Introduction
Increasing evidence has suggested that specific
types of cancer may contain their own stem-like cells, known as
cancer stem cells (CSCs), which have key roles in the initiation,
maintenance and recurrence of tumors (1–3). In
particular, attention has been paid to a subset of CSCs, termed the
side population (SP), which was identified by flow cytometry. These
SP cells are able to exclude the DNA binding dye, Hoechst 33342,
and are highly enriched for stem cells in numerous types of tissue
(4–6).
SP cells have been isolated from multiple solid tumors, and studies
have suggested that they may have significant roles in
tumorigenesis and cancer therapy. A SP of cells in nasopharyngeal
carcinoma (NPC) were found to exhibit characteristics of stem-like
cancer cells (7–12). However, the molecular mechanisms
underlying the modulation of these stem-like cell populations in
NPC have remained elusive.
Cellular proliferation is a critical process
underlying the growth, development and regeneration of eukaryotic
organisms, and appropriate control of the cell cycle is required
for the proliferation of normal cells (13,14).
Deregulation of the cell cycle is responsible for the aberrant cell
proliferation characteristic of cancer, and the loss of cell cycle
checkpoint control, which promotes genetic instability. The cell
cycle machinery, which functions as an integration point for
information transduced via upstream signaling networks, is a target
for potential diagnostic and therapeutic interventions (13–15).
Apoptosis is a physiological cell death process that has key
functions in normal development, as well as in the pathophysiology
of various diseases (16,17). A balance between the expression of
anti-apoptotic and pro-apoptotic factors underlies apoptosis; and
this balance may be altered by certain extracellular signals.
Significant alterations to this regulatory pathway may result in
the development of various diseases, including autoimmune and
neurodegenerative diseases, as well as certain types of cancer
(16–19). The
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/Akt pathway
is known to have key roles in cell proliferation, apoptosis and
survival in various cell types (20).
The PI3K/Akt signaling pathway has been shown to regulate
metastasis in multiple cancer cells (21,22).
In the present study, SP cells were identified in
the HK-1 NPC cell line, and the SP and NSP cells within this
population were sorted for analysis of the cell cycle and apoptosis
at differential time-points. In addition, the expression levels of
key molecules associated with the PI3K/Akt signaling pathway,
including PI3K and Akt, were evaluated by western blotting at the
corresponding time-points. The results of the present study may aid
the elucidation of the involvement of dysregulation of the PI3K/Akt
signaling pathway in cell cycle and apoptosis of SP cells in
NPC.
Materials and methods
Cell culture
HK-1 human NPC cells, a highly differentiated NPC
cell line, were provided by the Chinese University of Hong Kong
(Hong Kong, China), and cultured in RPMI-1640 (Gibco Life
Technologies, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco Life Technologies), 100 U/ml penicillin
and 100 µg/ml streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) at 37°C in a 5% CO2 incubator.
Identifying and sorting of SP cells by
flow cytometry (FCM)
HK-1 cells were cultured in RPMI-1640 with 10% FBS
until they reached ~70% confluence. The cells were trypsinized with
0.25% Trypsin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a 5%
CO2 cell incubator. Following centrifugation at 500 × g
for 5 min at room temperature, the single cell suspension was
resuspended in prewarmed RPMI-1640 culture medium containing 2% FBS
at a concentration of 1×106 cells/ml. Hoechst 33342 (10
mg/ml; Biotium Inc., Hayward, CA, USA) was added at a final
concentration of 5 µg/ml with or without 50 µmol/l verapamil (5
mmol/l; Sigma-Aldrich), an adenosine triphosphate binding cassette
(ABC) transporter inhibitor, to determine whether the fluorescent
efflux effect was altered. The cell suspensions were incubated in a
37°C circulating water bath for 90 min with gentle shaking every 15
min. Subsequently, the cells were washed twice with pre-cooled
phosphate-buffered saline (PBS; Solarbio Science and Technology
Co., Ltd., Beijing, China), resuspended in iced PBS with 2% FBS
buffer and 1 µg/ml propidium iodide (PI; Sigma-Aldrich) was added
to exclude dead cells. The entire protocol was performed in the
dark. A MoFlo™ XDP high-performance cell sorter (Beckman Coulter,
Brea, CA, USA) was used for analysis of the SP profile and
subsequent cell sorting. In the flow cytometry graphs, SP cells
displayed a low Hoechst staining intensity. Finally, SP and NSP
cells were sorted from the HK-1 cell line for further experiments.
Data and images were acquired using Summit v.5.2 software (Beckman
Coulter).
CSC marker assay in SP and NSP
cells
The total expression and cell surface expression
levels of various CSC markers were evaluated in sorted SP and NSP
cells by flow cytometric analysis (MoFlo™ XDP). CSC cell surface
marker expression was determined by washing freshly sorted SP and
NSP cells with PBS, prior to incubation with the following
fluorescent conjugated antibodies (5
µg/105-107 cells): ABC superfamily G member 2
(ABCG2)-phycoerythrin (PE) (eBioscience, Inc., San Diego, CA, USA),
CD133-PE [Miltenyi Biotec Technology & Trading (Shanghai) Co.,
Ltd. Shanghai, China], CD34-electron-coupled dye [ECD (PE-Texas
Red)] (Beckman Coulter), CD26-fluorescein isothiocyanate (FITC;
Beckman Coulter), cytokeratin 14-FITC (Biological, Swampscott, MA,
USA) for 1 h at 4°C. PE mouse immunoglobulin G (IgG)2b
isotype control (eBioscience, Inc.), mouse IgG2b isotype
control FITC (eBioscience, Inc.) and mouse IgG2b isotype
control ECD (eBioscience, Inc.) were used as negative controls for
non-specific background signals.
