Introduction
Breast cancer is the most frequently-diagnosed type
of cancer in women and results in over half a million mortalities
worldwide each year (1).
Advanced-stage breast cancer can metastasize to other tissues of
the body, such as the lymph nodes, reducing the effectiveness of
radiation therapy or chemotherapy. Metastatic disease is
responsible for >90% of cancer-associated mortalities (2). Thus, controlling or even reversing the
process of metastasis would be a significant breakthrough in the
treatment of cancer.
Metastasis is a complex cascade process, in which
tumor cell invasion through the extracellular matrix (ECM) is one
of the key early events. Matrix metalloproteinases (MMPs) are
zinc-dependent endopeptidases that are able to degrade numerous
types of ECM proteins (3). At
present, 24 types of MMP have been identified, and MMP-9 (also
termed gelatinase-B, a 92-kDa type IV collagenase) is considered to
be essential in tumor invasion and migration, particularly in
breast cancer (4). The expression and
secretion of MMP-9 is regulated by various stimuli, including
inflammatory cytokines, growth factors, tumor necrosis factor-α and
12-O-tetradecanoylphorbol-13-acetate (TPA) (5,6). TPA is a
well-known inflammatory stimulus that induces tumor invasion and
metastasis by directly activating protein kinase C isoforms
(7). The activity of MMP-9 is
controlled primarily at the transcriptional level. Nuclear
factor-κB (NF-κB) is a transcription factor that binds to the
promoter region of MMP-9 to regulate the expression of MMP-9
(8).
Hispolon was initially isolated as a yellow pigment
from Inonotus hispidus and then identified in a number of
traditional medicinal mushrooms, including Phellinus linteus
and Phellinus igniarius, which have been used to treat
various cancer types in East Asia (9–11).
Hispolon has been reported to possess analgesic, anti-inflammatory
and anticancer activities (10,12–14). A
previous study demonstrated that hispolon may induce apoptosis in
breast and bladder cancer cells via the MDM2-recruited ERK1/2
activity, and downregulate the level of MDM2 through the
chaperone-mediated autophagy pathway (15,16). Human
gastric cancer cells, rather than normal gastric cells, were
selectively killed by hispolon due to the abrogation of the
glutathione antioxidant system and the resultant excessive
accumulation of reactive oxygen species (10). However, only a limited number of
studies have investigated the antimetastatic effects of hispolon,
while the underlying mechanism of the process has not been
elucidated. In the present study, TPA-treated MDA-MB-231 human
breast cancer cells were used to evaluate the antimetastatic
potential of hispolon. In addition, the effect of hispolon
treatment on the secretion and expression of MMP-9 at the
transcriptional and translational levels, as well as the effect on
the NF-κB signaling pathway, were investigated. Finally, the study
examined whether hispolon can be used as an antimetastatic
drug.
Materials and methods
Reagents and chemicals
Hispolon was synthesized as previously described
(10,17), and its purity was detected on the
basis of the NMR and mass spectra. A stock solution of 40 mM
hispolon was prepared in dimethyl sulfoxide (DMSO) and diluted to
the appropriate concentrations prior to use. The final
concentration of DMSO was kept below 0.1% (v/v) in all assays.
Doxorubicin (Dox), MTT, DMSO and Matrigel were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Bay 11–7082 (an NF-κB
inhibitor) and TPA were purchased from Beyotime Institute of
Biotechnology (Haimen, Jiangsu, China). Primary antibodies
[polyclonal rabbit anti-human MMP-9 (catalog no., #3852);
monoclonal mouse IgG1 anti-human IκBα (catalog no.,
#4814); monoclonal rabbit IgG anti-human phospho-IκBα (catalog no.,
#2859); monoclonal rabbit IgG anti-human NF-κB p65 (catalog no.,
#8242); monoclonal rabbit IgG anti-human phospho-NF-κB p65 (catalog
no., #3033); monoclonal rabbit IgG anti-human histone H3 (catalog
no., #4499); and monoclonal rabbit IgG anti-human β-actin (catalog
no., #4970)], and horseradish peroxidase-conjugated secondary
antibodies [goat anti-rabbit IgG (catalog no., #7074) and horse
anti-mouse IgG (catalog no., #7076)] were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture and reagents
The human MDA-MB-231 breast cancer cell line was
obtained from the Institute of Biochemistry and Cell Biology
(Chinese Academy of Sciences, Shanghai, China). Cells were cultured
in Dulbecco's modified Eagle's medium (Gibco Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco
Life Technologies), 100 units/ml penicillin and 100 units/ml
streptomycin (Beyotime Institute of Biotechnology). All cells were
cultured in a humidified cell incubator with an atmosphere of 5%
CO2 at 37°C and subcultured with 0.25% trypsin and 0.02%
EDTA (Beyotime Institute of Biotechnology).
Cell viability assay
Briefly, cells were seeded in 96-well microtiter
plates at a density of 5×103 cells/well and left for 24
h to adhere. The cells were cultured with the indicated
concentrations of hispolon (0, 5, 10, 20, 40 or 60 µM) in the
absence or presence of 160 nM TPA for 24 h. Next, the cells were
incubated with MTT (0.5 mg/ml) for an additional 4 h at 37°C. The
resulting formazan precipitate was dissolved in 150 µl DMSO and the
absorbance was measured at 490 nm with a Sunrise microplate reader
(Tecan Group Ltd, Männedorf, Switzerland). Dox was used as a
positive control for all the assays. Each experiment was repeated
at least three times.
