Introduction
Gastric cancer is one of the most prevalent types of
malignant tumors in South America, Eastern Europe and Asian
countries and adenocarcinoma is the most common form of gastric
cancer (1–3). It has been well-established that the
pathogenesis of gastric cancer occurs through a multistep
progression from chronic gastritis to atrophic gastritis,
intestinal metaplasia and dysplasia, finally resulting in cancer
(4). Loss of cell polarity and
disruption of intracellular adhesion are frequently observed during
this process and have been reported to have a critical role in
cancer progression (5,6).
Tight junctions are the most apical type of cellular
junction, which function as a selective barrier and establish
cellular polarity in epithelial cells (7–9). In
addition, tight junctions are involved in the regulation of cell
proliferation and differentiation, among other cellular functions
(10). Tight junctions are typically
lost in cancer, which was reported to be involved in the invasive
and metastatic phenotype of tumor cells (11–13).
Claudins are a family of integral membrane proteins
and are the major protein components of tight junctions. Of the
numerous tight junction proteins, claudins are key functional
proteins and are expressed in various types of tissues and cells.
In addition, claudins were reported to have a marked impact on the
biological behavior of tumor progression (14,15). Of
note, the expression of claudin-1 and claudin-4 was demonstrated to
be frequently altered in various tumor tissues.
The expression of claudin-1 was reported to be
significantly increased in intestinal-type carcinomas compared with
diffuse-type gastric carcinomas (15). However, another study demonstrated
that the claudin-1 expression was significantly reduced in
intestinal-type gastric carcinomas compared with the diffuse-type
(16). Furthermore, transformation of
claudin-1 expression was identified in gastric carcinoma (17).
Studies into the function of claudin-4 have not
provided consistent results. It was reported that claudin-4
expression was significantly correlated with improved rates of
patient survival in gastric cancer (16,18).
However, Resnick et al (19)
suggested that moderate to strong staining for claudin-4 in gastric
cancer was associated with decreased survival rates. Soini et
al (15) found that claudin-4 was
not associated with patient survival. Overexpression of claudin-4
was demonstrated to be significantly associated with reduced
invasiveness in pancreatic carcinoma (20). However, overexpression of claudin-3
and claudin-4 was reported to result in increased invasion,
motility and survival of tumor cells (21).
Therefore the biological functions of claudin-1 and
claudin-4 have not been clarified and studies on the role of their
expression in gastric carcinomas have been limited. Further
investigations are required for clarification of these
controversial results and to fully elucidate the function of
claudin-1 and claudin-4. The present study aimed to evaluate the
clinicopathological associations of claudin-1 and claudin-4
expressions in gastric adenocarcinoma.
Patients and methods
Patients
Tissues were obtained from 94 patients with gastric
adenocarcinoma who underwent surgical resection between January
2010 and April 2013 at Kagawa University Hospital (Kagawa, Japan).
The patients' histological findings, along with their lymph node
metastases, venous invasion and tumor, node, metastasis (TNM)
stages were evaluated based on the Japanese Classification of
Gastric Adenocarcinoma (22,23). All subjects provided written informed
consent. The study was conducted with the approval of the
Institutional Research Ethics Committee of Kagawa Prefectural
University of Health Sciences (Kagawa, Japan).
Immunohistochemistry
Tissues were obtained from primary tumors (slides, 4
µm) and were deparaffinized in 99% xylene (Muto Pure Chemicals Co.,
Ltd., Tokyo, Japan) for 15 min, then rehydrated in a graded series
of ethanol (Muto Pure Chemicals Co., Ltd.), followed by antigen
retrieval by microwave heating for 15 min at 2 kW in 0.01 M citrate
buffer (pH 6.0) containing 38 mg/dl citric acid monohydrate and 241
mg/dl trisodium citrate dehydrate (Wako Pure Chemical Industries,
Ltd., Osaka, Japan). Endogenous peroxidase activity was blocked
using 3% hydrogen peroxide (Wako Pure Chemical Industries, Ltd.).
