Introduction
Hemangiopericytoma (HPC) is a soft tissue tumor of
vascular origin and can originate from anywhere in the human body.
HPC cases that arise in the head and neck account for ~15–25% of
cases, 5% of which develop in the nasal and sinus area (1,2). Sinonasal
HPC accounts for ≤1% of sinonasal tumors, which often reoccur
locally but rarely metastasize (3).
Surgery is the main treatment method for primary and recurrent
sinonasal HPC (4). Open surgical
methods and endoscopic techniques have been considered as standard
care of sinonasal HPC. Surgical removal resulted in no recurrence
in 79.7% of the cases (4). Due to the
complexity of nasal anatomy it is difficult to completely excise
the HPC lesion by open or endoscopic surgery. The residual tumor is
a major cause of local recurrence of sinonasal HPC, therefore,
post-surgical rehabilitation for HPC remains a challenge. Only two
cases have previously reported the use of adjuvant radiotherapy for
residual or recurrent lesions and very few reports of chemotherapy
for sinonasal HPC have been recorded (1,5). The
present case study reports that adjuvant radiotherapy and
chemotherapy were effective to control the recurrent and
intracranial invasion of one case of sinonasal HPC.
Case report
Patient presentation
In October 2011, a 42-year-old man diagnosed with
recurrent and intracranial invasion of sinonasal HPC, was admitted
to Xuanwu Hospital. The patient underwent multiple surgeries to
remove the tumors, however, no adjuvant therapy was adopted during
this period and the tumors reoccurred within 1 year. In December
2012, on admittance to Tangshan People's Hospital (Tangshan,
China), the patient presented with limited mouth opening and
chewing ability, and hearing loss. Maxillary sinus puncture was
performed and the biopsy specimens were fixed with 10%
formalin.
Pathological examination
The formalin-fixed, paraffin-embedded tissue
sections (4 µm thick) were deparaffinized in xylene and dehydrated
through a graduated alcohol series into water. The sections were
then used for hematoxylin and eosin (HE) staining or
immunohistochemical analysis. For HE staining, briefly, the
sections were incubated in working solution of Mayer's hematoxylin
for 10 min (stain nuclear blue) and then rinsed in water followed
by counterstain in eosin-phloxine solution for 1 min (stain
cytoplasm pink). For immunohistochemical assay, endogenous
peroxidase activity was blocked with 3% H2O2
in methanol for 20 min. Antigen retrieval was performed by
microwaving sections in 0.01 M sodium citrate (pH 6.0).
Non-specific binding was blocked by incubating sections with 5%
bovine serum albumin in phosphate-buffered saline (PBS) for 30 min
at room temperature. Without washing, these sections were incubated
with mouse anti-human CD34 monoclonal antibody (catalog no.
sc-65261; dilution, 1:500), rabbit anti-human monoclonal CD31
antibody (catalog no. sc-8306; dilution, 1:500) or mouse anti-human
monoclonal actin antibody (catalog no. sc-58673; dilution, 1:500)
in PBS at 4°C overnight in a moist box, respectively. After the
wash steps, the sections were incubated with corresponding horse
radish peroxidase conjugated anti-mouse or anti-rabbit secondary
antibodies (Dako Cytomation). The antigen was visualized with
substrate chromogen (Dako liquid DAB chromogen; Dako Cytomation).
Finally, tissue specimens were stained with Mayer's haematoxylin to
discriminate the nucleus from the cytoplasm. Images captured for
all sections were acquired using an Olympus CX 31 microscope
(Olympus, Tokyo, Japan). Positive cells were indicated by the
presence of a distinct brown color in the nuclei or cytoplasm.
Normal tissues were used as control tissues, and non-immune IgG was
also used as a negative control antibody for immunohistochemical
staining. All of the antibodies were purchased from Santa Cruz
Biotechnology (Tokyo, Japan).