Matrigel-based Transwell invasion
assay
Cell invasion assays were carried out as previously
described (5). Briefly, 24-well
Transwell chambers with 8 µm polycarbonate nucleopore filters
(Corning Incorporated, Corning, NY, USA) were coated with 20
µg/well Matrigel (Sigma-Aldrich). A total of 2×105 cells
were seeded onto the upper part of the Matrigel-coated filter, and
serum-free medium supplemented with 0–40 µM hispolon was incubated
in the lower part for 2 h prior to the addition of 160 nM TPA.
Following incubation at 37°C for 24 h, the cells that had invaded
on the lower side of the membrane were fixed with 4%
paraformaldehyde for 15 min, and then stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology)for 10 min.
Subsequently, the stained cells were counted under a light
microscope (BX51; Olympus Corporation, Tokyo, Japan). The
percentage inhibition of invasive cells was expressed relative to
the TPA alone-treated group. Each experiment was repeated at least
three times.
Wound-healing assay
MDA-MB-231 cells were seeded in a 24-well plate
(10,000 cells/well) and grown to 80–90% confluence. The monolayer
of cells was scratched with white pipette tips, washed twice with
phosphate-buffered saline and replaced with serum-free medium.
Next, the cells were treated with the indicated concentrations of
hispolon (0, 10, 20 or 40 µM) for 2 h and incubated with TPA for
another 24 h. Images of the cell cultures were captured using a
light microscope (BX51, Olympus Corporation, Tokyo, Japan) at 0 and
24 h. The migration of cells was assessed by counting the cells
that crossed the blank lines and the percentage inhibition of the
migration of the cells was expressed relative to the TPA
only-treated group. Each experiment was repeated at least three
times.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
A total of 5×105 MDA-MB-231 cells/well
were seeded in a 6-well plate and pretreated with hispolon at the
indicated concentrations (0, 10, 20 or 40 µM) for 2 h, followed by
TPA stimulation for a further 24 h. Total RNA was isolated with
TRIzol reagent (Sangon Biotech Co., Ltd., Shanghai, China)
according to the manufacturer's instructions, and the concentration
of RNA was measured using a UV-2550 spectrophotometer (Shimadzu
Corporation, Kyoto, Japan). A total of 2 µg RNA was converted to
complementary DNA (cDNA) using a PrimeScript 1st Strand cDNA
Synthesis kit (Takara Bio, Inc., Otsu, Japan) and the cDNA was
amplified with Takara rTaq (Takara Bio, Inc.). The amplification
conditions were one cycle of denaturation at 95°C for 3 min,
followed by 33 cycles of 94°C for 15 sec, 60°C for 30 sec and 72°C
for 60 sec, with one extension cycle at 72°C for 10 min. The primer
sequences used were as follows: MMP-9 forward,
5′-CAACATCACCTATTGGATCC-3′, and reverse, 5′-CTGTAGAGTCTCTCGCT-3′;
GAPDH forward, 5′-CATGTAGGCCATGAGGTCCACCAC-3′, and reverse,
5′-TGAAGGTCGGTGTGAACGGATTTGGC-3′. The PCR products were subjected
to electrophoresis on a 1% (w/v) agarose gel. The bands were
visualized by GoldView staining (SBS Genetech Co., Ltd., Beijing,
China).
Western blot analysis
Cell lysates were obtained and protein
concentrations were determined using the BCA protein assay kit
(Pierce Biotechnology, Inc., Rockford, IL, USA). Equal quantities
of proteins (30–100 µg protein/lane) were subjected to 10–15%
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). The membranes were
blocked in 5% (w/v) nonfat dry milk for 1 h and incubated with
polyclonal rabbit anti-human MMP-9 (1:1,000), monoclonal mouse
anti-human IκBα (1:1,000), monoclonal rabbit anti-human p-IκBα
(1:1,000), monoclonal rabbit anti-human p65 (1:2,000), monoclonal
rabbit anti-human p-p65 (1:1,000), monoclonal rabbit anti-human
histone H3 (1:2,000), monoclonal rabbit anti-human GAPDH (1:1,000)
and monoclonal rabbit anti-human β-actin (1:1,000) primary
antibodies at 4°C overnight. The membranes were then incubated with
secondary goat anti-rabbit (1:2,000) or horse anti-mouse (1:8,000)
IgG antibodies for 1 h. Bands were detected using the SuperSignal™
West Pico Chemiluminescent Substrate (Thermo Fisher Scientific,
Waltham, MA, USA).
Gelatin zymography
MDA-MB-231 cells were incubated in serum-free medium
pretreated with the indicated concentrations of hispolon or Bay
11–7082 for 2 h, followed by addition of 160 nM TPA to the medium
and incubation for 24 h. Next, the conditioned medium was
collected. Equal volumes were mixed with non-reducing sample buffer
and subjected to 10% SDS-PAGE containing 0.1% (w/v) gelatin at 4°C.
