Introduction
Hematopoietic stem cell transplantation (HSCT) is a
successful treatment strategy for children with potentially fatal
diseases, such as hematological malignancies, severe aplastic
anemia, inherited diseases or neuroblastoma (1). Prior to undergoing HSCT, high-dose
chemotherapy and total-body irradiation (TBI) are administered in
an attempt to eradicate residual malignant cells and cure the
patient; however, the high-dose chemotherapy and radiotherapy may
increase the risk of adverse effects in almost every organ or
system in the body. In particular, the major skeletal complications
observed in TBI survivors are osteoporosis, avascular necrosis and
benign or malignant bone tumors.
Osteochondroma is a benign cartilage-capped tumor
that predominantly develops at the juxtaepiphyseal region of the
long bones. There have been few reports of osteochondroma as a
secondary bone tumor following the treatment of childhood
neuroblastoma (2–4); however, there are considerable
experimental and clinical data linking osteochondroma to localized
high-dose and TBI (5). The rate of
malignant transformation in primary osteochondroma, principally
into chondrosarcoma, is estimated to be 1–5% (6), and transformation into osteosarcoma is
rare. The simultaneous occurrence of osteosarcoma and
osteochondroma following treatment of neuroblastoma with
chemotherapy, radiotherapy and autologous peripheral HSCT rescue
has been reported previously (7).
However, the current literature indicates a low incidence of
malignant degeneration in radiation-induced osteochondroma
(8–10)
and only one case of chondrosarcoma arising within
radiation-induced osteochondroma following childhood TBI has been
reported thus far (11).
Herein, the present study reports a case of
osteosarcoma arising from osteochondroma 11 years after the patient
underwent surgery, local irradiation, chemotherapy, TBI and HSCT
for neuroblastoma. Due to the radiographic characteristics and
localization of the tumor, it is postulated that the osteosarcoma
arose from osteochondroma and developed following TBI. To the best
of our knowledge, this is the first reported case of its type.
Written informed consent was obtained from the patient's
family.
Case report
A 5-year-old boy was admitted to Niigata University
Hospital (Niigata, Japan) in January 1998 with a 2-week history of
fever, cough, fatigue, headache and abdominal pain. On physical
examination, a gross abdominal mass was palpable. Abdominal
computed tomography (CT) demonstrated a retroperitoneal mass
located adjacent to the left kidney and multiple enlarged lymph
nodes along the aorta. The patient underwent an open biopsy, and
pathological examination revealed a diagnosis of neuroblastoma,
revealing undifferentiated unfavorable histology, DNA diploidy and
MYCN oncogene amplification (12 copies). Multiple metastases to the
occipital bone, left humerus, left tibia and bone marrow were
indicated upon metaiodobenzylguanidine (123I-MIBG)
scanning. In addition, urine vanillylmandelic acid (VMA),
homovanillic acid (HVA) and serum neuron-specific enolase (NSE)
levels were elevated to 86.8 mg/g creatinine (Cr; normal range VMA,
5.8–19.1 mg/g Cr), 103.7 mg/g Cr (normal range HVA, 9.4–23.4 mg/g
Cr) and >200 ng/ml (normal range NSE, <16.3 ng/ml),
respectively. On the basis of these findings, the patient was
diagnosed with stage IV neuroblastoma and considered to belong to
the high-risk group, according to the International Neuroblastoma
Staging System (12).
Following the diagnosis of neuroblastoma, the
patient was treated according to the nationwide standard protocol
established by the Japan Study Group for Advanced Neuroblastoma
(13). Induction therapy with one
cycle of regimen A1, consisting of cyclophosphamide (CPA; 1,200
mg/m2; day 1), etoposide (100 mg/m2; days
1–5), tetrahydropyranyl-Adriamycin® (THP-ADM; 40 mg/m2;
day 3) and cisplatin (90 mg/m2; day 5), and three cycles
of regimen A3, consisting of CPA (1,200 mg/m2; days
1–2), etoposide (100 mg/m2; days 1–5), THP-ADM (40
mg/m2; day 3) and cisplatin (25 mg/m2, days 1
5), was repeated every 4 weeks. Following completion of these 4
cycles, residual metastatic lesions were identified by performing a
CT scan. Therefore, ifosfamide-based salvage chemotherapy was
immediately undertaken, including one cycle of ifosfamide,
carboplatin (IC) and etoposide (ICE), containing 4.5
g/m2 ifosfamide (day 2), 385 mg/m2
carboplatin (day 2) and 100 mg/m2 etoposide (days 1–5),
and two cycles of IC, containing 6 mg/m2 ifosfamide and
500 mg/m2 carboplatin, followed by administration of
high-dose melphalan (70 mg/m2 on consecutive days) with
concomitant preoperative autologous peripheral HSCT. Following
induction chemotherapy, tumor markers were still not normalized
(VMA, 47.8 mg/g Cr; HVA, 31.2 mg/g Cr; NSE, 9.7 ng/ml) and an MIBG
hotspot indicated that a small lesion remained in the left tibia.
