, heart and
synovium (11). In total, two types
of DCs have been identified in the blood, namely myeloid DCs
(CD11C+/CD123−) and plasmacytoid DCs
(CD123+/CD11C−). DCs are able to
differentiate from a diverse range of progenitors and precursors
under appropriate culture conditions (11,12).
Preliminary studies concerning DCs have been
hampered by the limited number of cells that are able to be
purified (13,14). In practice, DCs can be generated from
two primary cell types: CD34+ cells and monocytes
(13). There are a number of ways to
isolate monocytes, including plastic adherence and specific
marker-based separation techniques, such as MACS and fluorescent
activated cell sorting (15).
However, DCs derived from CD34+ cells or monocytes
differ in their differentiation pathways, phenotype and functions
(16–18). A study by Delirezh et al
(19) compared the phagocytic
activity of two types of cells. The results revealed that the
phagocytic activity of DCs obtained by the MACS method was higher
than that of the cells isolated by the adherent method. This may be
explained by differences in the maturity of the two types of
DCs.
It has been established that populations of human
monocytes can be divided into two different subsets, namely
CD14− CD16+ (5–10%) and CD14+
CD16− (90–95%) (20,21). These
two types of monocytes differ in their phagocytic activity. The
majority of previous studies have used the MACS method to separate
CD34 + cells from cord blood in order to induce DCs
(13,16–18,22).
Others have used an adherent method to induce the differentiation
of DCs from peripheral blood or bone marrow (23,24). The
present study compared the two methods using cord blood and
concluded that the adherent method is a simple technique that is
able to induce the formation of DCs. Despite this, the DCs derived
from CD34+ cells by MACS were phenotypically stable and
could be maintained for a longer period of time in culture; a
feature suitable for research purposes. In terms of clinical
applications, the adherence method may be preferable, due to less
manipulation and the absence of exposure to the magnetic field of
the MACS apparatus (19).
DCs isolated by the MACS method demonstrated higher
homogeneity. Furthermore, the yield and viability were markedly
increased compared with the DCs isolated using the adherent
methods. Differentiating DCs using MACS is, however, more
technically demanding than adherence. Monocyte isolation methods
affect the phenotypes and functions of resultant DCs due to the
different compositions of the monocyte subsets that are isolated.
Additional studies should be conducted in order to establish a
clear understanding of DCs. Isolation methods should be selected
according to the specific clinical or experimental purpose.
References
|
1
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banchereau J, Briere F, Caux C, et al:
Immunobiology of dendritic cells. Annu Rev Immunol. 18:767–811.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuler G, Schuler-Thurner B and Steinman
RM: The use of dendritic cells in cancer immunotherapy. Curr Opin
Immunol. 15:138–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu CS, Boyer J, Schullery DS, et al:
Phase I/II randomized trial of dendritic cell vaccination with or
without cyclophosphamide for consolidation therapy of advanced
ovarian cancer in first or second remission. Cancer Immunol
Immunother. 61:629–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coosemans A, Vanderstraeten A, Tuyaerts S,
et al: Wilms' Tumor Gene 1 (WT1) - loaded dendritic cell
immunotherapy in patients with uterine tumors: a phase I/II
clinical trial. Anticancer Res. 33:5495–5500. 2013.PubMed/NCBI
|
|
6
|
Mody N, Dubey S, Sharma R, Agrawal U and
Vyas SP: Dendritic cell-based vaccine research against cancer.
Expert Rev Clin Immunol. 11:213–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GanjiBakhsh M, Nejati V, Delirezh N, Asadi
M and Gholami K: Mixture of fibroblast, epithelial and endothelial
cells conditioned media induce monocyte-derived dendritic cell
maturation. Cell Immunol. 272:18–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Delirezh N, Majedi L, Asri Rezaei S and
Ranjkeshzadeh H: Generation of mature monocyte-derived dendritic
cells in the presence of heparin and monocyte conditioned medium:
phenotypic and functional comparison. Iran Biomed J. 15:79–84.