To determine the total expression of these CSC
markers, the sorted SP and NSP cells were fixed in 4%
paraformaldehyde (Solarbio Science and Technology Co., Ltd.) for 30
min, washed in PBS (3 × 30 sec) and incubated with 0.1% Triton-X
100 (Solarbio Science and Technology Co., Ltd.) for 20 min.
Subsequently, the cells were suspended in PBS and the corresponding
aforementioned antibodies were added according to the
manufacturer's instructions. Mouse IgG2b isotype control
antibodies were used as the negative control. The results were
analyzed by flow cytometry (MoFlo™ XDP).
RNA isolation and
reverse-transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis
Total RNA was extracted from the SP and NSP cells
using an RNeasy® kit (Qiagen, Inc., Valencia, CA, USA) and
complemetary (c)DNA synthesis was performed using the RevertAid
First Strand cDNA Synthesis kit (CWBio, Beijing, China) according
to the manufacturer's instructions. Subsequently, qPCR was
conducted using the GoTaq qPCR master mix (Promega Corp., Madison,
WI, USA). The primers used for RT-qPCR are presented in Table I. RT-qPCR was performed using the
BIO-RAD CFK96TM Real-Time System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The data were analyzed with Bio-Rad CFK Manager
2.0 software (Bio-Rad Laboratories, Inc.). Messenger (m)RNA
expression was assessed by evaluating the threshold cycle (CT)
values. GAPDH was used as an internal control.
 | Table I.Human-specific primer sequences used
in the present study. To avoid false positive signals originating
from DNA contamination, all human-specific polymerase chain
reaction primers were designed with known amplicon size, and where
possible flanking a region that contained a minimum of one
intron. |
Table I.
Human-specific primer sequences used
in the present study. To avoid false positive signals originating
from DNA contamination, all human-specific polymerase chain
reaction primers were designed with known amplicon size, and where
possible flanking a region that contained a minimum of one
intron.
| Target gene | Forward primer | Reverse primer |
|---|
| ABCG2 |
AGCTGCAAGGAAAGATCCAA |
TGCCCATCACAACATCATCT |
| CD133 |
TTGTGGCAAATCACCAGGTA |
TCAGATCTGTGAACGCCTTG |
| CD34 |
CAAGCCACCAGAGCTATTCC |
TCCACCGTTTTCCGTGTAAT |
| CD26 |
CAAATTGAAGCAGCCAGACA |
CACACTTGAACACGCCACTT |
| CK14 |
TTCTGAACGAGATGCGTGAC |
GCAGCTCAATCTCCAGGTTC |
Flow cytometric analysis of the cell
cycle
The SP and NSP cells of the HK-1 cell line were
sorted by flow cytometry as previously described, and divided into
two groups, respectively. One group was for analysis of the cell
cycle of sorted SP and NSP cells (0 h). The other was for the
analysis of the cell cycle of SP and NSP cells following culture in
RPMI-1640 supplemented with 10% fetal bovine serum for 24 or 48 h.
Cells were harvested at 0, 24 and 48 h and fixed in 70% ethanol at
4°C. The cells were then washed with cold PBS and stained with PI
in working solution (0.5 mg/ml RNase and 0.1 mg/ml PI in PBS). The
cell cycle distribution was determined by flow cytometric analysis
using a MoFloTM XDP High-Performance Cell Sorter
(Beckman Coulter) and the data were analyzed using Summit v.5.2
software.
Flow cytometric analysis of
apoptosis
The SP and NSP cells of the HK-1 cell line were
sorted by flow cytometry. The sorted cells were cultured in
RPMI-1640 supplemented with 10% FBS for 24 or 48 h, prior to
harvest. The cell apoptosis ratio was analyzed using an Alexa
Fluor® 488 Annexin V/Dead Cell Apoptosis kit (Invitrogen
Life Technologies, Carlsbad, CA, USA). Briefly, 5×105
cells were stained with Annexin V-FITC (5 µl) and 100 µg/ml PI (1
µl) in 100 µl binding buffer and incubated at room temperature for
15 min in the dark. Subsequently, 400 µl of binding buffer was
added and mixed gently, and the stained cells were analyzed using a
MoFlo™ XDP flow cytometer. The data were evaluated using Summit
v.5.2 software.
Western blot analysis
SP and NSP cells at 0 and 48 h following sorting,
were lysed in radioimmunoprecipitation buffer (CWBio, Beijing,
China) and total protein concentration was determined using a
Pierce® BCA Protein Assay kit (Thermo Fisher Scientific,
Waltham, MA, USA). Extracts containing 50 µg protein were separated
with 10% SDS-PAGE and electroblotted onto nitrocellulose membranes
(HyClone; GE Healthcare Life Sciences). The membranes were
inhibited using Tris-buffered saline/Tween-20 (25 mM Tris-HCl, 150
mM NaCl and 0.05% Tween-20; pH 7.5; Solarbio Science and Technology
Co., Ltd.) containing 5% non-fat milk followed by overnight
incubation at 4°C with the following primary antibodies: rabbit
anti-PI3K polyclonal antibody (catalog no. 4292; Cell Signaling
Technology, Inc., Danvers, MA, USA; dilution, 1:500); rabbit
anti-Akt polyclonal antibody, (catalog no. 9272; Cell Signaling
Technology, Inc.; dilution, 1:300) and mouse anti-14-3-3σ
monoclonal antibody (E-11; catalog no. sc-166473; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following three washes, the
membranes were incubated with horseradish peroxidase-conjugated
mouse anti-rabbit (catalog no. sc-2491; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA; dilution, 1:5,000) and goat anti-mouse
(catalog no. sc-2039; Santa Cruz Biotechnology, Inc.; dilution,
1:5,000) IgG secondary antibodies for 1 h at room temperature and
the signals were visualized using an enhanced chemiluminescence
detection system (Universal Hood II; Bio-Rad Laboratories, Inc.)
with Image Lab™ software. Anti-GAPDH antibody (Santa Cruz
Biotechnology, Inc.; 1:3,000) was used as a loading control.