Thus, gross total resection to remove the entire macroscopic tumor
from the primary site, including the regional lymph nodes, and
combined resection of the left kidney were performed. This was
followed by 12 Gy of intraoperative radiation therapy of the tumor
bed and para-aortic lymph nodes. One cycle of regimen C
chemotherapy, containing cyclophosphamide (1,500 mg/m2;
day 1) and dacarbazine (250 mg/m2; days 1–5), was
administered postoperatively. Peripheral blood stem cells were
collected by apheresis using positive cluster of differentiation
34+ selection and the presence of tumor cells was
detected by immunofluorescence. High-dose consolidation therapy was
then administered, consisting of thiotepa (200 mg/m2
intravenously, twice daily for 2 days; total dose, 800
mg/m2) and 12 Gy of fractionated TBI, followed by
autologous peripheral HSC rescue. TBI was delivered using 12 Gy
radiation in 6 doses of 2-Gy fractions twice-daily for 3
consecutive days. Tumor markers, including serum NSE, and urinary
HVA and VMA became normalized and were maintained within the normal
ranges immediately after completion of the radiotherapy.
The patient developed multiple osteochondromas ~6
years after being treated for neuroblastoma in February 2004,
despite having no clinically detectable bony masses prior to the
neuroblastoma treatment and no family history of hereditary
multiple exostoses. At this time, the patient did not have any
experience pain or discomfort and did not receive any treatment
following this diagnosis. At the age of 17 years in August 2009,
~11 years after treatment for neuroblastoma, the patient presented
to our clinic with a 1-week history of intermittent stabbing pain
in the upper left arm. Physical examination revealed swelling along
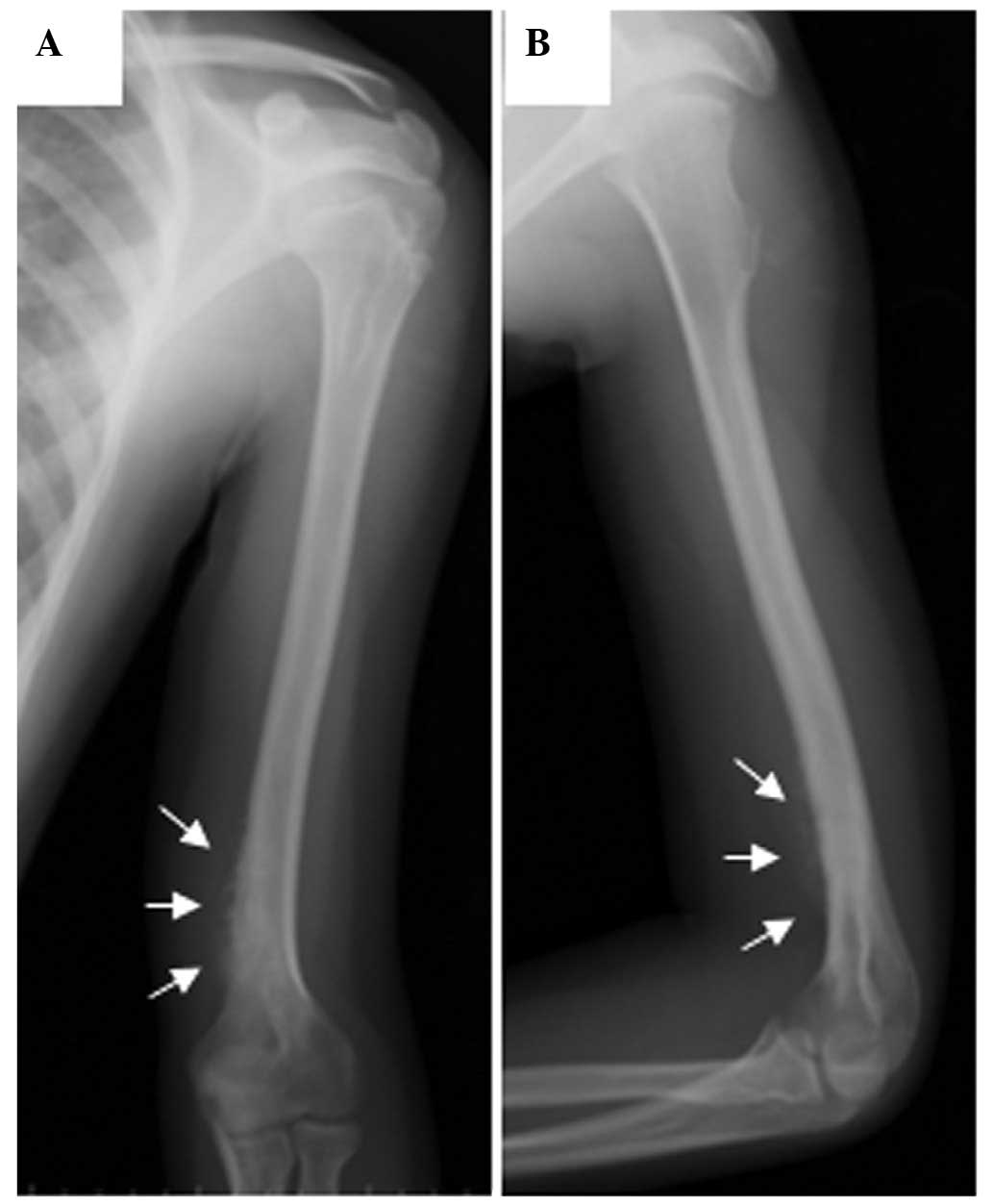
the distal aspect of the upper left arm, and radiography of the
left elbow identified a periosteal reaction in the distal humeral
shaft and an associated medial soft-tissue mass (Fig. 1). 99mTc-MDP radionuclide
bone scanning revealed a single focus of increased activity in the
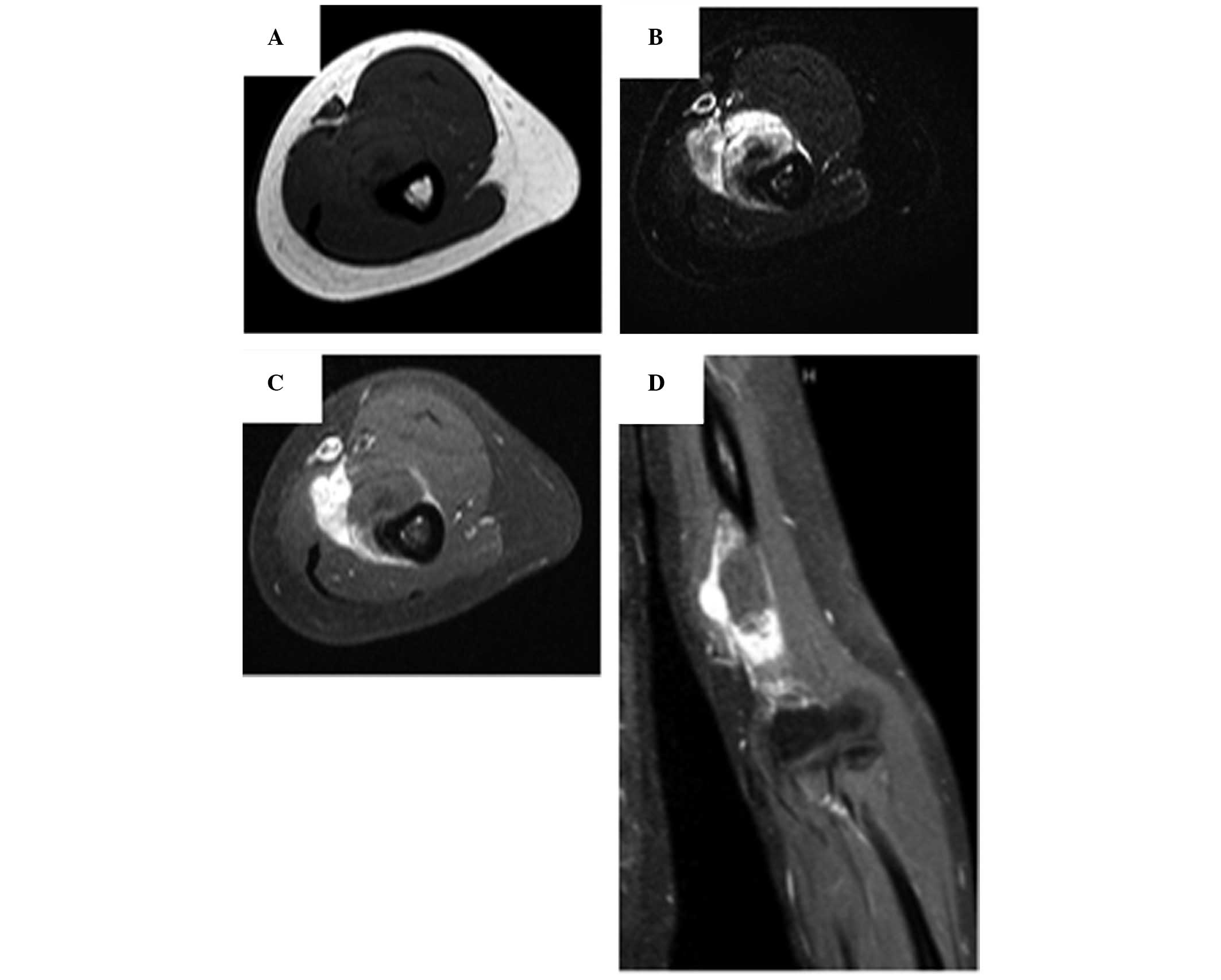
region of the left distal humerus. Subsequent magnetic resonance
imaging of the tumor demonstrated an absence of medullary
involvement, an irregular cortical surface and a soft-tissue mass
(Fig. 2). The subperiosteal location
of the tumor was clear and the imaging features were typical of
osteosarcoma arising from osteochondroma. The remainder of the
metastatic work-up, including chest radiography, whole-body CT and
24-h monitoring of urinary VMA, dopamine and catecholamine levels,