2011.PubMed/NCBI
|
|
9
|
Takamizawa M, Rivas A, Fagnoni F, et al:
Dendritic cells that process and present nominal antigens to naive
T lymphocytes are derived from CD2+ precursors. J
Immunol. 158:2134–2142. 1997.PubMed/NCBI
|
|
10
|
Ito T, Inaba M, Inaba K, et al: A
CD1a+/CD11c+ subset of human blood dendritic cells is a direct
precursor of Langerhans cells. J Immunol. 163:1409–1419.
1999.PubMed/NCBI
|
|
11
|
Cella M, Jarrossay D, Facchetti F, et al:
Plasmacytoid monocytes migrate to inflamed lymph nodes and produce
large amounts of type I interferon. Nat Med. 5:919–923. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oehler L, Berer A, Keil F, et al:
Generation of dendritic cells from human chronic myelomonocytic
leukemia cells in fetal calf serum-free medium. Leuk Lymphoma.
38:577–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferlazzo G, Wesa A, Wei WZ and Galy A:
Dendritic cells generated either from CD34+ progenitor
cells or from monocytes differ in their ability to activate
antigen-specific CD8+ T cells. J Immunol. 163:3597–3604.
1999.PubMed/NCBI
|
|
14
|
Broxmeyer HE, Hangoc G, Cooper S, et al:
Growth characteristics and expansion of human umbilical cord blood
and estimation of its potential for transplantation in adults. Proc
Natl Acad Sci USA. 89:4109–4113. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Brussel I, Ammi R, Rombouts M, et al:
Fluorescent activated cell sorting: An effective approach to study
dendritic cell subsets in human atherosclerotic plaques. J Immunol
Methods. 417:76–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caux C, Massacrier C, Vanbervliet B, et
al: CD34+ hematopoietic progenitors from human cord blood
differentiate along two independent dendritic cell pathways in
response to granulocyte-macrophage colony-stimulating factor plus
tumor necrosis factor alpha: II. Functional analysis. Blood.
90:1458–1470. 1997.PubMed/NCBI
|
|
17
|
Canque B, Camus S, Dalloul A, et al:
Characterization of dendritic cell differentiation pathways from
cord blood CD34(+) CD7(+)CD45RA(+) hematopoietic progenitor cells.
Blood. 96:3748–3756. 2000.PubMed/NCBI
|
|
18
|
Ferlazzo G, Klein J, Paliard X, Wei WZ and
Galy A: Dendritic cells generated from CD34+ progenitor cells with
flt3 ligand, c-kit ligand, GM-CSF, IL-4 and TNF-alpha are
functional antigen-presenting cells resembling mature
monocyte-derived dendritic cells. J Immunother. 23:48–58. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delirezh N, Shojaeefar E, Parvin P and
Asadi B: Comparison the effects of two monocyte isolation methods,
plastic adherence and magnetic activated cell sorting methods, on
phagocytic activity of generated dendritic cells. Cell J.
15:218–223. 2013.PubMed/NCBI
|
|
20
|
Delirezh N and Shojaeefar E: Phenotypic
and functional comparison between flask adherent and magnetic
activated cell sorted monocytes derived dendritic cells. Iran J
Immunol. 9:98–108. 2012.PubMed/NCBI
|
|
21
|
Mucci I, Legitimo A, Compagnino M, et al:
The methodological approach for the generation of human dendritic
cells from monocytes affects the maturation state of the resultant
dendritic cells. Biologicals. 37:288–296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ackermann B, Tröger A, Glouchkova L,
Körholz D, Göbel U and Dilloo D: Characterization of
CD34+ progenitor-derived dendritic cells pulsed with
tumor cell lysate for a vaccination strategy in children with
malignant solid tumors and a poor prognosis. Klin Padiatr.
216:176–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schott M, Seissler J, Feldkamp J, von
Schilling C and Scherbaum WA: Dendritic cell immunotherapy induces
antitumour response in parathyroid carcinoma and neuroendocrine
pancreas carcinoma. Horm Metab Res. 31:662–664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
DeMatos P, Abdel-Wahab Z, Vervaert C,
Hester D and Seigler H: Pulsing of dendritic cells with cell
lysates from either B16 melanoma or MCA-106 fibrosarcoma yields
equally effective vaccines against B16 tumors in mice. J Surg
Oncol. 68:79–91. 1998. View Article : Google Scholar : PubMed/NCBI
|