Sections were then incubated for 2 h at room temperature with the
following primary antibodies: Anti-claudin-1 antibody (mouse
monoclonal IgG2a; Abcam, Cambridge, UK; catalog no. ab56417;
dilution, 1:100) and anti-claudin-4 antibody (rabbit polyclonal;
Abcam; catalog no. ab15104; dilution, 1:200). Slides were rinsed
three times with phosphate-buffered saline (PBS; Wako Pure Chemical
Industries, Ltd.) and incubated for 15 min at room temperature with
secondary antibodies with Histofine Simple Stain MAX PO (MULTI)
(universal immuno-peroxidase polymer, anti-mouse and anti-rabbit;
Nichirei Biosciences Inc., Tokyo, Japan) according to the
manufacturer's instructions and stained with 3,3′-diaminobenzidine
tetrahydrochloride (DAB) using a DAB substrate kit (Nichirei
Biosciences Inc.). The sections were counterstained with Meyer's
hematoxylin and then dehydrated, cleared with 99% xylene for 15 min
and mounted in malinol (Muto Pure Chemicals, Co., Ltd.). Colon
cancer samples and normal gastric mucosa samples obtained from
Kagawa University Hospital were used as positive controls. The
expression of claudin-1 and claudin-4 in the tissues was observed
under microscope (BX53; Olympus Corporation, Tokyo, Japan) with
photographs taken on a microscope camera (DP20-5; Olympus
Corporation).
The classification of claudin expression was based
on the criteria of Jung et al (14). Briefly, the immunostaining for
claudin-1 or claudin-4 was assessed using the following scoring: No
staining, 0; <25% cells positive and incomplete membranous
staining, 1+; 25–50% cells positive and incomplete membranous
staining, 2+; 50–75% cells positive and complete or incomplete
membranous staining, 3+; >75% cells positive and complete
membranous staining, 4+. In the evaluation, the expression of
claudin-1 and claudin-4 were grouped into negative (0, 1+) and
positive (2+, 3+, 4+) groups.
Statistical analysis
Univariate analysis was performed using the
Chi-squared or Fisher's exact tests for categorical data. All
statistical analyses were performed using SPSS 21.0 software
(International Business Machines, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Table I summarizes the
clinical parameters of patients with gastric adenocarcinoma. A
total of 67 (71.3%) patients were men and 27 (28.7%) patients were
women, with a median age of 72-years (range, 50–91 years).