revealed no abnormalities. In addition, there was no family history
of malignancy. Fine-needle aspiration cytology revealed oval- to
spindle-shaped cells, a number of which had a high
nucleocytoplasmic ratio with hyperchromatic nuclei, irregular
nuclear border and prominent nucleoli. Additionally, osteoblasts
with pronounced nuclear atypia were observed and diagnosed as
osteosarcoma. Due to the heminephrectomy and chemotherapy
previously undergone for the treatment of neuroblastoma, renal and
liver dysfunction, as well as prolonged myelosuppression, were
observed following two cycles of preoperative chemotherapy,
comprising cisplatin (100 mg/m2; day 1) and doxorubicin
(25 mg/m2; days 1–2). Furthermore, severe neuralgic pain
in the jaw and abdomen was precipitated by an additional cycle of
single agent chemotherapy comprising 2 mg vincristine.
One month after completion of three cycles of
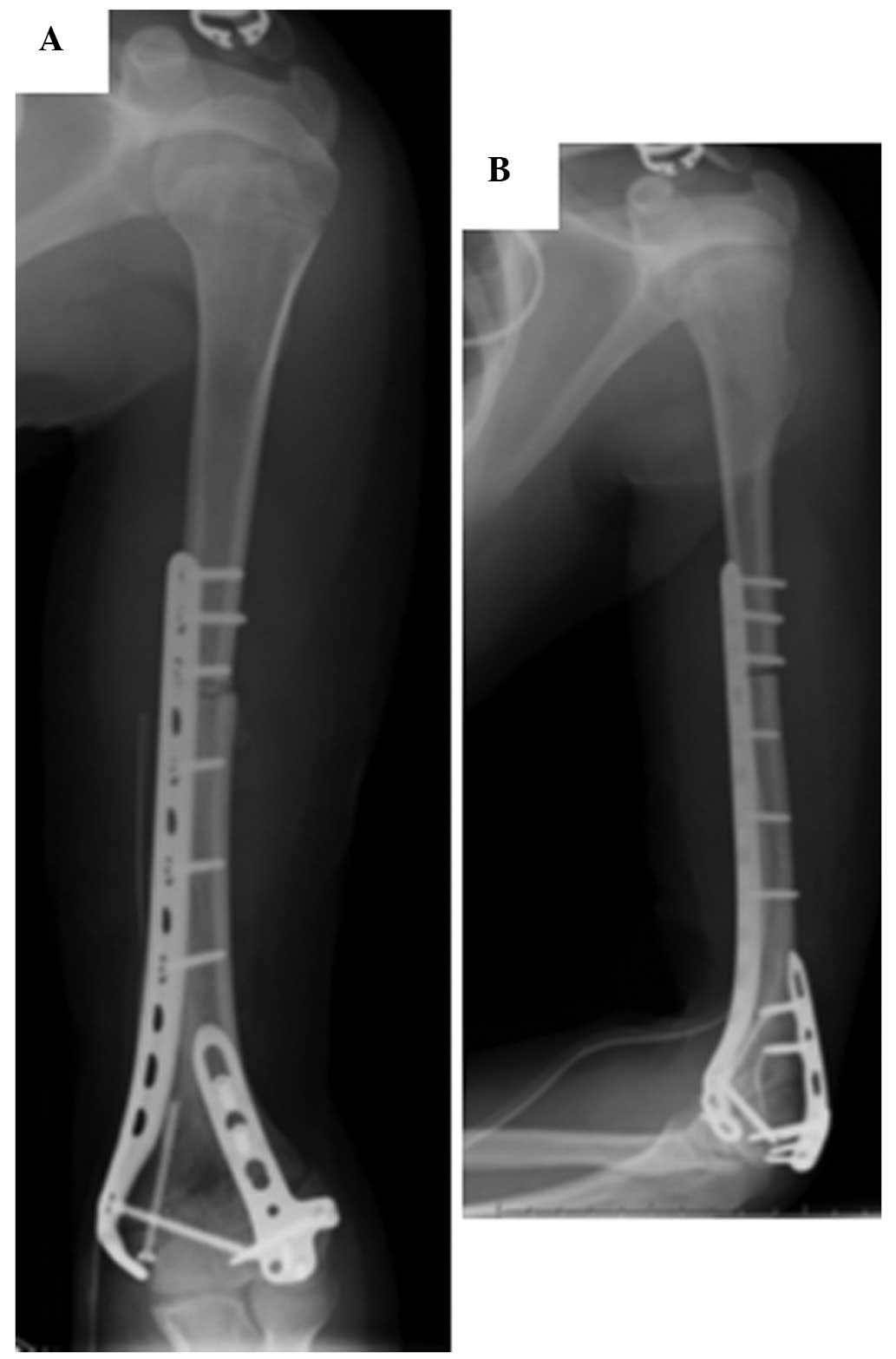
preoperative chemotherapy, the patient underwent wide local
excision of the tumor followed by reconstruction with an
extracorporeally irradiated autograft combined with an iliac bone
graft (Fig. 3). All bone segments
were administered with a single fraction of 70 Gy of radiation.
Following resection, the patient received postoperative
chemotherapy, including nine cycles of high-dose methotrexate(7
