Introduction
The antitumoral immune response is a complex
physiological process involving a variety of immune cells and
molecules, including membrane molecules and dissolubility factors
(1). As well as the engagement of T
cell receptors, costimulation with immune regulatory molecules is
required for the optimal activation of T cells (2). The B7 family, a group of costimulatory
and coinhibitory proteins, encompasses critical ligands that
interact with known and unknown receptors on the surface of T
lymphocytes, regulating stimulation or inhibition (3). The aberrant expression of members of the
B7 family is considered to be a mechanism by which human
malignancies may escape host immune surveillance (3–5).
B7-H3 [also known as cluster of differentiation (CD)
276], a member of the B7 family, is expressed on the surface of
lymphoid cells, including dendritic cells, monocytes/macrophages
and activated T cells. In addition, it is expressed on the surface
of non-lymphoid tissue cells, including epithelial, anterior
pituitary progenitor and muscle cells, as well as fibroblast-like
synoviocytes (3,4). At present, the regulatory role of B7-H3
in tumor immunity remains controversial. A number of studies
considered B7-H3 to be a costimulatory molecule promoting T-cell
activation and proliferation and, thus, resulting in enhanced tumor
immunity (4,5). However, other studies identified that a
higher expression of B7-H3 in prostate, colon, ovarian, pancreatic
and lung cancer tumors was positively correlated with more advanced
tumor stages and poorer prognosis (6–12).
The immunoglobulin superfamily, triggering receptor
expressed on myeloid cells (TREM), includes members such as the
TREM-like transcript-2 (TLT-2). TLT-2 is expressed on B cells,
granulocytes and macrophages, and is constitutively expressed on
CD8+ T cells. Furthermore, TLT-2 expression is induced
on CD4+ T cells following their activation (11). Hashiguchi et al (13) proposed that TLT-2 may be a
counter-receptor for B7-H3 and that the TLT-2/B7-H3 pathway
costimulates CD8+ T cell activation.
Oral squamous cell carcinoma (OSCC) presents a major
health problem worldwide; a total of 274,300 new cases are
diagnosed each year and the disease accounts for 128,000
mortalities, annually (14). OSCC is
the most common type of cancer in the oral cavity, accounting for
>90% of malignant neoplasms in this area (15). Approximately 96% of cases of OSCC are
preceded by dysplasia, which presents as white epithelial lesions
on the oral mucosa (leukoplakia). Dysplastic lesions in the form of
erythroplakia (red lesions)are also commonly observed, which
exhibit a 90% risk of malignant conversion (16). The majority of OSCC cases are
diagnosed by oral examination and ~50% of patients diagnosed with
OSCC succumb to the disease (17). At
present, treatment modalities include surgery, radiotherapy and
chemotherapy. However, despite the radical nature of treatment,
recurrences are common (18). Thus,
the identification of novel prognostic indicators is required to
aid diagnosis and selection of the most effective treatment methods
(19). To the best of our knowledge,
thus far, no studies have been conducted investigating the roles of
B7-H3 and TLT-2 in human OSCC. Therefore, the aim of the present
study was to investigate and compare the gene and protein
expression levels of B7-H3 and TLT-2 in human OSCC and healthy
mucosal tissue samples.
Materials and methods
Patient selection
The present cross-sectional study was conducted in
accordance with the principles outlined in the Declaration of
Helsinki (20). Tissue specimens from
patients and healthy subjects were collected according to the
procedures approved by the Ethics Committee of Peking University
Shenzhen Hospital (Shenzhen, China). Furthermore, all the patients
and healthy individuals provided written informed consent prior to
enrolment in this study.