Following analysis of the tumor samples, it was reported that 46
(48.9%) patients had stage I, 25 (26.6%) patients had stage II, 16
(17.0%) patients had stage III and 7 patients (7.5%) had stage IV
gastric cancer.
 | Table I.Clinical characteristics of 94
gastric adenocarcinoma patients. |
Table I.
Clinical characteristics of 94
gastric adenocarcinoma patients.
|
Characteristics | Patients, n
(%) |
|---|
| Age, years (mean ±
standard deviation) | 72.1±8.9 |
| Gender |
|
|
Male | 67 (71.3) |
|
Female | 27 (28.7) |
| Histological
type |
|
| Well to
moderately differentiated | 47 (50.0) |
| Poorly
differentiated | 47 (50.0) |
| Lymphatic
invasion |
|
|
Negative | 24 (25.5) |
|
Positive | 70 (74.5) |
| Venous
invasion |
|
|
Negative | 30 (31.9) |
|
Positive | 64 (68.1) |
| Lymph node
metastasis |
|
| N0 | 64 (68.1) |
| N1 | 12 (12.8) |
| N2 | 4 (4.2) |
| N3 | 14 (14.9) |
| Depth of tumor
invasion |
|
| T1 | 37 (39.4) |
| T2 | 11 (11.7) |
| T3 | 18 (19.1) |
| T4 | 28 (29.8) |
| Stage |
|
| I | 46 (48.9) |
| II | 25 (26.6) |
|
III | 16 (17.0) |
| IV | 7 (7.5) |
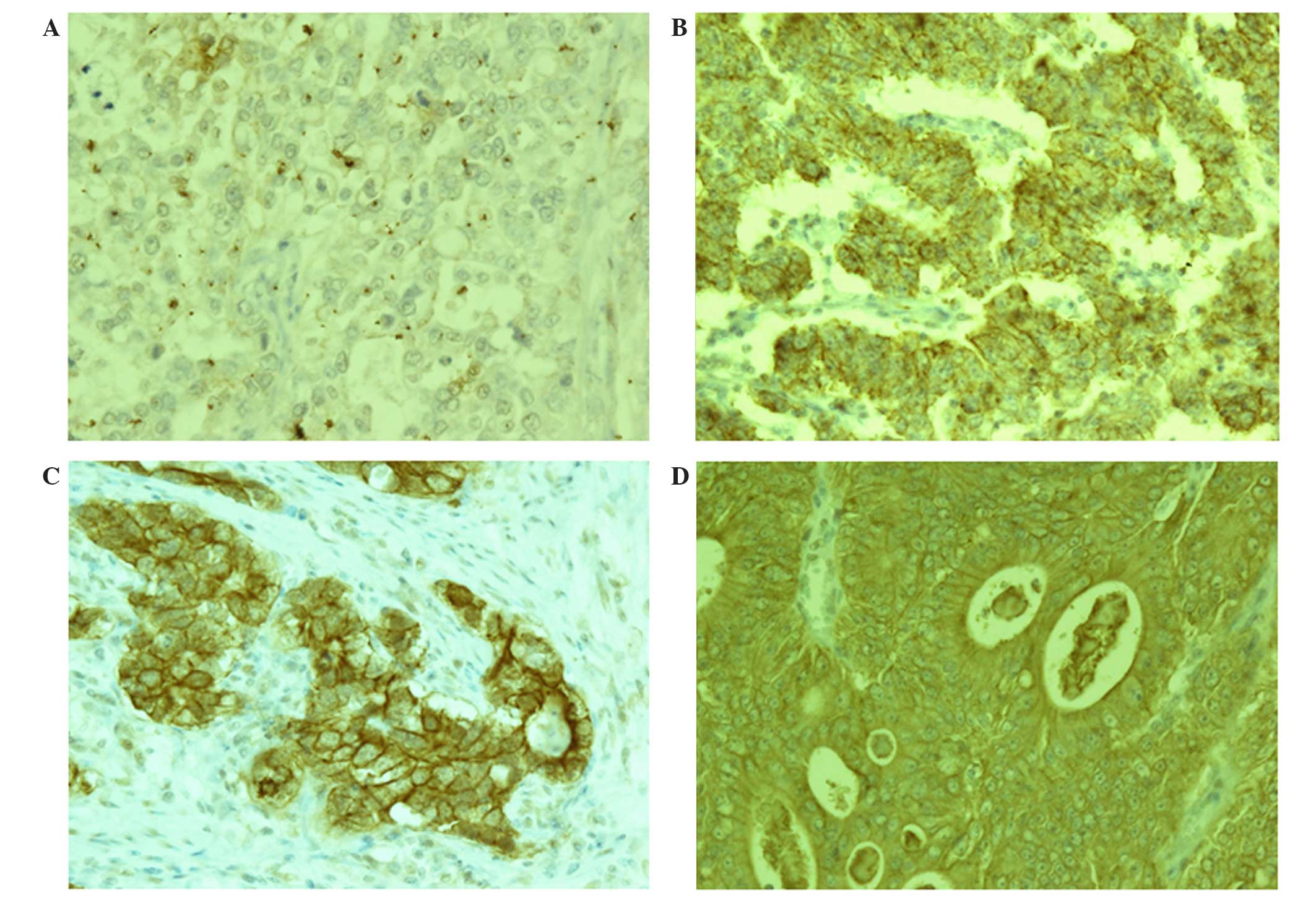
As shown in Fig. 1,
claudin-1 and claudin-4 were primarily expressed in the cell
membrane of gastric adenocarcinoma cells; in addition, certain
samples displayed a low level of cytoplasmic staining. The
expression rate of claudin-1 was 43.6% and that of claudin-4 was
87.2% (Table II). Claudin-1
expression levels were revealed to by associated with histological
type, as they were significantly higher in well- to moderately-
differentiated gastric adenocarcinomas compared with
poorly-differentiated adenocarcinomas (P<0.01) (Fig. 1, Table
III). However, no significant associations were determined
between the expression of claudin-1 and age, gender, lymphatic
invasion, venous invasion, depth of tumor invasion, lymph node
metastasis or stage of gastric cancer in patients (Table III). The expression rate of
claudin-4 in poorly-differentiated gastric adenocarcinomas was
comparable to that of the well- to moderately-differentiated
gastric adenocarcinomas; therefore, claudin-4 was not significantly
associated with any clinicopathological factors (Fig. 1, Table
III).
 | Table II.Immunohistochemical staining for the
expression rate of claudins in 94 gastric adenocarcinoma tissue
samples. |
Table II.