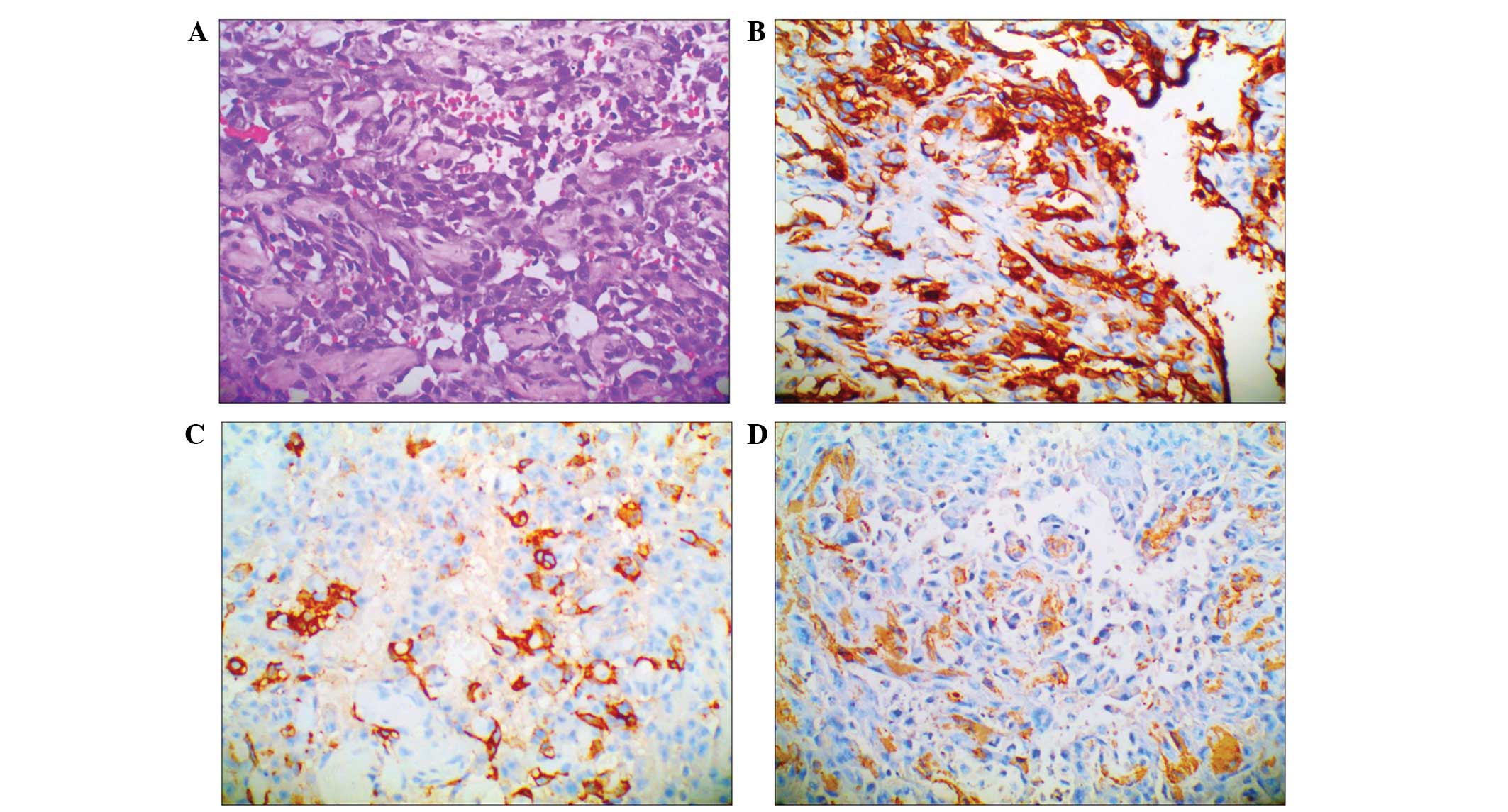
Under observation, HPC usually consists of
spindle-shaped cells with elongated nuclei, and it displays
characteristic staghorn-like vascular channels (6,7). In the
present study, the tumor cells expressed CD34, CD68(+/-),
epithelial membrane antigen, CD31, α-actin, desmin, CD99, S-100,
B-cell lymphoma-2 (Bcl-2) and Ki-67(30%), but were negative for
creatine kinase (CK) (Fig. 1).