g/m2), two cycles of ifosfamide (3 mg/m2;
days 1–2) and doxorubicin (30 mg/m2; days 1–2), one
cycle of ifosfamide (1 g/m2; days 1–4), 4.5 mg vindesine
(day 1) and carboplatin (100 mg/m2; days 1–4), and one
cycle of IC, containing 1 mg/m2 ifosfamide (days 1–2)
and 100 mg/m2 carboplatin (days 1–2). Doses of
nephrotoxic cytostatic agents were reduced due to renal impairment.
Postoperatively, immobilization with long arm brace was continued
until there was radiographic evidence of union. A final
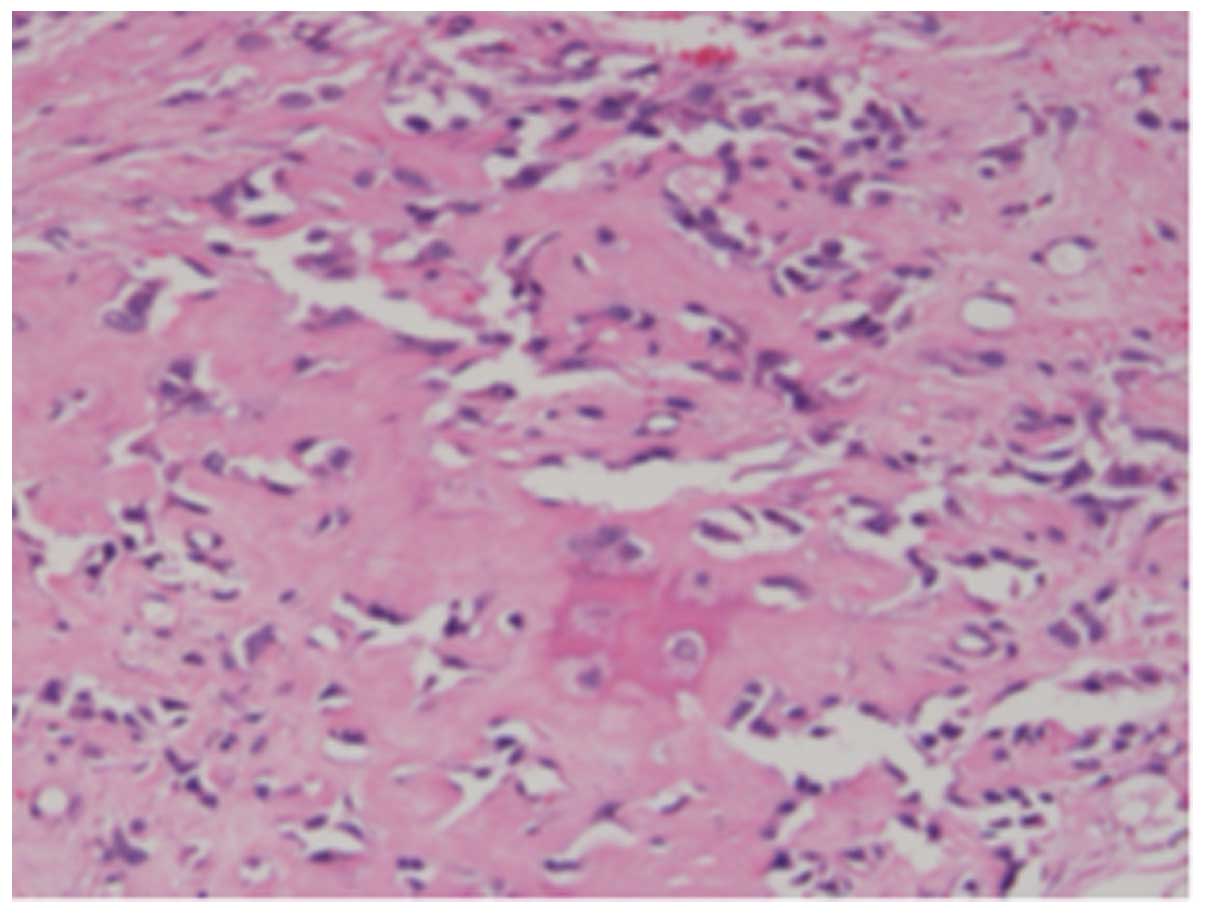
pathological examination identified disease-free margins and
<50% necrosis of high-grade osteosarcoma (Fig. 4). After 40 months of follow-up, no
clinical or radiographic evidence of recurrence or metastasis were
observed in the current patient.
Discussion
Advances in the diagnosis and management of
pediatric cancer have led to increasing long-term survival rates
(14). The initial follow-up of
off-treatment patients is aimed at detecting relapse and
treatment-associated morbidity (15).
Over time, surveillance includes monitoring for late effects of
therapy, including secondary malignancies, the risk of which is
higher in childhood cancer survivors compared with the general
population (16,17). A study of 14,358 childhood cancer