Statistical analysis
Results were statistically analyzed by Student's
unpaired t-test using SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Putative CSC markers are
differentially expressed in SP and NSP cells of the HK-1 cell
line
The SP fraction of the NPC cell line, HK-1, was
determined using a flow cytometric SP discrimination assay. The
percentage of SP cells was found to be 3.65±1.51%. The proportion
of SP cells was significantly blocked by verapamil (Fig. 1A and B). Subsequently, the
MoFloTM XDP High-Performance Cell sorter was used to
isolate the SP and NSP cells from the HK-1 cells and the cell
surface expression levels of CSC markers were evaluated. The
expression levels of putative CSC markers, including ABCG2, CD133,
CD34 and CD26, were higher in SP cells compared with those of NSP
cells (P<0.05). ABCG2 expression was higher in SP cells (4.27%)
than NSP cells (0.41%). CD133 was 3.28 and 1.90% in SP and NSP
cells, respectively. CD34 and CD26 exhibited analogous expression
patterns. However, no differential expression of CK14 was detected
between SP and NSP cells (Fig.
1C).
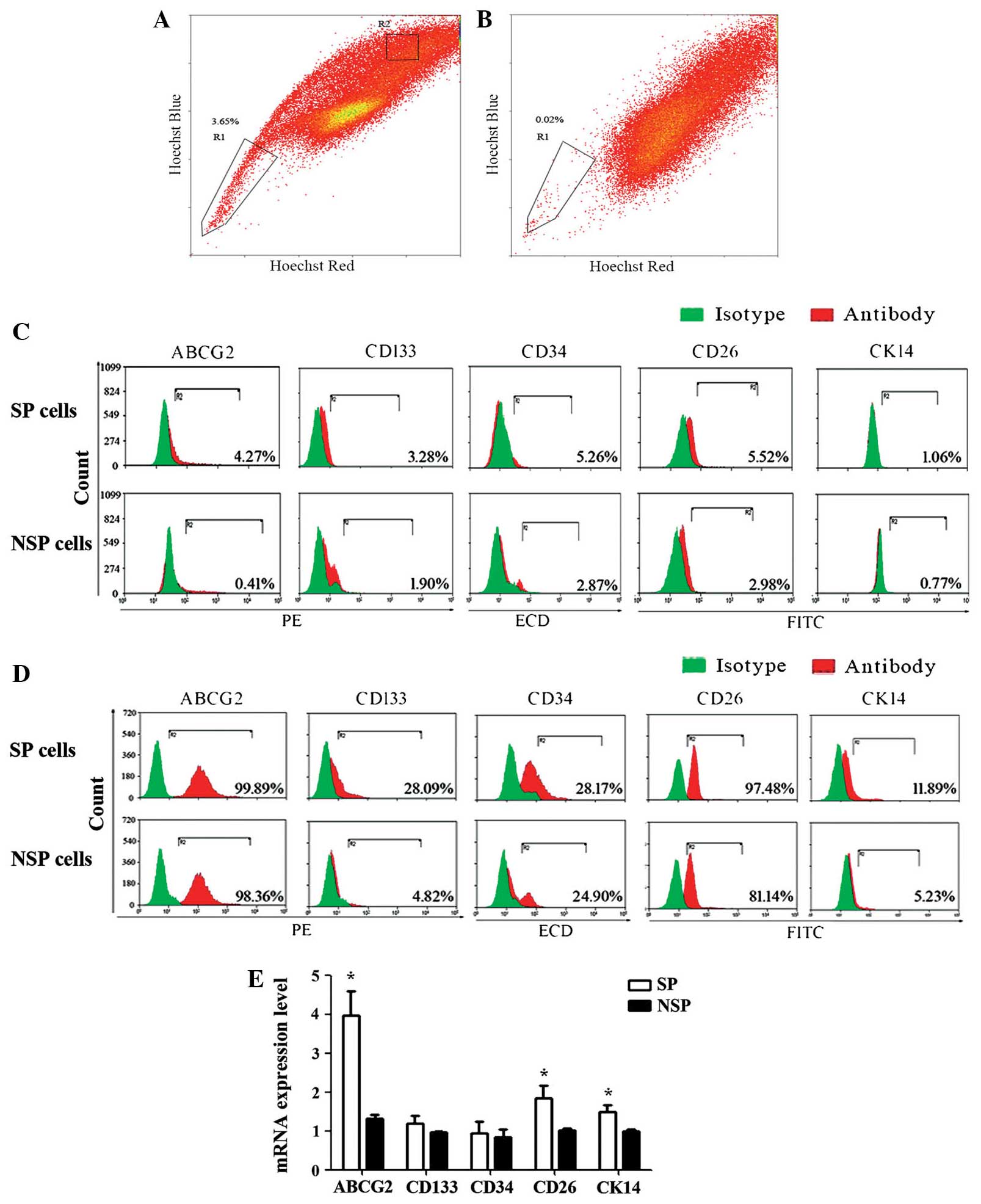 | Figure 1.Human nasopharyngeal carcinoma cell
lines contain a fraction of SP cells and CSC-associated markers are
differentially expressed in SP and NSP cells. (A) Scatter-blot
analysis of HK-1 cells stained with Hoechst 33342. (B) Scatter-blot
analysis of HK-1 cells stained with Hoechst 33342 plus verapamil
treatment. SP and NSP cells are indicated in boxes R1 and R2,
respectively. (C) CSC marker, ABCG2, CD133, CD34, CD26 and CK14,
expression on the cell surface of sorted SP and NSP cells was
evaluated by flow cytometry. (D) Total expression levels of CSC
markers, ABCG2, CD133, CD34, CD26 and CK14, in SP and NSP cells.