Human OSCC samples were obtained from 76 patients
(female, 32; male, 44; median age, 50.9 years; age range, 23–81
years) who had undergone tumor surgery for OSCC at Peking
University Shenzhen Hospital between 2007 and 2010. All tumors were
staged according to the the Union for International Cancer Control
TNM classification system for head and neck tumors (21). A total of 46 samples exhibited
high-grade histological differentiation, 26 cases exhibited
moderate differentiation and 4 cases exhibited low-grade
differentation. Furthermore, the study included 19 stage T1 tumors,
31 stage T2 tumors, 21 stage T3 tumors and 5 stage T4 tumors. The
present study excluded patients with chronic disease, including
diabetes, liver disease, tuberculosis or autoimmune diseases (such
as rheumatoid arthritis or systemic lupus erythematosus), and
patients that had previously undergone chemotherapy, radiotherapy
or preoperative hormonal treatment. In addition, healthy oral
mucosal samples were obtained from 76 control subjects (female, 36;
male, 40; median age, 45.3 years; age range, 21–62 years) during
wisdom tooth extraction.
Tissue sampling
Immediately after surgical resection, all the tissue
samples were snap-frozen in liquid nitrogen (for RNA extraction) or
fixed in 10% buffered formalin solution and stored at −80°C, prior
to embedding in paraffin (for histopathological and
immunohistochemical analysis).
Immunohistochemistry
Immunohistochemical analyses were conducted using
monoclonal goat anti-human B7-H3 (1:200; cat no. AF1027; R&D
Systems, Inc., Minneapolis, MN, USA) and polyclonal goat anti-human
TLT-2 (1:200; cat. no. 032737R; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) primary antibodies. Antigen retrieval was achieved
by microwave pretreatment at 92°C in 0.01 mol/l ethylene diamine
tetraacetic acid buffer. Subsequently, xylene was used to
deparaffinize the paraffin-embedded tissue sections (4-µm),
followed by rehydration through graded alcohol into distilled
water. Endogenous peroxidase activity was quenched by incubating
the slides in 3% hydrogen peroxide in methanol for 10–15 min and
the samples were blocked using an avidin/biotin blocking kit
(Vector Laboratories Inc., Burlingame, CA, USA) for 5 min. The OSCC
sections were incubated with the primary antibodies overnight at
4°C; however, the antibodies were omitted from the negative control
samples. Next, a goat avidin-biotin complex staining kit (Santa
Cruz Biotechnology, Inc.) was used to incubate the samples with a
biotinylated rabbit anti-goat IgG secondary antibody (1:20; cat.
no. BA-9001; Beijing Zhongshan Golden Bridge Biotechnology, Co.,
Ltd., Beijing, China) and horseradish peroxidase enzyme-avidin
conjugate, according to the manufacturer's instructions. The
sections were subsequently counterstained with hematoxylin
(Sigma-Aldrich, St. Louis, MO, USA). The B7-H3 and TLT-2
immunoreactivity of all samples was independently evaluated by two
investigators in a blinded manner, based on the intensity of
staining as follows: 0, no staining; 1+, weak diffuse cytoplasmic
staining (<10% of the cancer cells may exhibit stronger
intensity); 2+, moderate to strong granular cytoplasmic staining,
present in 10–90% of the cancer cells; 3+, >90% of the tumor
cells exhibited strong intensity. All specimens were evaluated by
the two independent and blinded investigators using a multiheaded
microscope (CX31; Olympus Corporation, Tokyo, Japan) and the
consensus score was used for subsequent analyses (22).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The frozen tissue was homogenized using a
Mikro-Dismembrator U (Sartorius BBI Systems GmbH, Göttingen,
Germany), according to the manufacturer's instructions. Total RNA
was isolated using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) and cDNA was synthesized using the SuperScript®
First-Strand Synthesis system (Invitrogen Life Technologies),
according to the manufacturer's instructions. The sequences of the
primers used for amplification were as follows: Forward, 5′-CCC ATC
CCA CCC ATA ATT CTT ACCC-3′, and reverse, 5′-AGG CTC TGC CTT TTC
TGC TGC TATG-3′, for B7-H3 (product length, 182 bp; GenBank
accession no., NM_025240.2); forward, 5′-CCC CTT TTA CCA CTG GTG
TGA TGGT-3′, and reverse, 5′-GTG GTG CTG GTA GCA GTG AAG CTGT-3′,
for TLT-2 (product length, 118 bp; GenBank accession no.,
NM_024807.2); and forward, 5′-AAA CTG GAA CGG TGA AGGTG-3′, and
reverse, 5′-AGT GGG GTG GCT TTT AGGAT-3′, for β-actin (product
length, 166 bp; GenBank accession no., NM_001101.3). Quantitative
RT-PCR (qRT-PCR) was conducted in a 20-µl reaction solution
containing 2 µl cDNA, 15 µl 2X Power SYBR® Green PCR master mix
(Applied Biosystems Life Technologies, Warrington, UK) and 200 nM
of each pair of primers. The β-actin gene was used as an endogenous
control. Amplification was performed for 38 cycles at 94°C for 30
sec, 62°C for 30 sec and 72°C for 30 sec. The amplified PCR
products were quantified by gel electrophoresis and visualized
using the AlphaImager Mini System (ProteinSimple, San Jose, CA,
USA). Each analysis was run in triplicate, the relative
quantification of gene expression was determined using the
comparative mean [standard deviation (SD)] and the results were
normalized using the human β-actin housekeeping gene.