Imaging analysis
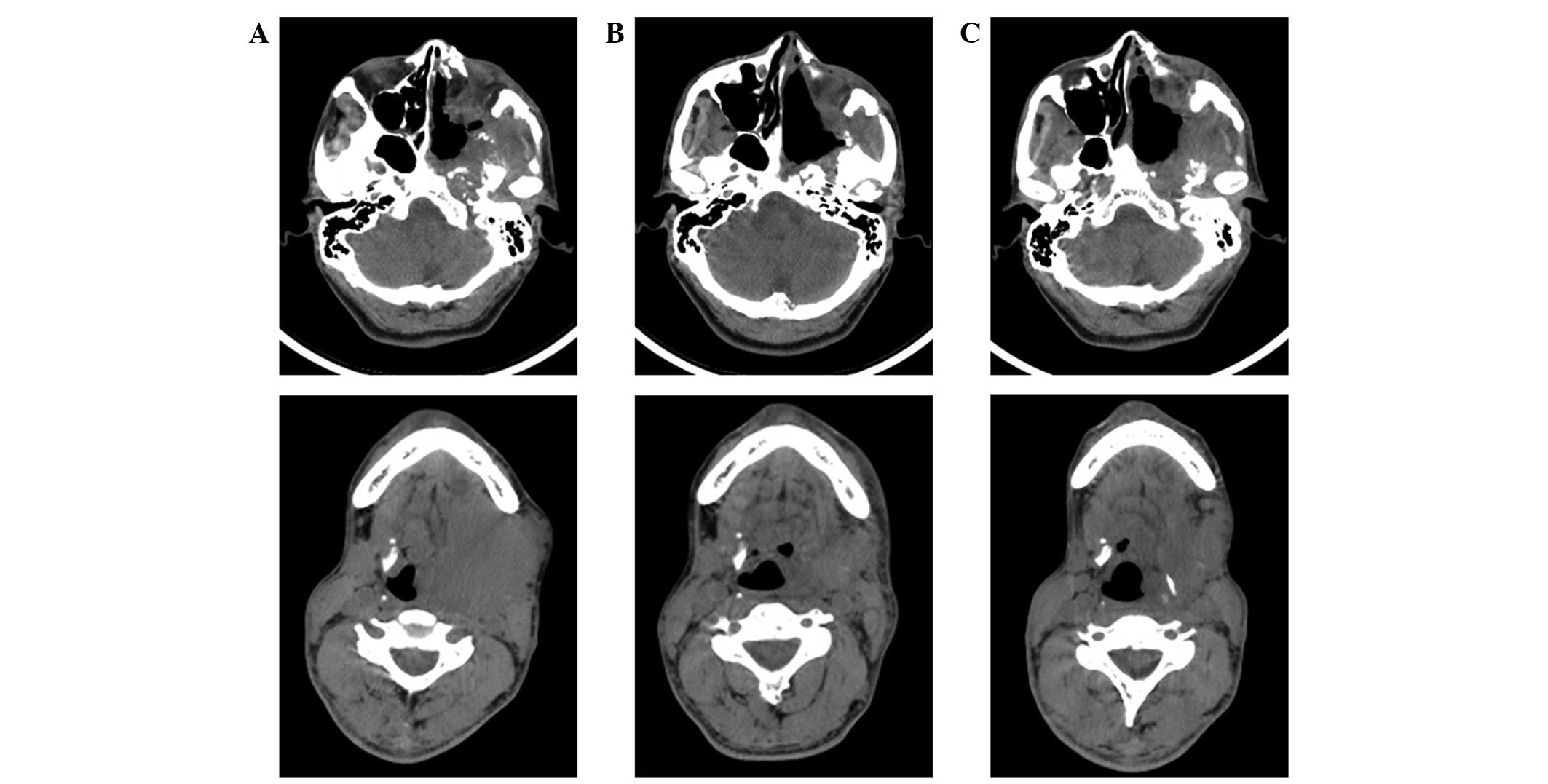
On admission, the head and neck CT scan showed a
lamellar high-density shadow in the left temporal lobe and bone
destruction in the left sphenoid wing and petrous tip. The left
sphenoid sinus wall, inside and outside of the board sphenoid wing
was absent. Masses were observed in the soft tissues, including the
left orbital tissue, temporal fossa, nasopharyngeal and
oropharyngeal walls, parapharyngeal space and the masseter gap,
indicating multiple recurrent and intracranial invasion of
sinonasal HPC. In addition, lymph node enlargement was identified
in the neck. The representative CT images are presented in Fig. 2.