Flow cytometric analysis revealed that CSCs markers were highly
expressed in SP cells. (E) Relative mRNA expression levels of
ABCG2, CD133, CD34, CD26 and CK14 in SP and NSP cells were
determined by reverse transcription-quantitative polymerase chain
reaction. Three independent experiments were performed. The results
are shown as the mean ± standard error and refer to freshly sorted
cells (0 h). *P<0.05 vs. NSP cells. SP, side population; CSC,
cancer stem cell; NSP, non-side population; mRNA, messenger RNA;
PE, phycoerythrin; ECD, electron-coupled dye (PE-Texas Red); FITC,
fluorescein isothiocyanate; ABCG2, adenosine triphosphate-binding
cassette transporter superfamily G member 2. |
Protein and mRNA expression levels of
putative CSC markers differ between SP and NSP cells in NPC
Subsequently, the total protein expression levels of
the putative CSC markers were examined in sorted SP and NSP cells
by flow cytometry. The results demonstrated that CD133, CD34, and
CK14 expression was higher in SP cells compared with that of NSP
cells. Expression levels were 28.09, 28.17 and 11.89% in SP cells,
and 4.82, 24.90 and 5.23% in NSP cells, respectively. The fraction
of ABCG2 and CD26 expression was high in SP and NSP cells (Fig. 1D). In addition, the expression levels
of these markers in SP and NSP cells were evaluated by RT-qPCR. It
was demonstrated that there were significant differences in the
mRNA expression levels of ABCG2, CD26, and CK14 between SP and NSP
cells of the HK-1 cell line (P<0.05). However, there was no
significant difference in the expression of CD133 and CD34
(Fig. 1E). In SP and NSP cells, the
mRNA and protein expression levels of CSC markers, including CD133
and CD34, is not consistent.
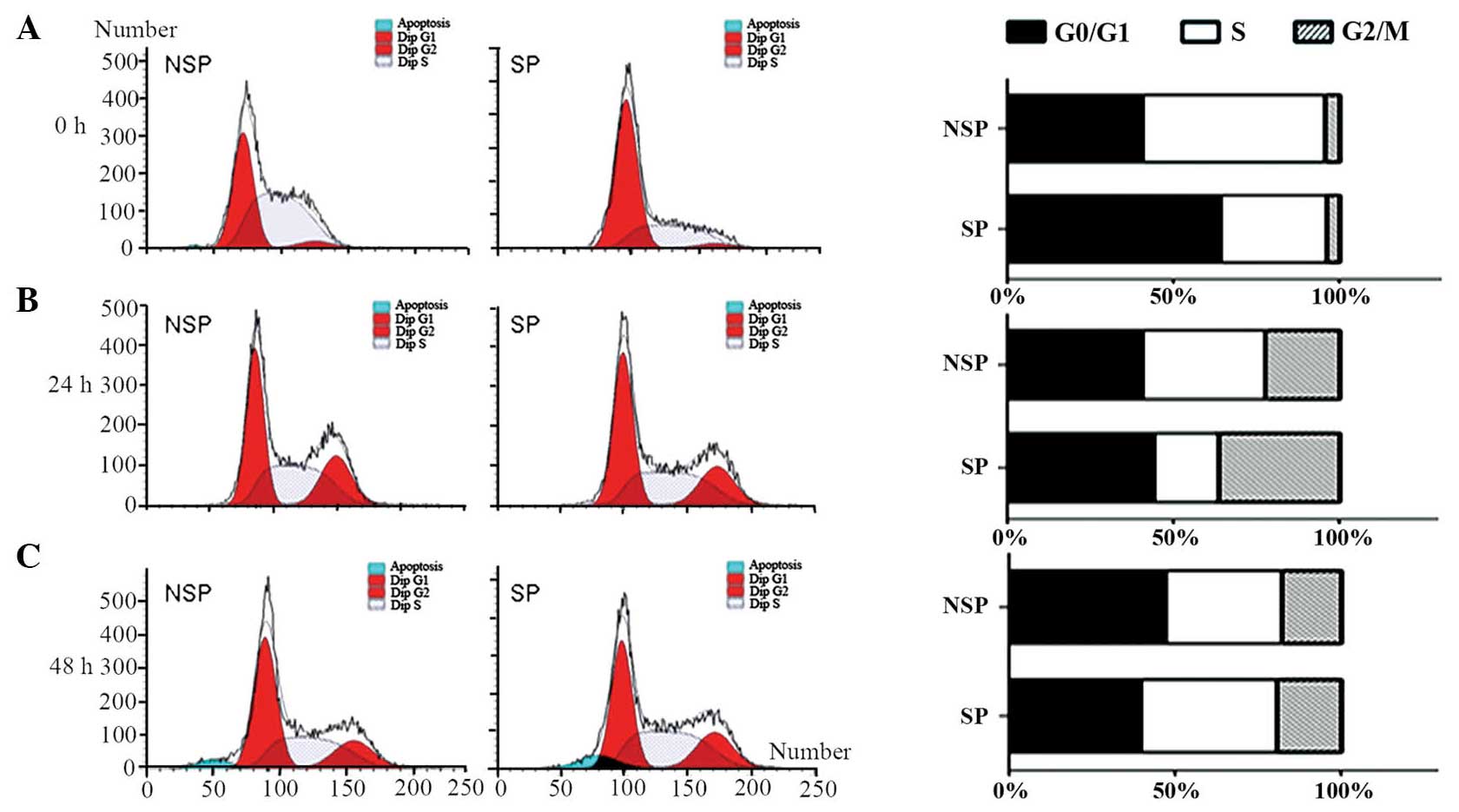
Cell cycle distribution differs
between freshly sorted SP and NSP cells of the HK-1 cell line
Cell cycle progression was compared between SP and
NSP cells 0, 24 and 48 h following sorting by flow cytometric
analysis. When cells were freshly sorted, SP cells revealed a
significant increase in the proportion of cells in G0/G1 phase and
a reduction in the percentage of cells in S phase (Fig. 2A). The percentages of G0/G1, S and
G2/M phases were 64, 32.22 and 3.78%, respectively. By contrast,
the majority of NSP cells were in the proliferative phase, and the
percentages of cells in G0/G1, S and G2/M phases were 40.76, 54.83
and 4.41%, respectively. Immediately following sorting, SP and NSP
cells demonstrated significant differences in cell cycle
distribution. However, following 24 h of culture, the differences
in cell cycle distribution between SP and NSP cells were abrogated.