Statistical analysis
mRNA expression values between the OSCC and control
groups were compared by performing two sample t-tests using SPSS
software (version 11.0; SPSS, Inc., Chicago, IL, USA). Comparisons
between B7-H3 and TLT-2 protein expression levels, and pathological
features were evaluated by performing a χ2 test and
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical staining
Protein expression levels of B7-H3 and TLT-2 were
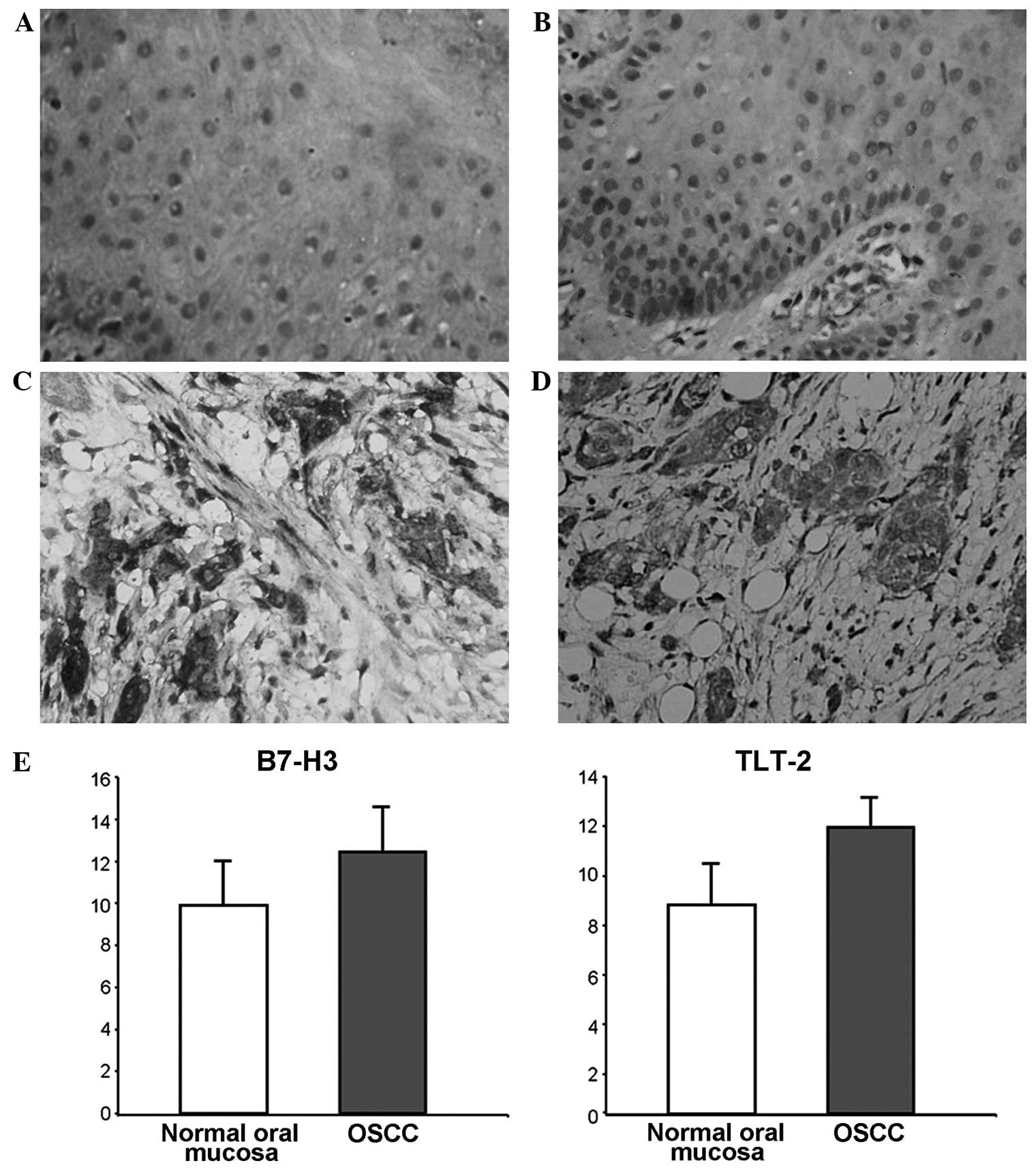
detected in healthy oral mucosa and OSCC tissue samples. In the
healthy oral mucosa, weak expression of B7-H3 (Fig. 1A) and TLT-2 (Fig. 1B) was sporadically detected within the
cell membrane of epithelial cells. However, elevated expression
levels of B7-H3 (Fig. 1C) and TLT-2
(Fig. 1D) protein were observed in
the OSCC tissue samples. The two proteins demonstrated similar
expression patterns, and were widely expressed within the cell
membrane and cytoplasm of malignant epithelial, vascular
endothelial and inflammatory cells.
RT-PCR
The mRNA expression levels of B7-H3 and TLT-2 were
detected in the OSCC and healthy oral mucosal tissue samples. As
expected, the RT-PCR products of B7-H3 and TLT-2 were 182 and 118
bp long, respectively.
qRT-PCR
The mRNA expression levels of B7-H3 and TLT-2 were
detected in all the OSCC and healthy oral mucosa specimens. The
expression levels of B7-H3 mRNA in the OSCC and healthy oral mucosa
tissues were 12.449 (SD, 2.14) and 9.899 (SD, 2.12), respectively.