Immunohistochemical staining for the
expression rate of claudins in 94 gastric adenocarcinoma tissue
samples.
| Protein | Positive expression
(%) |
|---|
| Claudin-1 | 41 (43.6) |
| Claudin-4 | 82 (87.2) |
 | Table III.Correlation between claudin-1 and
claudin-4 expression and clinicopathologic characteristics of
gastric adenocarcinoma in 94 tissue samples from patients. |
Table III.
Correlation between claudin-1 and
claudin-4 expression and clinicopathologic characteristics of
gastric adenocarcinoma in 94 tissue samples from patients.
|
| Claudin-1 | Claudin-4 |
|---|
|
|
|
|
|---|
|
Characteristics | (–) | (+) | P-value | (–) | (+) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
≤60 | 8 | 1 | 0.073 | 3 | 6 | 0.087 |
|
>60 | 45 | 40 |
| 9 | 76 |
|
| Gender |
|
|
|
|
|
|
|
Male | 35 | 32 | 0.295 | 9 | 58 | 1.000 |
|
Female | 18 | 9 |
| 3 | 24 |
|
| Histological
type |
|
|
|
|
|
|
| Well to
moderately differentiated | 17 | 30 | <0.001 | 4 | 43 | 0.354 |
| Poorly
differentiated | 36 | 11 |
| 8 | 39 |
|
| Lymphatic
invasion |
|
|
|
|
|
|
|
Negative | 15 | 9 | 0.644 | 4 | 20 | 0.495 |
|
Positive | 38 | 32 |
| 8 | 62 |
|
| Venous
invasion |
|
|
|
|
|
|
|
Negative | 13 | 17 | 0.081 | 4 | 26 | 1.000 |
|
Positive | 40 | 24 |
| 8 | 56 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
| N0 | 34 | 30 | 0.107 | 8 | 56 | 0.139 |
| N1 | 9 | 3 |
| 0 | 12 |
|
| N2 | 4 | 0 |
| 0 | 4 |
|
| N3 | 6 | 8 |
| 4 | 10 |
|
| Depth of tumor
invasion |
|
|
|
|
|
|
| T1 | 17 | 20 | 0.345 | 4 | 33 | 0.612 |
| T2 | 6 | 5 |
| 1 | 10 |
|
| T3 | 11 | 7 |
| 4 | 14 |
|
| T4 | 19 | 9 |
| 3 | 25 |
|
| Stage |
|
|
|
|
|
|
| I | 22 | 24 | 0.071 | 5 | 41 | 0.420 |
| II | 14 | 11 |
| 2 | 23 |
|
|
III | 10 | 6 |
| 4 | 12 |
|
| IV | 7 | 0 |
| 1 | 6 |
|
Discussion
The present study examined the expression of
claudin-1 and claudin-4 in 94 patients with gastric adenocarcinoma.
In order to evaluate the altered protein expression and whether it
was associated with clinicopathological parameters,
immunohistochemical staining was conducted using primary antibodies
for claudin-1 and claudin-4. The expression of claudin-1
demonstrated a significant correlation with histological type, with
significantly increased levels in well- to
moderately-differentiated gastric adenocarcinomas. However, no
significant correlations were observed between claudin-4 expression
in gastric adenocarcinoma and clinicopathological parameters. These
results may therefore provide evidence for the development of a
useful molecular marker for predicting cancer progression and
prognosis in gastric adenocarcinoma, as claudin-1 expression may be
a phenotypic feature in well- to moderately-differentiated gastric
adenocarcinoma.
Tumor cells undergo epithelial-to-mesenchymal
transition (EMT) in order to execute the multi-step process of
tumorigenesis and metastasis (5).
Tight junction proteins, including claudins, cadherins and
vimentins are essential for the process of EMT; these proteins are
crucial for the preservation of the cell layer integrity and
regulation of cell proliferation (24). In addition, the role of tight junction
proteins in tumor progression has been associated with numerous
other protein interactions (25,26).
However, numerous studies have reported that the expression of
tight junction proteins was decreased in cancer cells (27,28).
Previous studies have identified the expression of
claudins in several cancer types, including breast, pancreatic,
liver and esophageal cancer (10,29–33).