The percentages of cells in G0/G1, S and G2/M phases in SP and NSP
cells at 24 h were 44.09, 19.55 and 36.37 vs. 40.5%, 37.27% and
22.23%, respectively (Fig. 2B). In
accordance, the cell cycle distribution of SP and NSP cells 48 h
following sorting also coincided. The percentages of cells in
G0/G1, S and G2/M phase in SP and NSP cells at 48 h were 39.5%,
41.05% and 19.45% vs. 47.17%, 35.3% and 17.53%, respectively
(Fig. 2C). These results suggested
that the differences in cell cycle distribution between SP and NSP
cells converged with time.
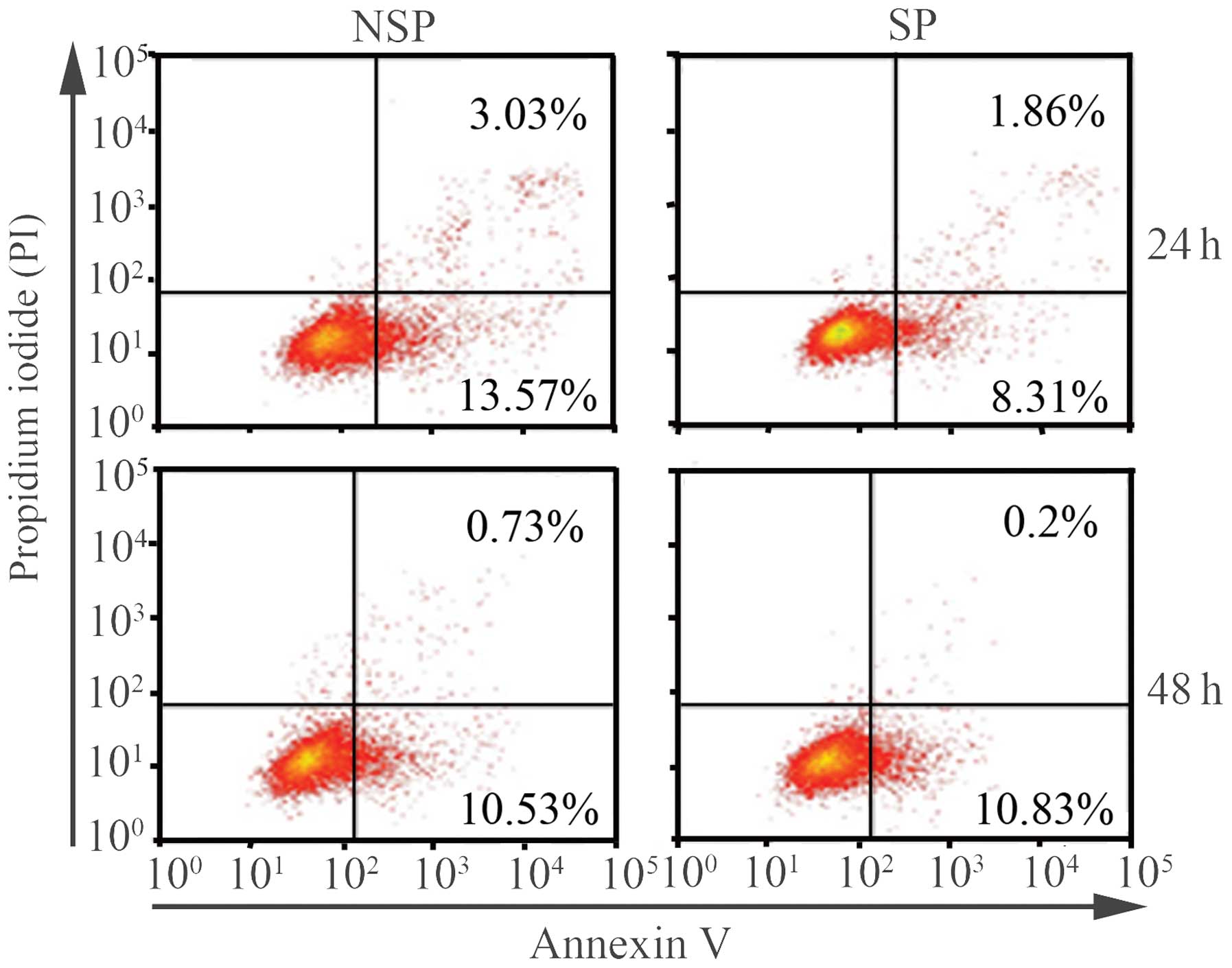
The apoptotic ratio of NSP cells is
higher than that of SP cells 24 h following sorting
Given that SP cells were found to be rich in CSCs
markers compared with NSP cells, whether SP cells had a lower
apoptotic ratio than NSP cells was determined. Annexin V-FITC and
PI staining were used to analyze the percentage of apoptotic cells
in SP and NSP cells at various time-points by flow cytometry. The
apoptotic ratio of NSP cells was higher than that of SP cells 24 h
following sorting, without any external stimuli to the cells. The
apoptotic ratios of SP and NSP cells were 10.17 and 16.6%,
respectively. As observed in cell cycle distribution, no
significant differences in the apoptotic proportion were detected
between SP and NSP cells 48 h following sorting. The apoptotic
ratios of SP and NSP cells at 48 h were 11.03 and 11.36%,
respectively (Fig. 3).