The gel was washed four times with denaturing buffer (2.5% Triton
X-100, pH 7.6, 50 mM Tris-HCl, 5 mM CaCl2 and 1 µM
ZnCl2) and equilibrated twice in developing buffer (pH
7.6, 50 mM Tris-HCl, 5 mM CaCl2 and 1 µM
ZnCl2). Subsequently, the gel was incubated with the
fresh developing buffer for 24 h at 37°C and stained with 0.05%
coomassie blue R-250 (Sangon Biotech Co., Ltd.). The MMP-9
gelatinolytic activity was measured by clear bands against the blue
background.
Electrophoretic mobility shift assay
(EMSA)
MDA-MB-231 cells were pretreated with 0–40 µM
hispolon for 2 h, followed by treatment with or without 160 nM TPA
for 2 h. The cultured cells were collected and the nuclear proteins
were prepared using a Nuclear Protein Extraction kit (Beyotime
Institute of Biotechnology). The double-stranded oligonucleotides
(Beyotime Institute of Biotechnology) containing the consensus
sequence for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were end labeled
with biotin and used as probes for the EMSA. Nuclear extracts (10
µg) were incubated with the binding buffer (10 mM Tris-HCl, pH 7.5,
50 mM NaCl, 0.5 mM dithiothreitol, 0.5 mM EDTA, 2.5% (v/v) glycerol
and 1 µg/µl poly dI-dC) for 10 min and then mixed with 0.5
pmol-labeled probes for 20 min at room temperature. The DNA-protein
complex was separated on a 6% native polyacrylamide gel in 0.5X
Tris/borate/EDTA buffer at 4°C and transferred onto positively
charged nitrocellulose membranes (GE Healthcare Bio-Sciences). The
competition assay was performed by adding 100-fold excess of an
unlabeled oligonucleotide as a specific competitor. Bands were
detected using the SuperSignal™ West Pico Chemiluminescent
Substrate, as described by the manufacturer.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Analysis was performed with Excel 2007 software
(Microsoft, Seattle, WA, USA) using a Student's two-tailed t-test
or one-way analysis of variance, in which P<0.05 vs. control was
considered to indicate a statistically significant difference.
Results
Effect of hispolon on the
proliferation of MDA-MB-231 cells
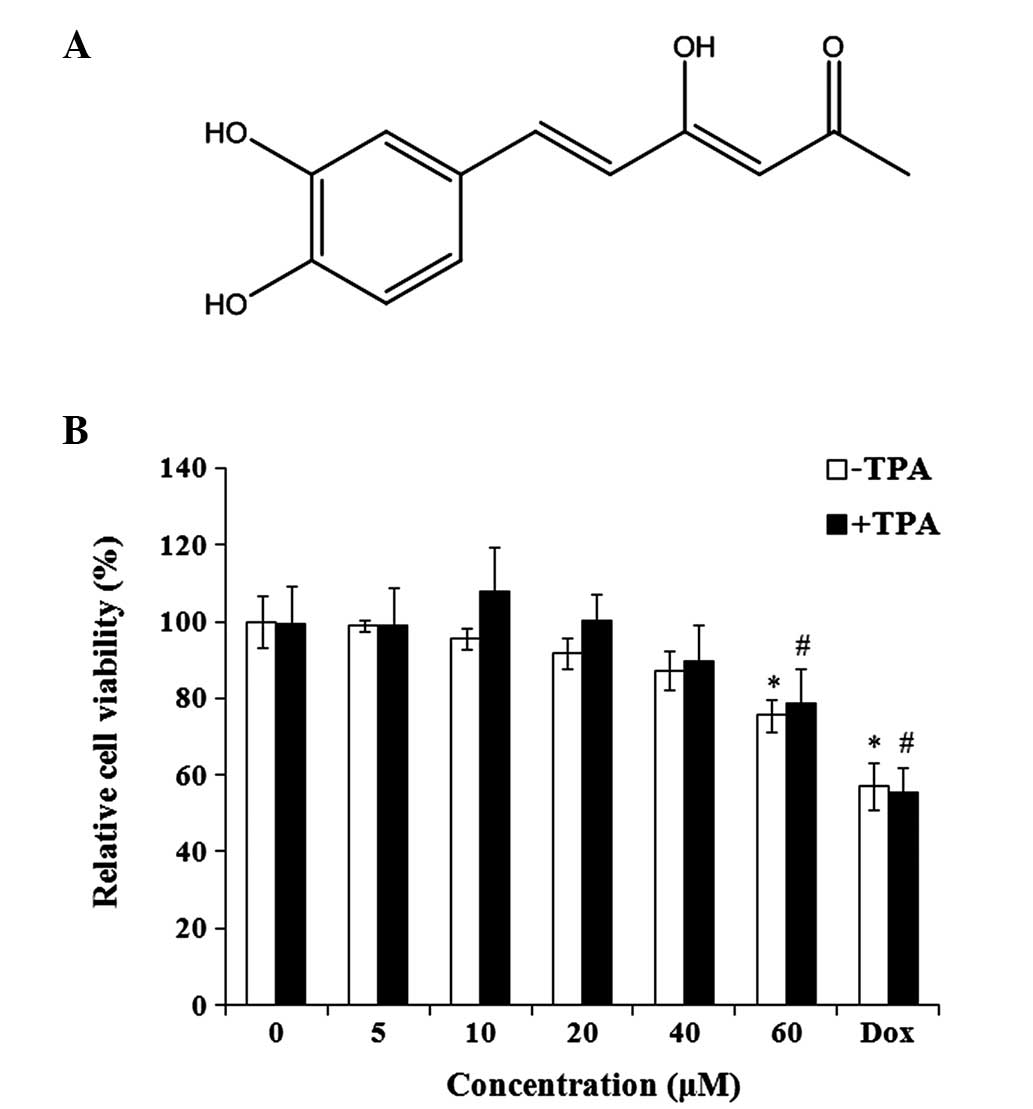
Hispolon is an active polyphenolic compound
(Fig. 1A). The effect of hispolon on
the viability of MDA-MB-231 cells was evaluated. Hispolon
demonstrated non-significant toxicity on TPA-treated and untreated
MDA-MB-231 cells at concentrations between 0 and 40 µM for 24 h
(Fig. 1B). To measure the
anti-invasive effect of hispolon, non-toxic concentrations (0–40
µM) were used for the subsequent experiments, avoiding the
interference resulting from the antiproliferative activity of
hispolon.