Claudin-1 expression was reported be attenuated in breast cancer as
well as colon cancer (31,34,35). In
addition, the expression levels of claudin-1, claudin-3, claudin-4
and claudin-5 were all significantly decreased in diffuse
adenocarcinoma and were essential for determining the phenotype and
loose cohesion of cells in diffuse gastric carcinoma (15). Claudin-3 expression levels were
significantly depleted in advanced tumor-stage (T3 and T4) gastric
adenocarcinoma cases (16); in
addition, the loss of claudin-7 was correlated with increased
cellular discohesion in breast carcinoma (36). Thus, the reduced expression of
claudins in cancer supported the hypothesis that tumorigenesis is
associated with tight junctions disruption and that this process is
critical for reduced cohesion and invasiveness as well as the
limited differentiation capacity of cancer cells. Decreased
expression of tight junction proteins, such as claudins, in cancer
results in reduced cell adhesion during the progression of cancer
to metastasis (37,38). The results of the present study
indicated that claudin-1 expression was reduced in
poorly-differentiated gastric adenocarcinomas compared with well-
to moderately-differentiated gastric adenocarcinomas; in addition,
the present findings confirmed that the loss or downregulation of
tight junction proteins in cancer cells was essential for
tumorigenesis.
Histologically, gastric adenocarcinomas may be
separated into two main categories according to their biologic
behaviors: Differentiated and undifferentiated adenocarcinoma. In
addition gastric adenocarcinomas may be catagorized into intestinal
or diffuse type, as well as expanding or infiltrative type
(39–41). In general, the intestinal type is
well-differentiated with cohesive, glandular-like tumor cells,
whereas the diffuse type is poorly-differentiated with
infiltrating, non-cohesive tumor cells. Those tumors classed as
differentiated include papillary, well-differentiated and
moderately-differentiated adenocarcinomas, while undifferentiated
tumors include poorly-differentiated adenocarcinomas, signet ring
cell carcinomas and mucinous adenocarcinomas. Several evaluation
studies of prognostic value regarding gastric carcinoma have been
performed. Adachi et al (42)
reported that the overall 5-year survival rate was increased in
patients with well-differentiated gastric carcinoma compared with
those patients with poorly-differentiated gastric carcinoma (76 vs.
67%, respectively). In addition, Park et al (43) reported that the overall cumulative
5-year survival rates for patients were 67% for well- to
moderately-differentiated and 54% for poorly-differentiated gastric
cancer. Therefore, patients with poorly-differentiated
adenocarcinoma may have a worse prognosis compared with those with
well- to moderately-differentiated types.
Immunohistochemical staining was performed for
claudin-1 and claudin-4 in the present study. The frequency of
claudin-1 expression was 43.6% (41/94), which was significantly
decreased in poorly-differentiated gastric adenocarcinoma tissue
compared with the well- to moderately-differentiated tumor tissue
(23.4 vs. 63.8%); however, no significant difference was observed
in other pathologic features. These results suggested that the
expression of claudin-1 is associated with poorly-differentiated
gastric adenocarcinoma and that the loss of claudin-1 expression
may be an efficient predictive marker for tumor recurrence and
survival outcome of patients.
In the present study, the expression of claudin-4
was not found to be significantly associated with the
clinicopathological factors assessed. However, the correlation
between claudin-4 and clinicopathological factors remains
controversial; Jung et al (16) reported that the expression of
claudin-4 was significantly lower in cases with positive lymphatic
invasion in gastric cancer and Zhu et al (44) demonstrated that claudin-4 expression
was significantly associated with tumor differentiation, gender,
age and tumor location (44). By
contrast, Kuo et al (45)
reported that claudin-4 expression was not associated with age,
gender, depth of wall invasion, lymph node metastasis or
differentiation; these results were comparable with those of the
present study.
Several studies have investigated claudin-4
expression in cancer. One study reported that claudin-4 levels were
markedly lower in diffuse-type gastric cancer compared with
intestinal-type gastric cancer (45).
Another study demonstrated that the expression of claudin-4 was
significantly reduced in patients with positive lymphatic invasion
in their gastric cancer tissue (16).
In addition, reduced expression of claudin-4 was reported to be
correlated with glandular structure and loss of differentiation in
gastric cancer (46). Furthermore, it
was suggested that the expression of claudin-4 attenuated
pancreatic cancer cell invasion (20). Paradoxically, overexpression of
claudin-4 was observed in breast and ovarian carcinoma (38,47); in
addition, claudin-4 overexpression in ovarian cells may be highly
associated with features of metastasis, including invasion,
motility and cell survival (21).