Radio- and chemotherapy regimens
As the patient was not able to tolerate surgery,
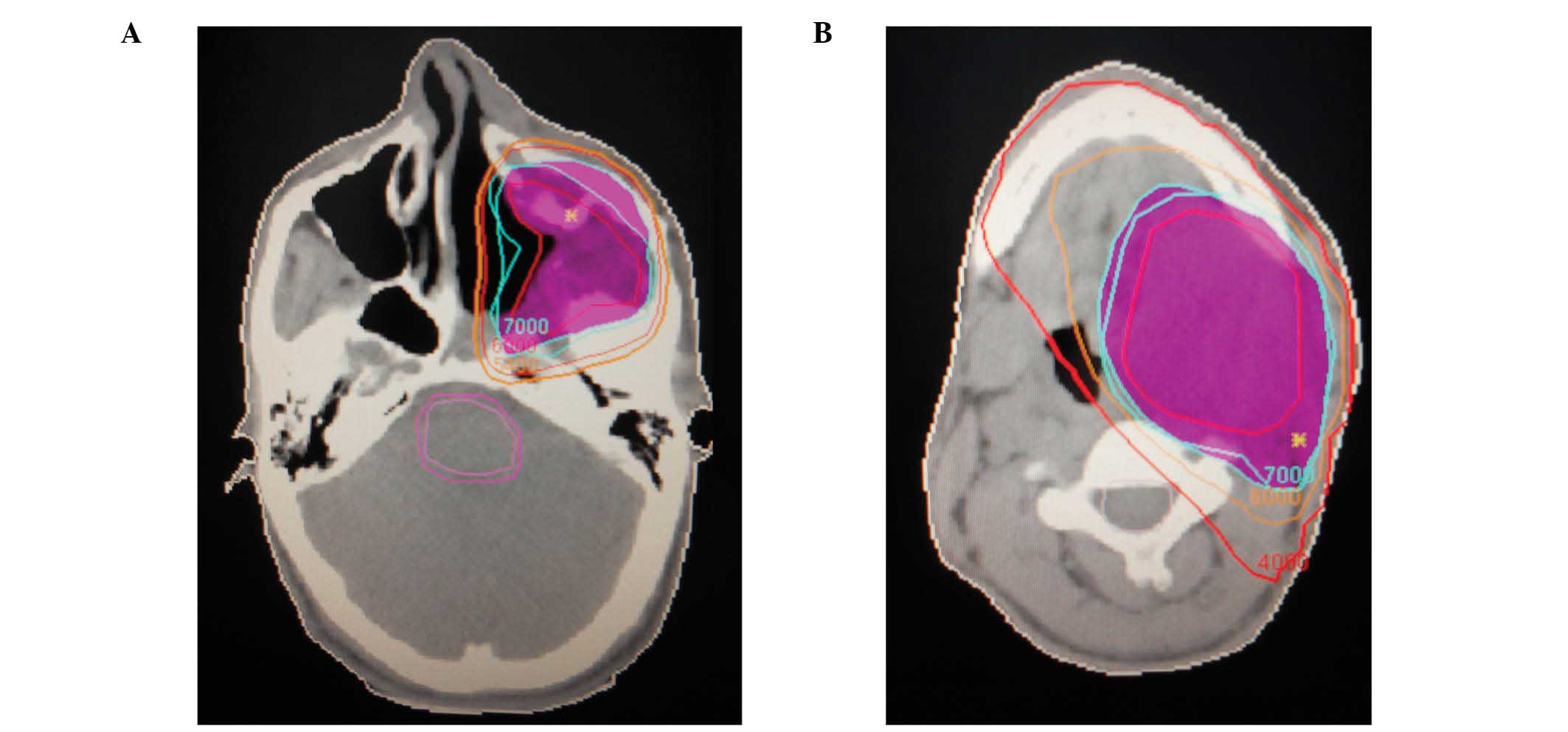
intensity-modulated radiotherapy was adopted. All treatment plans
(70 Gy, 2 Gy per fraction, 35 fractions) produced adequate target
coverage (ensuring at least 95% geometrical coverage of the
planning target volume). The dose distribution is presented in
Fig. 3, in which pink indicates the
70 Gy dose response curve. A CT scan was performed following 30
cycles of radiotherapy and exhibited a reduction of masses in the
left temporal fossa and partial restoration of the destructed skull
base bones (Fig. 2B). The symptoms
were markedly relieved. These findings demonstrated that the tumors
responded to radiation therapy.
Following radiation therapy, adjuvant chemotherapy
was adopted. Pirarubicin (Shenzhen Main Luck Pharmaceuticals Inc.,
Shenzhen, China) was administered intravenously on day 1 (50
mg/m2), and cisplatin was administered intravenously
(Qilu Pharmaceutical Co., Ltd., Jinan, China) on days 2–4 (75
mg/m2). As demonstrated in Fig. 2C, a further tumor response was
observed following 2 cycles of chemotherapy. No recurrence and
metastasis was observed at the 1 year follow-up subsequent to the
combined therapy.