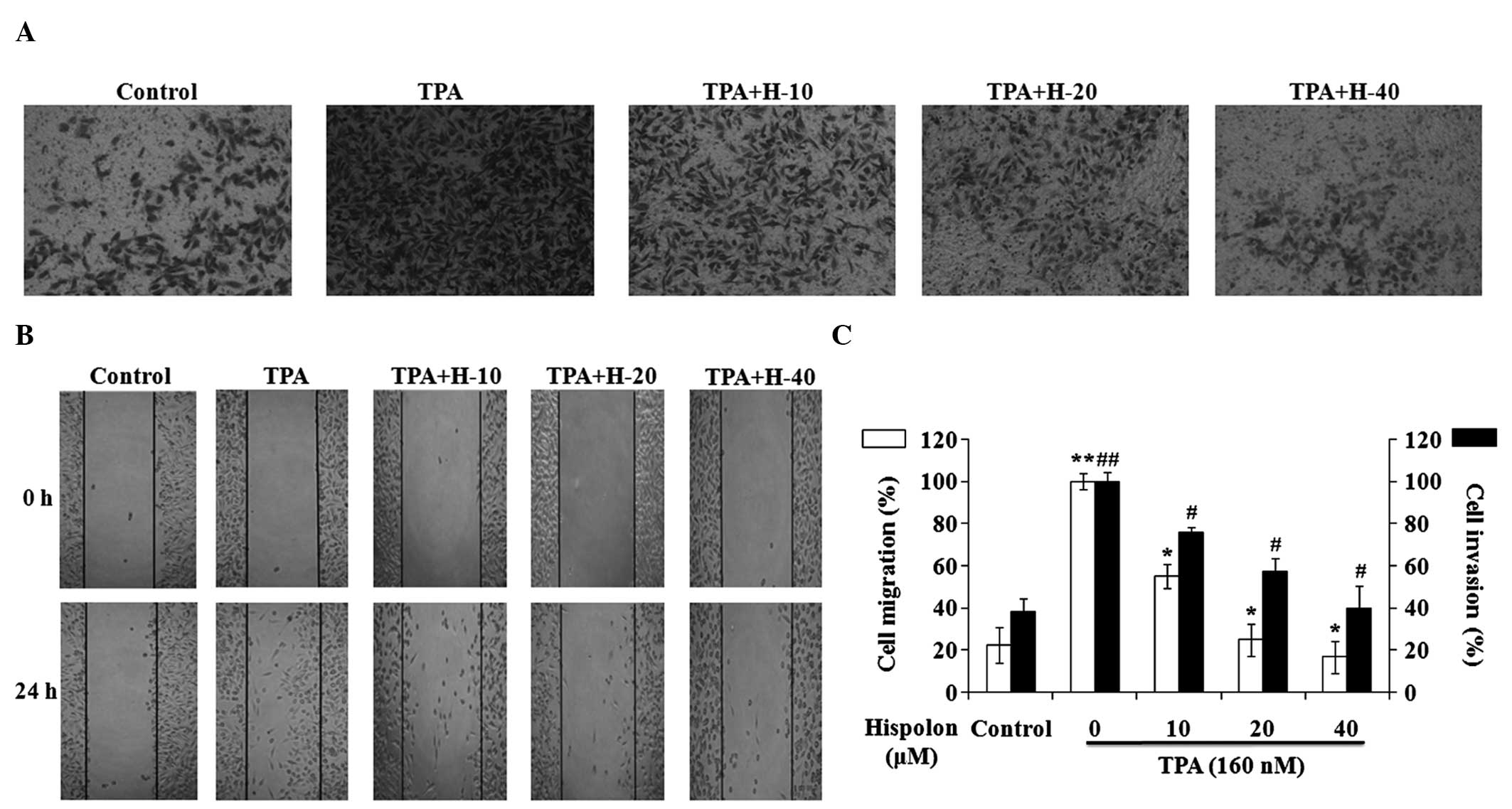
Hispolon inhibits TPA-induced
migration and invasion of MDA-MB-231 cells
A wound-healing assay and a Matrigel-based Transwell
invasion assay were performed in TPA-induced MDA-MB-231 cells. TPA
treatment resulted in a marked increase in cell invasion and
migration, while hispolon inhibited the TPA-induced cell invasion
and migration in a dose-dependent manner (Fig. 2A and B). A concentration of 40 µM
hispolon inhibited the cell invasion and migration significantly,
and the levels of cell invasion and migration in the 40 µM
hispolon-treated group were similar to the DMSO control group
(Fig. 2C). Collectively, these
results indicate that hispolon effectively prevented TPA-induced
migration and invasion in the MDA-MB-231 cells.