Thus, the expression patterns of claudin-4 in various types of
cancer were diverse and provided contradictory results. The
mechanisms for the upregulation or downregulation of claudin-4
expression in tumorigenesis remain to be fully elucidated and these
paradoxical points require further investigation.
In conclusion, downregulation of claudin-1
expression in poorly-differentiated gastric adenocarcinoma is
involved in the biological transformation of tumor behavior. Based
on these results, claudin-1 was suggested to be an important
protein associated with histological type and may have potential
for use as a prognostic marker. Further studies, with a greater
number of subjects, are required in order to elucidate the
association of claudin-1 expression with tumor progression and to
perform a long-term clinical survival analysis.
References
|
1
|
Yamamoto S: Stomach cancer incidence in
the world. Jpn J Clin Oncol. 31:4712001.PubMed/NCBI
|
|
2
|
Ahn YO, Park BJ, Yoo KY, et al: Incidence
estimation of stomach cancer among Koreans. J Korean Med Sci.
6:7–14. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American Cancer
Society award lecture on cancer epidemiology and prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
5
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Cell Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matter K and Balda MS: Signalling to and
from tight junctions. Nat Rev Mol Cell Biol. 4:225–236. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitic LL, Van Itallie CM and Anderson JM:
Molecular physiology and pathophysiology of tight junctions I.
Tight junction structure and function: lessons from mutant animals
and proteins. Am J Physiol Gastrointest Liver Physiol.
279:G250–G254. 2000.PubMed/NCBI
|
|
9
|
Tsukita S, Furuse M and Itoh M:
Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol.
2:285–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mitic LL and Anderson JM: Molecular
architecture of tight junctions. Annu Rev Physiol. 60:121–142.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin TA and Jiang WG: Tight junctions
and their role in cancer metastasis. Histol Histopathol.
16:1183–1195. 2001.PubMed/NCBI
|
|
12
|
Langbein L, Pape UF, Grund C, et al: Tight
junction-related structures in the absence of a lumen: occludin,
claudins and tight junction plaque proteins in densely packed cell
formations of stratified epithelia and squamous cell carcinomas.
Eur J Cell Biol. 82:385–400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itoh M and Bissell MJ: The organization of
tight junctions in epithelia: implications for mammary gland
biology and breast tumorigenesis. J Mammary Gland Biol Neoplasia.
8:449–462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turksen K and Troy TC: Barriers built on
claudins. J Cell Sci. 117:(12 Pt). 2435–2447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soini Y, Tommola S, Helin H and
Martikainen P: Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of
claudin expression associates with the diffuse subtype. Virchows
Arch. 448:52–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung H, Jun KH, Jung JH, Chin HM and Park
WB: The expression of claudin-1, claudin-2, claudin-3 and claudin-4
in gastric cancer tissue. J Surg Res. 167:e185–e191. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YL, Zhang S, Wang GR and Chen YP:
Expression transformation of claudin-1 in the process of gastric
adenocarcinoma invasion. World J Gastroenterol. 14:4943–4948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohtani S, Terashima M, Satoh J, et al:
Expression of tight-junction-associated proteins in human gastric
cancer: downregulation of claudin-4 correlates with tumor
aggressiveness and survival. Gastric Cancer. 12:43–51. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Resnick MB, Gavilanez M, Newton E, et al:
Claudin expression in gastric adenocarcinomas: a tissue microarray
study with prognostic correlation. Hum Pathol. 36:886–892. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Michl P, Barth C, Buchholz M, et al:
Claudin-4 expression decreases invasiveness and metastatic
potential of pancreatic cancer. Cancer Res. 63:6265–6271.
2003.PubMed/NCBI
|
|
21
|
Agarwal R, D'Souza T and Morin PJ:
Claudin-3 and claudin-4 expression in ovarian epithelial cells
enhances invasion and is associated with increased matrix
metalloproteinase-2 activity. Cancer Res. 65:7378–7385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sano T and Aiko T: New Japanese
classifications and treatment guidelines for gastric cancer:
Revision concepts and major revised points. Gastric Cancer.