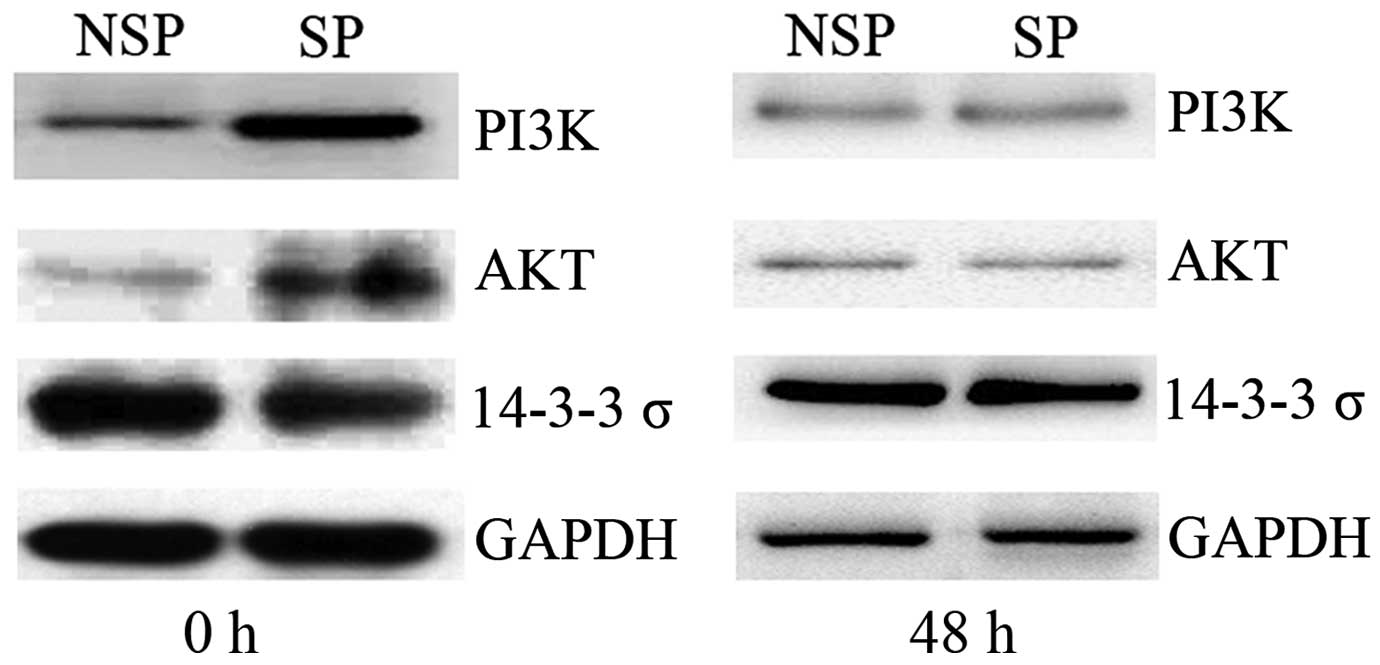
Dysregulation of The PI3K/Akt
signaling pathway is dysregulated in SP cells of HK-1 cells
immediately following sorting
To elucidate the potential mechanism underlying the
mediation of the cell cycle and apoptosis in SP cells, the
expression levels of key molecules associated with the PI3K/Akt
signaling pathway were detected by western blot analysis.
Immediately following cell sorting by flow cytometry (0 h), PI3K
and Akt expression levels were upregulated in SP cells compared
with those of NSP cells of the HK-1 cell line, whereas 14-3-3σ
protein expression was downregulated in SP cells. Once the sorted
cells had been cultured for 48 h, no significant difference in
PI3K, Akt or 14-3-3σ protein was detected between SP and NSP cells
of HK-1 cells (Fig. 4). Combined with
the aforementioned results of cell cycle and apoptosis analysis, it
was hypothesized that dysregulation of the PI3K/Akt signaling
pathway was associated with the alterations in the cell cycle and
apoptosis of SP cells in NPC.
Discussion
NPC is an endemic disease with an incidence rate of
15–50/100,000 individuals in southern China and Southeast Asia, and
represents one of the most significant public health issues in
these regions (23). Although studies
regarding the tumorigenesis of NPC have previously been published
(24–29), the molecular basis for NPC is not
fully understood. Recent studies have demonstrated that CSCs have a
significant role in the pathophisiology of head and neck squamous
cell carcinomas (30–32). In particular, research has focused on
a specific subset of CSCs, termed SP cells, which were identified
by FCM. Therefore, elucidation of the molecular mechanisms of SP in
NPC is urgently required for the improvement of clinical diagnosis
and therapy.
In the present study, the fraction of SP cells in
the HK-1 NPC cell line, was found to be 3.65±1.51%. The proportion
of SP cells in this cell line was significantly decreased following
verapamil treatment. SP cells expressed high levels of CSC markers
compared with those of NSP cells. For example, ABCG2 expression was
higher in SP cells (4.27%) than in NSP cells (0.41%), and CD133
expression was 3.28 and 1.90% in SP and NSP cells, respectively.
Wang et al (7) revealed that
SP cells represented ~2.6% of the total cells in the NPC cell line,
CNE-2. Another four human NPC cell lines, C-666-1, SUNE-1, HONE-1
and CNE-1, were also found to contain small subpopulations of SP
cells and their proportions were 0.1, 6.8, 1.8 and 0.7%,
respectively. Certain putative CSC markers are highly expressed in
SP cells (7–9), and the results of these studies
corroborate the results presented in the present study.