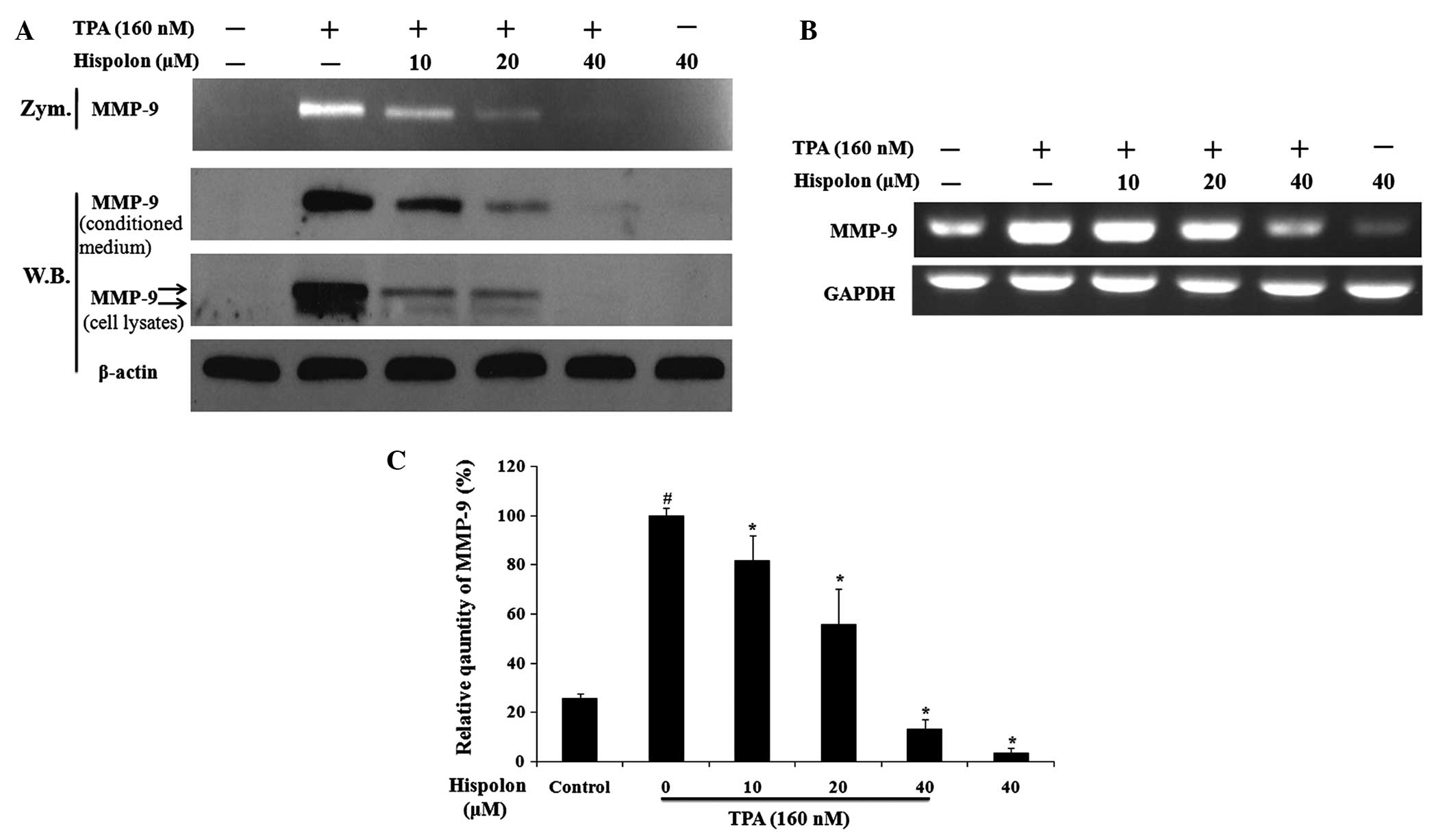
Hispolon inhibits TPA-induced MMP-9
expression and secretion
A previous study demonstrated that MMP-9 is an
important ECM-degrading enzyme and that activation of MMP-9 may
contribute to the process of cancer metastasis (18,19). In
the present study, the effects of hispolon treatment on TPA-induced
MMP-9 expression and secretion in MDA-MB-231 cells were
investigated. Hispolon was found to significantly reduce
TPA-induced MMP-9 gelatinolytic activity as assessed by gelatin
zymography (Fig. 3A). In addition,
western blot analysis of the conditioned medium demonstrated that
hispolon inhibited the secretion of MMP-9 elicited by TPA in a
dose-dependent manner. Furthermore, analysis of the whole cell
lysates demonstrated that hispolon treatment resulted in a
reduction in TPA-induced intracellular expression of MMP-9. It
should be noted that the precursor (85 kDa, lower band) and mature
forms (92 kDa, upper band) of MMP-9 were detected in cell lysates
(Fig. 3A).