This study was approved by the Research Ethics
Committee of Tangshan People's Hospital (Tangshan, China). Written
informed consent was obtained from the patient. All specimens were
handled and anonymized according to ethical and legal
standards.
Discussion
HPC is a relatively indolent neoplasm and commonly
behaves in a benign manner, but HPC in the nasal and sinus area
often recurs (1,5). Metastasis of sinonasal HPC is rare. The
prognosis of sinonasal-type HPC is closely associated with tumor
grade, and a higher grade results in a higher mortality rate
(5). Although the expression of a
number of immunohistochemical markers, such as α-actin, CD31 and
CD34, has been detected in sinonasal HPC tissues (5,8), no
specific immunohistochemical markers have been identified. However,
staghorn-like vascular channels are considered a histological
feature that is specific to sinonasal HPC (5). Although sinonasal HPC is regarded as
radioresistant, it has previously been reported that adjuvant
radiotherapy may be used for positive surgical margins or recurrent
lesions (1,5). In addition, chemotherapy appears to be
useful in disseminated HPC (4,9,10), whilst there is not sufficient evidence
of the effectiveness of radiotherapy for sinonasal HPC. It has been
previously reported that adriamycin-based chemotherapy was adopted
for patient with spleen HPC following simple splenectomy (9). It was recommended that resection coupled
with chemotherapy should be performed in cases of resectable
recurrence, as it still has a good chance of being curative
(10,11). For the first time, pirarubicin and
cisplatin were adopted as chemotherapy drugs for the patient with
sinonasal HPC and had obvious curative effect.
In the current study, radiotherapy followed by
chemotherapy (pirarubicin combined with cisplatin) demonstrated
positive efficacy. Therefore, for cases of multiple recurrent and
invasive sinonasal HPC, combined treatment of radiotherapy and
chemotherapy is recommended, but additional cases are required to
evaluate the clinical efficacy and impact on survival.
References
|
1
|
Billings KR, Fu YS, Calcaterra TC and
Sercarz JA: Hemangiopericytoma of the head and neck. Am J
Otolaryngol. 21:238–243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terada T and Kato T: Sinonasal-type
hemangiopericytoma of the nasal cavity and paranasal sinus. Int J
Clin Oncol. 17:169–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duman FU, Ayhan S, Işısağ A, Eskıızmır G
and Tarhan S: Sinonasal-type haemangiopericytoma: A case report.
Turk Patoloji Derg. Mar 18–2014.(Epub ahead of print) (In
Turkish).
|
|
4
|
Dahodwala MQ, Husain Q, Kanumuri VV,
Choudhry OJ, Liu JK and Eloy JA: Management of sinonasal
hemangiopericytomas: a systematic review. Int Forum Allergy Rhinol.
2013.3(7): 581–7. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson LD, Miettinen M and Wenig BM:
Sinonasal-type hemangiopericytoma: a clinicopathologic and
immunophenotypic analysis of 104 cases showing perivascular myoid
differentiation. Am J Surg Patho1. 27:737–749. 2003. View Article : Google Scholar
|
|
6
|
Fletcher CD1: Distinctive soft tissue
tumors of the head and neck. Mod Pathol. 2002.15(3): 324–30.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agaimy A1, Barthelmeß S, Geddert H, Boltze
C, Moskalev EA, Koch M, Wiemann S, Hartmann A and Haller F:
Phenotypical and molecular distinctness of sinonasal
haemangiopericytoma compared to solitary fibrous tumour of the
sinonasal tract. Histopathology. 2014.65(5): 667–73. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yokoi H, Arakawa A, Kuribayashi K,
Inoshita A, Haruyama T and Ikeda K: An immunohistochemical study of
sinonasal hemangiopericytoma. Auris Nasus Larynx. 38:743–746. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Illuminati G, Pizzardi G, Calio F, Pacilè
MA, Carboni F, Palumbo P and Vietri F: Hemangiopericytoma of the
spleen. Int J Surg. 15:6–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamuro M, Nakamura S, Shiraha H, et al: A
case of primary intracranial hemangiopericytoma with hepatic
metastases: successful treatment with radiofrequency ablation and
transcatheter arterial chemoembolization. Clin J Gastroenterol.
2:30–35. 2009. View Article : Google Scholar
|
|
11
|
Maria PS, Mauri M and Carmignani L: A
unique cause of hemoperitoneum: spontaneous rupture of a splenic
hemangiopericytoma. Int J Emerg Med. 4:132011. View Article : Google Scholar : PubMed/NCBI
|