In order to reveal the characteristics of the cell
cycle and apoptosis in SP cells, the cells were evaluated at
differential time-points following sorting (0, 24 or 48 h). The
results of the present study revealed that freshly sorted SP cells
demonstrated a significant increase in the number of cells in G0/G1
phase. However, following 48 h of culture, differences in cell
cycle distribution between SP and NSP cells were abrogated. In
addition, the apoptotic ratio of NSP cells was higher than that of
SP cells 24 h following sorting, whereas no significant differences
were detected following 48 h of culture. We hypothesize that
culturing the SP and NSP cells in complete medium after sorting may
have caused the SP cells to differentiate, subsequently losing
their stem cell properties. Previous studies have revealed that
normal and neoplastic stem cells obtained from neural and
epithelial organs only exhibit initial tumor-specific properties
when cultured in serum-free medium containing epidermal growth
factor (EGF) and fibroblast growth factor (FGF)-2 (33–35). In
addition, adherent cells expanded in Laminin-coated culture plates
in serum free medium containing N2-supplement, EGF and basic FGF
maintain initial tumor-specific properties (36). However, when the cells were cultured
in traditional complete medium, stem cells differentiated and lost
their stem cell phenotype (37,38). In
contrast to embryonic stem cells, a characteristic feature of adult
stem cells is their proliferative quiescence. It is widely accepted
that this quiescent state is a functionally significant feature of
adult stem cells (39–41).
To reveal the potential mechanisms underlying the
cell cycle and apoptosis in SP cells, the expression levels of key
molecules associated with the PI3K/Akt signaling pathway were
detected. PI3K and Akt expression was upregulated, while 14-3-3σ
protein expression was downregulated in freshly sorted SP cells (0
h). However, there was no significant difference in the expression
of these molecules in SP and NSP cells following 48 h of culture.
14-3-3σ, a potential tumor suppressor protein, is able to
negatively regulate cell cycle progression by inducing G2-M phase
arrest (42,43). It has previously been demonstrated
that 14-3-3σ is transactivated by p53 in response to DNA damage
and, in turn, interacts with p53 and positively regulates p53
activity (44). p53 is known to be
involved in mediating the complex response to ionizing radiation,
inducing irreversible growth arrest and apoptosis (45). The results of the present study are in
accordance with those of previous reports.
In conclusion, the results of the present study
suggested that dysregulation of the PI3K/Akt signaling pathway may
be associated with mediation of the cell cycle and apoptosis of SP
cells in NPC. However, elucidation of the detailed mechanisms
underlying this process requires further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81272975), the Key Project
of Hunan Provincial Natural Science Foundation (no. 12JJ2044), the
Project of Hunan Provincial Natural Science Foundation (no.
12JJ3121), the Project of Hunan Provincial Development and Reform
Commission and the Planned Science and Technology Project of Hunan
Province (nos. 2010FJ3088 and 2012FJ2014).
Abbreviations:
|
CSC
|
cancer stem cell
|
|
NPC
|
nasopharyngeal carcinoma
|
|
SP
|
side population
|
|
NSP
|
non side population
|
|
ABCG2
|
adenosine triphosphate-binding
cassette transporter superfamily G member 2
|
|
PI3K
|
phosphatidylinositol-4,5-bisphosphate
3-kinase
|
|
FCM
|
flow cytometry
|
References
|
1
|
Kristoffersen K, Villingshøj M, Poulsen HS
and Stockhausen MT: Level of Notch activation determines the effect
on growth and stem cell-like features in glioblastoma multiforme
neurosphere cultures. Cancer Biol Ther. 14:625–637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cetin I and Topcul M: Cancer stem cells in
oncology. J BUON. 17:644–648. 2012.PubMed/NCBI
|
|
3
|
Muñoz P, Iliou MS and Esteller M:
Epigenetic alterations involved in cancer stem cell reprogramming.
Mol Oncol. 6:620–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsutake N, Iwao A, Nagai K, et al:
Characterization of side population in thyroid cancer cell lines:
Cancer stem-like cells are enriched partly but not exclusively.
Endocrinology. 148:1797–1803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao JX, Cui YX, Long ZJ, et al:
Pluripotency-associated genes in human nasopharyngeal carcinoma
CNE-2 cells are reactivated by a unique epigenetic
sub-microenvironment. BMC Cancer. 10:682010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiraga T, Ito S and Nakamura H: Side
population in MDA-MB-231 human breast cancer cells exhibits cancer
stem cell-like properties without higher bone-metastatic potential.
Oncol Rep. 25:289–296. 2011.PubMed/NCBI
|
|
7
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kong QL, Hu LJ, Cao JY, et al:
Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal
transition and increases the number of side population stem-like
cancer cells in nasopharyngeal carcinoma. PLoS Pathog.
6:e10009402010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang Y, Zhong Z, Huang Y, Deng W, Cao J,
Tsao G, Liu Q, Pei D, Kang T and Zeng YX: Stem-like cancer cells
are inducible by increasing genomic instability in cancer cells. J
Biol Chem. 285:4931–4940. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang HB, Ren CP, Yang XY, Wang L, Li H,
Zhao M, Yang H and Yao KT: Identification of label-retaining cells
in nasopharyngeal epithelia and nasopharyngeal carcinoma tissues.
Histochem Cell Biol. 127:347–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Peng J, Zhang H, Zhu Y, Wan L,
Chen J, Chen X, Lin R, Li H, Mao X and Jin K: Notch1 signaling is
activated in cells expressing embryonic stem cell proteins in human
primary nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg.
39:157–166. 2010.PubMed/NCBI
|
|
12
|
Xia H, Cheung WK, Sze J, Lu G, Jiang S,
Yao H, Bian XW, Poon WS, Kung HF and Lin MC: miR-200a regulates
epithelial-mesenchymal to stem-like transition via ZEB2 and
beta-catenin signaling. J Biol Chem. 285:36995–37004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diaz-Moralli S, Tarrado-Castellarnau M,
Miranda A and Cascante M: Targeting cell cycle regulation in cancer
therapy. Pharmacol Ther. 138:255–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams GH and Stoeber K: The cell cycle
and cancer. J Pathol. 226:352–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aarts M, Linardopoulos S and Turner NC:
Tumour selective targeting of cell cycle kinases for cancer
treatment. Curr Opin Pharmacol. 13:529–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sankari SL, Masthan KM, Babu NA,
Bhattacharjee T and Elumalai M: Apoptosis in cancer - an update.