RT-PCR was then used to investigate the effect of
hispolon treatment on the regulation of TPA-induced MMP-9
transcription. Hispolon treatment resulted in a reduction in the
MMP-9 mRNA expression levels in a dose-dependent manner (Fig. 3B). The relative quantity of MMP-9 mRNA
indicated that 40 µM hispolon suppressed TPA-induced MMP-9 gene
expression by 90%. In addition, the mRNA expression of MMP-9 in the
40 µM hispolon-treated group was reduced compared with the DMSO
control group (Fig. 3C). These
results demonstrated that hispolon inhibited TPA-induced MMP-9
expression and secretion.
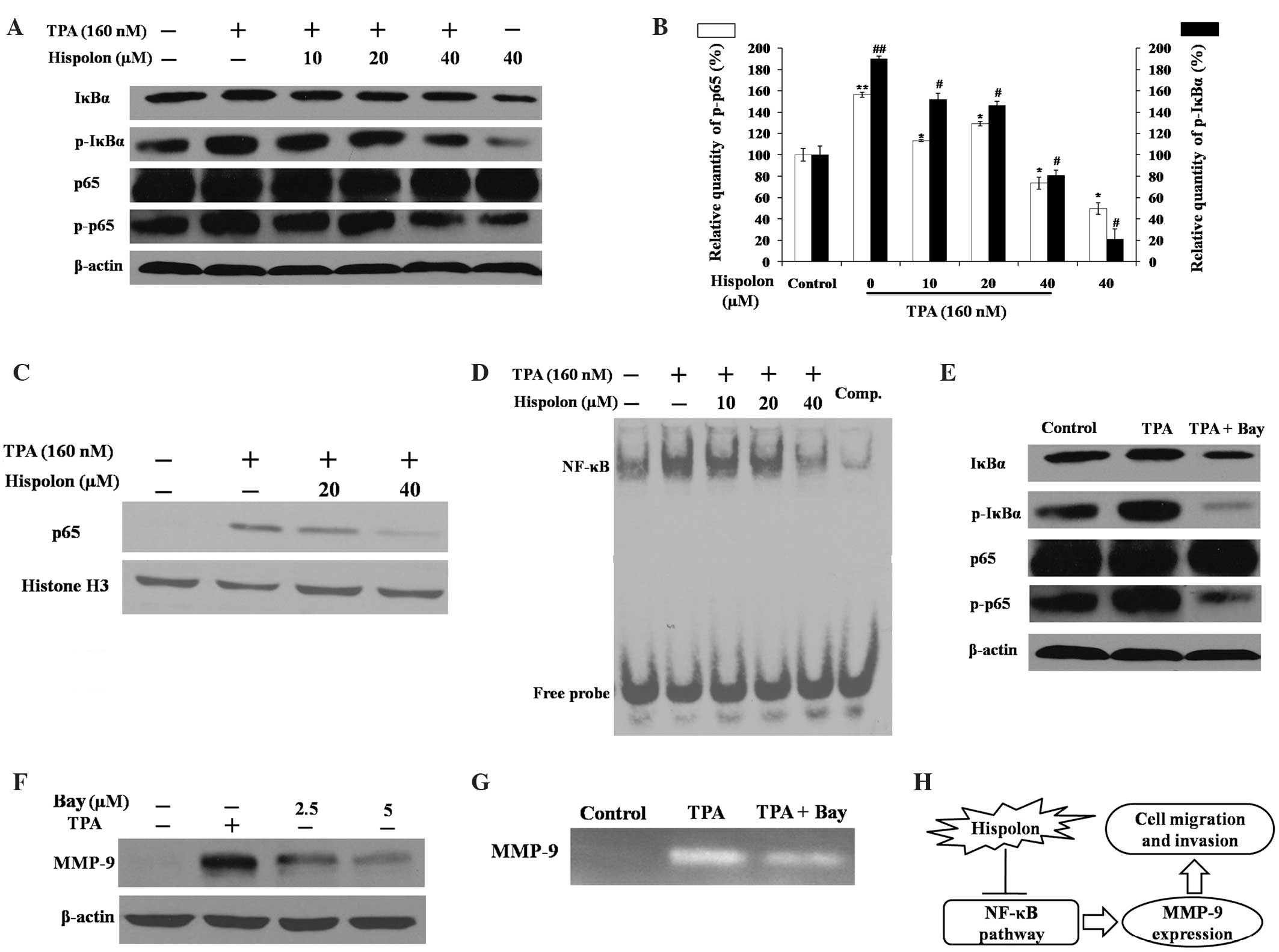
Hispolon inhibits the TPA-induced
NF-κB signaling pathway
Previous studies have demonstrated that NF-κB is
important in TPA-induced MMP-9 gene transcription and promotes
breast cancer cell migration and metastasis (16,17). The
effects of hispolon on the NF-κB signaling pathway were also
assessed in the present study (Fig.