survivors by the Childhood Cancer Survivor Study cohort identified
an increased risk of second malignant neoplasm (SMN) among all
primary childhood cancer diagnoses. When compared with the general
population, the overall standardized incidence ratio of developing
SMN was 6.4, with an estimated 30-year cumulative incidence rate of
9.3% (17). The use of high-dose
chemotherapy to eradicate disease in these aggressive pediatric
malignancies, particularly alkylating agents, anthracyclines, and
epipodophyllotoxins, has been shown to increase the risk of
developing SMN (18). Furthermore,
radiation and autologous HSCT are also known to increase the risk
of SMN in children. In one of the largest studies to date, the
Center for International Blood and Marrow Transplant Research
assembled a cohort of 1,487 pediatric autologous transplant
recipients with a median age of 8 years (range, 1–21 years). SMN
was reported in 35 patients [AML/myelodysplastic syndrome (MDS),
n=13; solid tumor, n=20; subtype unknown, n=2] and the overall
cumulative incidence of SMN 10 years after autologous HSCT was
2.60% (AML/MDS, 1.06%; solid tumor, 1.30%). When compared with an
age- and gender-matched general population, autologous HSCT
recipients exhibited a 24-fold greater risk of developing SMN (95%
confidence interval, 16.0–33.0) (19).