Asian Pac J Cancer Prev. 13:4873–4878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zielinski RR, Eigl BJ and Chi KN:
Targeting the apoptosis pathway in prostate cancer. Cancer J.
19:79–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Beesoo R, Neergheen-Bhujun V, Bhagooli R
and Bahorun T: Apoptosis inducing lead compounds isolated from
marine organisms of potential relevance in cancer treatment. Mutat
Res Fundam Mol Mech Mutagen. 768:84–97. 2014. View Article : Google Scholar
|
|
19
|
Jia LT, Chen SY and Yang AG: Cancer gene
therapy targeting cellular apoptosis machinery. Cancer Treat Rev.
38:868–876. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiao M, Sheng S and Pardee AB: Metastasis
and AKT activation. Cell Cycle. 7:2991–2996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho JH: An epidemiologic and clinical study
of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
4:182–198. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Wang W, Zheng D, Peng S, Xiong W,
Ma J, Zeng Z, Wu M, Zhou M, Xiang J, et al: Risk of nasopharyngeal
carcinoma associated with polymorphic lactotransferrin haplotypes.
Med Oncol. 29:1456–1462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Zeng Z, Zhang W, Xiong W, Wu M,
Tan Y, Yi W, Xiao L, Li X, Huang C, et al: Lactotransferrin, a
candidate tumor suppressor, deficient expression in human
nasopharyngeal carcinoma and inhibits NPC cell proliferation by
modulating the mitogen-activated protein kinase pathway. Int J
Cancer. 123:2065–2072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou Y, Zeng Z, Zhang W, Xiong W, Li X,
Zhang B, Yi W, Xiao L, Wu M, Shen S, et al: Identification of
candidate molecular markers of nasopharyngeal carcinoma by
microarray analysis of subtracted cDNA libraries constructed by
suppression subtractive hybridization. Eur J Cancer Prev.
17:561–571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zeng Z, Zhou Y, Xiong W, Luo X, Zhang W,
Li X, Fan S, Cao L, Tang K, Wu M and Li G: Analysis of gene
expression identifies candidate molecular markers in nasopharyngeal
carcinoma using microdissection and cDNA microarray. J Cancer Res
Clin Oncol. 133:71–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan
SQ, Li XL, Luo XM, Wu MH, Yang YX, Huang C, et al: Gene expression
profiling of nasopharyngeal carcinoma reveals the abnormally
regulated WNT signaling pathway. Hum Pathol. 38:120–133. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng Z, Zhou Y, Zhang W, Li X, Xiong W,
Liu H, Fan S, Qian J, Wang L, Li Z, et al: Family-based association
analysis validates chromosome 3p21 as a putative nasopharyngeal
carcinoma susceptibility locus. Genet Med. 8:156–160. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Szafarowski T and Szczepanski MJ: Cancer
stem cells in head and neck squamous cell carcinoma. Otolaryngol
Pol. 68:105–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qian X, Wagner S, Ma C, Coordes A, Gekeler
J, Klussmann JP, Hummel M, Kaufmann AM and Albers AE: Prognostic
significance of ALDH1A1-positive cancer stem cells in patients with
locally advanced, metastasized head and neck squamous cell
carcinoma. J Cancer Res Clin Oncol. 140:1151–1158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han J, Fujisawa T, Husain SR and Puri RK:
Identification and characterization of cancer stem cells in human
head and neck squamous cell carcinoma. BMC Cancer. 14:1732014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
34
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li
S and Yao K: Aldehyde dehydrogenase 1, a functional marker for
identifying cancer stem cells in human nasopharyngeal carcinoma.
Cancer Lett. 330:181–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pollard SM, Yoshikawa K, Clarke ID, Danovi
D, Stricker S, Russell R, Bayani J, Head R, Lee M, Bernstein M, et
al: Glioma stem cell lines expanded in adherent culture have
tumor-specific phenotypes and are suitable for chemical and genetic
Screens. Cell Stem Cell. 4:568–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ,
Shim HE, Yoon S, Baek SY, Kim BS, Kang CD and Oh SO: Cancer spheres
from gastric cancer patients provide an ideal model system for
cancer stem cell research. Cell Mol Life Sci. 68:3589–3605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Singh S, Trevino J, Bora-Singhal N,
Coppola D, Haura E, Altiok S and Chellappan SP: EGFR/Src/Akt
signaling modulates Sox2 expression and self-renewal of stem-like
side-population cells in non-small cell lung cancer. Mol Cancer.
11:732012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Orford KW and Scadden DT: Deconstructing
stem cell self-renewal: Genetic insights into cell-cycle
regulation. Nat Rev Genet. 9:115–128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horsley V, Aliprantis AO, Polak L,
Glimcher LH and Fuchs E: NFATc1 balances quiescence and
proliferation of skin stem cells. Cell. 132:299–310. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Malumbres M: Physiological relevance of
cell cycle kinases. Physiol Rev. 91:973–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mhawech P: 14-3-3 proteins - an update.
Cell Res. 15:228–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Laronga C, Yang HY, Neal C and Lee MH:
Association of the cyclin-dependent kinases and 14-3-3σ negatively
regulates cell cycle progression. J Biol Chem. 275:23106–23112.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang HY, Wen YY, Chen CH, Lozano G and Lee
MH: 14-3-3σ positively regulates p53 and suppresses tumor growth.
Mol Cell Biol. 23:7096–7107. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gudkov AV and Komarova EA: The role of p53
in determining sensitivity to radiotherapy. Nat Rev Cancer.
3:117–129. 2003. View
Article : Google Scholar : PubMed/NCBI
|