4). The activity of NF-κB is primarily regulated by the
inhibitor of κBα (IκBα) protein. Western blot analysis demonstrated
that TPA-induced phosphorylation of IκBα was prevented by hispolon
treatment in a dose-dependent manner (Fig. 4A). Hispolon also resulted in a
reduction in phosphorylated NF-κB p65 induced by TPA, while the
level of total p65 remained the same in whole cell lysates.
Phosphorylation of IκBα and p65 is critical for p65 nuclear
translocation; thus, the effect of hispolon treatment on p65
nuclear translocation was assessed via western blot analysis of the
nuclear protein fraction. TPA induced p65 translocation to the
nucleus, and translocation was markedly reduced by 40 µM hispolon
treatment (Fig. 4C).
The EMSA assay results demonstrated that hispolon
significantly reduced TPA-induced NF-κB DNA binding activity
(Fig. 4D). The specificity of NF-κB
binding ability was confirmed using a competition assay with excess
unlabeled NF-κB probes. Bay 11–7082, an NF-κB-specific inhibitor,
inhibited the activity of MMP-9 and the phosphorylation of IκBα and
p65, which was in accordance with the results obtained following
hispolon treatment (Fig. 4E-G).
Therefore, these findings indicate that hispolon prevented
TPA-induced MMP-9 gene expression by blocking the NF-κB signaling
pathway in MDA-MB-231 cells.
Discussion
In China, traditional Chinese medicine has been used
to treat patients for centuries. Hispolon is an active compound
isolated from the fungi of Phellinus linteus (10), which is a traditional Chinese medicine
and is also known as ‘Sanghuang’. Extracts of Phellinus
linteus have previously been reported to have
immunostimulatory, anti-inflammatory and anticancer activities
(10,20–22).
Numerous studies have demonstrated that hispolon induces apoptosis
in human cancer cells (13–16,22). In
the present study, a non-toxic concentration of hispolon that
prevented TPA-induced migration and invasion of MDA-MB-231 cells
was established. The anticancer effects of hispolon may, therefore,
be attributed to its ability to induce apoptosis in cancer cells in
addition to its antimetastatic activities.
Overexpression of MMP-9 in human breast cancer has
been previously observed, and hispolon has been demonstrated to
prevent the metastasis of SK-Hep1 hepatoma cells by inhibiting
MMP-2/9 expression (14,23). The present study also indicated that
hispolon suppressed the secretion and expression of MMP-9 in a
dose-dependent manner in TPA-induced MDA-MB-231 cells. The mature
form of MMP-9 (92 kDa, upper band) possessed gelatinolytic activity
and was detected outside the cells, while the precursor form (85
kDa, lower band) remained inside the cells; these findings were
consistent with the results of previous studies (24,25).
Transcriptional regulation of MMP-9 has been proposed to be vital
in its activation (26). The present
study demonstrated that hispolon treatment reduced the MMP-9 mRNA
expression levels in TPA-treated and untreated MDA-MB-231
cells.
NF-κB is a critical transcription factor in the
regulation of MMP-9 gene expression and can be activated by TPA
(25). The present study investigated
whether hispolon treatment inhibited TPA-induced MMP-9 activity
through the NF-κB signaling pathway. When IκBα is phosphorylated it
is degraded by the proteasome, which is required for the activation
of NF-κB; in the present study, hispolon treatment resulted in a
reduction in this process. Phosphorylation of p65, which resulted
in an optimal induction of NF-κB target genes, was also reduced by
hispolon treatment. The nuclear fraction was isolated, while the
nuclear translocation of p65 and NF-κB DNA-binding activity were
demonstrated to be blocked by hispolon. A specific NF-κB inhibitor,
Bay 11–7082, was then used to confirm the effects of the NF-κB
signaling pathway in the metastasis of TPA-induced MDA-MB-231
cells. Similar to treatment with hispolon, the secretion and
expression of MMP-9 were prevented by Bay 11–7082. These results
indicate that NF-κB may be an upstream regulator of MMP-9 and that
hispolon inhibited TPA-induced metastasis of MDA-MB-231 cells via
the NF-κB signaling pathway.
In conclusion, the present study initially
determined non-toxic concentrations of hispolon that inhibited
TPA-induced migration and invasion in MDA-MB-231 human breast
cancer cells. Hispolon treatment was found to suppress the
secretion and expression of MMP-9 by blocking the activation of the
transcription factor, NF-κB (summarized in Fig. 4H). Therefore, hispolon, which is
regarded as a natural product, is notable for further development
as a potential antimetastasis agent for clinical use.
Acknowledgements
The authors would like to thank Dr Yong-Ting Luo
(Institute of Biophysics, Chinese Academy of Sciences, Beijing,
China) for his helpful discussion and editing of this manuscript.
This work was supported by a grant from the Natural Science
Foundation of Zhejiang Province (no. LQ14H160016).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin CW, Hou WC, Shen SC, et al: Quercetin
inhibition of tumor invasion via suppressing PKC
delta/ERK/AP-1-dependent matrix metalloproteinase-9 activation in
breast carcinoma cells. Carcinogenesis. 29:1807–1815. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pellikainen JM, Ropponen KM, Kataja VV, et
al: Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in
breast cancer with a special reference to activator protein-2,
HER2, and prognosis. Clin Cancer Res. 10:7621–7628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laulan NB and St-Pierre Y: Bone
morphogenetic protein 4 (BMP-4) and epidermal growth factor (EGF)
inhibit metalloproteinase-9 (MMP-9) expression in cancer cells.