Undergoing autologous HSCT also increases the risk
of developing osteochondroma. Nine Italian HSCT centers of the
Italian Association of Pediatric Hematology and Oncology assembled
a cohort of 1,632 pediatric transplant recipients; among them, a
total of 27 cases of osteochondroma (1.6%) were reported. Analysis
of cumulative risk stratified by various risk factors revealed that
male gender, autologous HSCT, age of <3 years at time of HSCT
and TBI significantly affected the risk of osteochondroma. However,
malignant degeneration of osteochondroma was not noted (20).
Malignant transformation of osteochondroma into
low-grade chondrosarcoma predominantly occurs in the cartilaginous
cap. This phenomenon is considered to occur stepwise, as follows:
̔Osteochondroma giving rise to peripheral low-grade chondrosarcoma
that in turn dedifferentiates into a high-grade sarcoma that may
appear as fibrosarcoma, malignant fibrous histiocytoma, and
osteosarcoma̓ (21). Low-grade
chondrosarcomas typically grow in the periphery, enlarging the
cartilaginous cap and, thus, producing clinical indicators that can
be identified on magnetic resonance imaging (22–24).
Certain cases of osteosarcoma secondary to osteochondroma
corroborate the stepwise theory and are composed of numerous types
of neoplastic tissue, such as normal osteochondroma, low-grade
chondrosarcoma and higher-grade osteosarcoma with a chondral
component, as described by Nojima et al (25). Other cases develop from the spongy
bone of the stalk and have no association with the cartilaginous
cap. In this type of secondary osteosarcoma, no thickening of the
cap is observed and a neoplastic cartilaginous component is not
present (22,26,27).
To the best of our knowledge, one previous report of
simultaneous occurrence of osteosarcoma and osteochondroma
following treatment of neuroblastoma with chemotherapy,
radiotherapy and BMT exists in the literature. The study reported
the case of an 11-year-old patient who presented with osteosarcoma
of the fourth right rib and osteochondroma of the left scapula 9.5
years after receiving radiation, chemotherapy, TBI and BMT for
abdominal neuroblastoma. However, the study did not report
malignant transformation of osteochondroma (7). Additionally, one study reported the case
of a 34-year-old man with chondrosarcoma arising from
radiation-induced osteochondroma of the left posterior eighth rib.
The osteochondroma developed following TBI received as part of a
conditioning regimen prior to undergoing BMT at the age of 8 years
(11).
The development of bone tumors in the current
patient may be attributed to more than one factor. For example, it
may be associated with the previous chemotherapy and radiotherapy
that were administered to treat the neuroblastoma 10 years earlier.
The present case illustrates the difficulty of assigning a single
factor to secondary tumors in a patient with cancer, particularly
when the patient has been treated with numerous therapeutic
modalities.
In conclusion, the current study presents the first
reported case of malignant transformation of osteochondroma into
osteosarcoma associated with TBI. Treatment with TBI prior to HSCT,
particularly in younger patients, results in a large number of
epiphyses at risk of injury and at an increased risk of malignant
degeneration, compared with osteochondroma arising in the absence
of radiotherapy. Considering that the average age of malignant
degeneration appears to be lower in this group of patients,
surveillance may have a role in clinical management in order to
avoid late complications of malignant degeneration and to improve
opportunities for curative treatment when this rare condition
occurs.
References
|
1
|
Faraci M, Barra S, Cohen A, et al: Very
late nonfatal consequences of fractionated TBI in children
undergoing bone marrow transplant. Int J Radiat Oncol Biol Phys.
63:1568–1575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paulino AC and Fowler BZ: Secondary
neoplasms after radiotherapy for a childhood solid tumor. Pediatr
Hematol Oncol. 22:89–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bordigoni P, Turello R, Clement L, et al:
Osteochondroma after pediatric hematopoietic stem cell
transplantation: Report of eight cases. Bone Marrow Transplant.
29:611–614. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paulino AC, Mayr NA, Simon JH and Buatti
JM: Locoregional control in infants with neuroblastoma: Role of
radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys.
52:1025–1031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcovici PA, Berdon WE and Liebling MS:
Osteochondromas and growth retardation secondary to externally or
internally administered radiation in childhood. Pediatr Radiol.
37:301–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bovée JVMG, Heymann D and Wuyts W:
OsteochondromaWHO Classification of Tumours of Soft Tissue and
Bone. Fletcher DM, Bridge JA, Hogendoorn PCW and Mertens F: IARC;
Lyon: pp. 250–251. 2013
|
|
7
|
Poustchi-Amin M, Leonidas JC and Elkowitz
SS: Simultaneous occurrence of osteosarcoma and osteochondroma
following treatment of neuroblastoma with chemotherapy,
radiotherapy, and bone marrow transplantation. Pediatr Radiol.