14:97–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Itoh M, Furuse M, Morita K, Kubota K,
Saitou M and Tsukita S: Direct binding of three tight
junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH
termini of claudins. J Cell Biol. 147:1351–1363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gottardi CJ, Arpin M, Fanning AS and
Louvard D: The junction-associated protein, zonula occludens-1,
localizes to the nucleus before the maturation and during the
remodeling of cell-cell contacts. Proc Natl Acad Sci USA.
93:10779–10784. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jechlinger M, Grunert S, Tamir IH, et al:
Expression profiling of epithelial plasticity in tumor progression.
Oncogene. 22:7155–7169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsukita S and Furuse M: Pores in the wall:
claudins constitute tight junction strands containing aqueous
pores. J Cell Biol. 149:13–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoevel T, Macek R, Swisshelm K and Kubbies
M: Reexpression of the TJ protein CLDN1 induces apoptosis in breast
tumor spheroids. Int J Cancer. 108:374–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuda Y, Semba S, Ueda J, et al: Gastric
and intestinal claudin expression at the invasive front of gastric
carcinoma. Cancer Sci. 98:1014–1019. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Resnick MB, Konkin T, Routhier J, Sabo E
and Pricolo VE: Claudin-1 is a strong prognostic indicator in stage
II colonic cancer: A tissue microarray study. Mod Pathol.
18:511–518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Satake S, Semba S, Matsuda Y, et al: Cdx2
transcription factor regulates claudin-3 and claudin-4 expression
during intestinal differentiation of gastric carcinoma. Pathol Int.
58:156–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xin S, Huixin C, Benchang S, et al:
Expression of Cdx2 and claudin-2 in the multistage tissue of
gastric carcinogenesis. Oncology. 73:357–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krämer F, White K, Kubbies M, Swisshelm K
and Weber BH: Genomic organization of claudin-1 and its assessment
in hereditary and sporadic breast cancer. Hum Genet. 107:249–256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tokés AM, Kulka J, Paku S, et al:
Claudin-1, -3 and -4 proteins and mRNA expression in benign and
malignant breast lesions: A research study. Breast Cancer Res.
7:R296–R305. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kominsky SL, Argani P, Korz D, et al: Loss
of the tight junction protein claudin-7 correlates with
histological grade in both ductal carcinoma in situ and invasive
ductal carcinoma of the breast. Oncogene. 22:2021–2033. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liebner S, Fischmann A, Rascher G, et al:
Claudin-1 and claudin-5 expression and tight junction morphology
are altered in blood vessels of human glioblastoma multiforme. Acta
Neuropathol. 100:323–331. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
40
|
Ming SC: Gastric carcinoma. A
pathobiological classification. Cancer. 39:2475–2485. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sugano H, Nakamura K and Kato Y:
Pathological studies of human gastric cancer. Acta Pathol Jpn.
32:(Suppl 2). 329–347. 1982.PubMed/NCBI
|
|
42
|
Adachi Y, Yasuda K, Inomata M, Sato K,
Shiraishi N and Kitano S: Pathology and prognosis of gastric
carcinoma: Well versus poorly differentiated type. Cancer.
89:1418–1424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park JM, Jang YJ, Kim JH, et al: Gastric
cancer histology: Clinicopathologic characteristics and prognostic
value. J Surg Oncol. 98:520–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu JL, Gao P, Wang ZN, et al:
Clinicopathological significance of claudin-4 in gastric carcinoma.
World J Surg Oncol. 11:1502013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kuo WL, Lee LY, Wu CM, et al: Differential
expression of claudin-4 between intestinal and diffuse-type gastric
cancer. Oncol Rep. 16:729–734. 2006.PubMed/NCBI
|
|
46
|
Lee SK, Moon J, Park SW, Song SY, Chung JB
and Kang JK: Loss of the tight junction protein claudin 4
correlates with histological growth-pattern and differentiation in
advanced gastric adenocarcinoma. Oncol Rep. 13:193–199.
2005.PubMed/NCBI
|
|
47
|
Kominsky SL, Vali M, Korz D, et al:
Clostridium perfringens enterotoxin elicits rapid and specific
cytolysis of breast carcinoma cells mediated through tight junction
proteins claudin 3 and 4. Am J Pathol. 164:1627–1633. 2004.
View Article : Google Scholar : PubMed/NCBI
|