Oncoscience. 2:309–316. 2015.PubMed/NCBI
|
|
6
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Patterns of MMP-2 and MMP-9 expression in
human cancer cell lines. Oncol Rep. 21:1323–1333. 2009.PubMed/NCBI
|
|
7
|
Han H, Du B, Pan X, et al: CADPE inhibits
PMA-stimulated gastric carcinoma cell invasion and matrix
metalloproteinase-9 expression by FAK/MEK/ERK-mediated AP-1
activation. Mol Cancer Res. 8:1477–1488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SK, Hwang YS, Park KK, et al:
Kalopanaxsaponin A inhibits PMA-induced invasion by reducing matrix
metalloproteinase-9 via PI3K/Akt- and PKCdelta-mediated signaling
in MCF-7 human breast cancer cells. Carcinogenesis. 30:1225–1233.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ali NAA, Pilgrim H, Liberra K, Lindequist
U and Jansen R: Hispolon, a yellow pigment from Inonotus hispidus.
Phytochemistry. 41:927–929. 1996. View Article : Google Scholar
|
|
10
|
Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou
H, Wang Y and Li YQ: Hispolon induces apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway. Free
Radic Biol Med. 45:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mo S, Wang S, Zhou G, et al: Phelligridins
C-F: cytotoxic pyrano[4,3-c][2]benzopyran-1,6-dione and
furo[3,2-c]pyran-4-one derivatives from the fungus Phellinus
igniarius. J Nat Prod. 67:823–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang HY, Sheu MJ, Yang CH, Lu TC, Chang
YS, Peng WH, Huang SS and Huang GJ: Analgesic effects and the
mechanisms of anti-inflammation of hispolon in mice. Evid Based
Complement Alternat Med. 2011:4782462011.PubMed/NCBI
|
|
13
|
Huang GJ, Deng JS, Huang SS, et al:
Hispolon induces apoptosis and cell cycle arrest of human
hepatocellular carcinoma Hep3B cells by modulating ERK
phosphorylation. J Agric Food Chem. 59:7104–7113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang GJ, Yang CM, Chang YS, et al:
Hispolon suppresses SK-Hep1 human hepatoma cell metastasis by
inhibiting matrix metalloproteinase-2/9 and urokinase-plasminogen
activator through the PI3K/Akt and ERK signaling pathways. J Agric
Food Chem. 58:9468–9475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu TL, Huang GJ, Lu TJ, et al: Hispolon
from Phellinus linteus has antiproliferative effects via
MDM2-recruited ERK1/2 activity in breast and bladder cancer cells.
Food Chem Toxicol. 47:2013–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu TL, Huang GJ, Wang HJ, et al: Hispolon
promotes MDM2 downregulation through chaperone-mediated autophagy.
Biochem Biophys Res Commun. 398:26–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Venkateswarlu S, Ramachandra MS, Sethuramu
K and Subbaraju GV: Synthesis and antioxidant activity of hispolon,
a yellow pigment from Inonotus hispidius. J Chem Sect B.
41:875–877. 2002.
|
|
18
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, et al: NF-kappaB promotes breast cancer cell
migration and metastasis by inducing the expression of the
chemokine receptor CXCR4. J Biol Chem. 278:21631–21638. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin Y, Yoon SH, Choe EY, et al:
PMA-induced up-regulation of MMP-9 is regulated by a
PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp Mol
Med. 39:97–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim GY, Oh YH and Park YM: Acidic
polysaccharide isolated from Phellinus linteus induces nitric
oxide-mediated tumoricidal activity of macrophages through protein
tyrosine kinase and protein kinase C. Biochem Biophys Res Commun.
309:399–407. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth, angiogenesis
and invasive behaviour of breast cancer cells through the
inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsiao PC, Hsieh YH, Chow JM, et al:
Hispolon induces apoptosis through JNK1/2-mediated activation of a
caspase-8, -9, and -3-dependent pathway in acute myeloid leukemia
(AML) cells and inhibits AML xenograft tumor growth in vivo. J
Agric Food Chem. 61:10063–10073. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scorilas A, Karameris A, Arnogiannaki N,
et al: Overexpression of matrix-metalloproteinase-9 in human breast
cancer: a potential favourable indicator in node-negative patients.
Br J Cancer. 84:1488–1496. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toth M, Gervasi DC and Fridman R: Phorbol
ester-induced cell surface association of matrix
metalloproteinase-9 in human MCF10A breast epithelial cells. Cancer
Res. 57:3159–3167. 1997.PubMed/NCBI
|
|
25
|
Ling H, Yang H, Tan SH, Chui WK and Chew
EH: 6-Shogaol, an active constituent of ginger, inhibits breast
cancer cell invasion by reducing matrix metalloproteinase-9
expression via blockade of nuclear factor-κB activation. Br J
Pharmacol. 161:1763–1777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jones CB, Sane DC and Herrington DM:
Matrix metalloproteinases: a review of their structure and role in
acute coronary syndrome. Cardiovasc Res. 59:812–823. 2003.
View Article : Google Scholar : PubMed/NCBI
|