26:155–157. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez CA, Vietti T, Ackerman LV, Eagleton
MD and Powers WE: Tumors of the sympathetic nervous system in
children. An appraisal of treatment and results. Radiology.
88:750–760. 1967. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Breslow NE, Takashima JR, Whitton JA, et
al: Second malignant neoplasms following treatment for Wilm's
tumor: A report from the National Wilms' Tumor Study Group. J Clin
Oncol. 13:1851–1859. 1995.PubMed/NCBI
|
|
10
|
Mahboubi S, Dormans JP and D'Angio G:
Malignant degeneration of radiation-induced osteochondroma.
Skeletal Radiol. 26:195–198. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagata S, Shen RK, Laack NN, et al:
Chondrosarcoma arising within a radiation-induced osteochondroma
several years following childhood total body irradiation: Case
report. Skeletal Radiol. 42:1173–1177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brodeur GM, Pritchard J, Berthold F, et
al: Revisions of the international criteria for neuroblastoma
diagnosis, staging, and response to treatment. J Clin Oncol.
11:1466–1477. 1993.PubMed/NCBI
|
|
13
|
Suita S, Tajiri T, Sera Y, et al: Improved
survival for patients with advanced neuroblastoma after high-dose
combined chemotherapy based in part on N-myc amplification. J
Pediatr Surg. 35:1737–1741. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mariotto AB, Rowland JH, Yabroff KR, et
al: Long-term survivors of childhood cancers in the United States.
Cancer Epidemiol Biomarkers Prev. 18:1033–1040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldsby RE, Taggart DR and Ablin AR:
Surviving childhood cancer: The impact on life. Paediatr Drugs.
8:71–84. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedman DL, Whitton J, Leisenring W, et
al: Subsequent neoplasms in 5-year survivors of childhood cancer:
The Childhood Cancer Survivor Study. J Natl Cancer Inst.
102:1083–1095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meadows AT, Friedman DL, Neglia JP, et al:
Second neoplasms in survivors of childhood cancer: Findings from
the Childhood Cancer Survivor Study cohort. J Clin Oncol.
27:2356–2362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Varan A and Kebudi R: Secondary malignant
neoplasms after childhood cancer. Pediatr Hematol Oncol.
28:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Danner-Koptik KE, Majhail NS, Brazauskas
R, et al: Second malignancies after autologous hematopoietic cell
transplantation in children. Bone Marrow Transplant. 48:363–368.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faraci M, Bagnasco F, Corti P, et al:
AIEOP-HSCT Group: Osteochondroma after hematopoietic stem cell
transplantation in childhood. An Italian study on behalf of the
AIEOP-HSCT group. Biol Blood Marrow Transplant. 15:1271–1276. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garrison RC, Unni KK, McLeod RA, Pritchard
DJ and Dahlin DC: Chondrosarcoma arising in osteochondroma. Cancer.
49:1890–1897. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamovec J, Spiler M and Jevtic V:
Osteosarcoma arising in a solitary osteochondroma of the fibula.
Arch Pathol Lab Med. 123:832–834. 1999.PubMed/NCBI
|
|
23
|
Staals EL, Bacchini P, Mercuri M and
Bertoni F: Dedifferentiated chondrosarcomas arising in preexisting
osteochondromas. J Bone Joint Surg Am. 89:987–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Engel EE, Nogueira-Barbosa MH, Brassesco
MS, et al: Osteosarcoma arising from osteochondroma of the tibia:
Case report and cytogenetic findings. Genet Mol Res. 11:448–454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nojima T, Yamashiro K, Fujita M, et al: A
case of osteosarcoma arising in a solitary osteochondroma. Acta
Orthop Scand. 62:290–292. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bovée JV, Sakkers RJ, Geirnaerdt MJ,
Taminiau AH and Hogendoorn PC: Intermediate grade osteosarcoma and
chondrosarcoma arising in an osteochondroma. A case report of a
patient with hereditary multiple exostoses. J Clin Pathol.
55:226–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meissner SA, Vieth V, August C and
Winkelmann W: Radiology-pathology conference: Osteosarcoma in a
cartilaginous exostosis of the femur. Clin Imaging. 30:206–209.
2006. View Article : Google Scholar : PubMed/NCBI
|