By contrast, the expression levels of TLT-2 mRNA in the OSCC and
healthy oral mucosa tissues were 11.567 (SD, 1.24) and 8.493 (SD,
1.67), respectively. Compared with the expression in healthy oral
mucosa samples, the expression levels of B7-H3 (P=0.0004) and TLT-2
(P<0.0001) were significantly higher in OSCC specimens (Fig. 1E).
Clinicopathological factors associated
with B7-H3 and TLT-2 protein expression
The statistical correlation between the B7-H3 and
TLT-2 protein status and specific clinicopathological parameters
was investigated using χ2 analysis, as indicated in
Table I. B7-H3 and TLT-2
overexpression were not associated with the location, size or
metastatic status of the tumor. However, the B7-H3 and TLT-2
expression levels were positively associated with lymph node
metastasis (P=0.026 and P=0.021, respectively) and negatively
correlated with histological differentiation (P=0.011 and P=0.016,
respectively).
 | Table I.Clinicopathological factors associated
with B7-H3 and TLT-2 protein expression levels. |
Table I.
Clinicopathological factors associated
with B7-H3 and TLT-2 protein expression levels.
|
| B7-H3 |
| TLT-2 |
|
|---|
|
|
|
|
|
|
|---|
| Factor | Normal | Increased | P-value | Normal | Increased | P-value |
|---|
| Tumor location |
|
| 0.499a |
|
| 0.371a |
|
Tongue | 14 | 24 |
| 16 | 22 |
|
Gingiva | 2 | 6 |
| 4 | 4 |
|
| Buccal
mucosa | 3 | 14 |
| 5 | 12 |
|
|
Palate | 2 | 2 |
| 1 | 3 |
|
| Floor
of the mouth | 4 | 5 |
| 1 | 8 |
|
| Tumor size |
|
| 0.135b |
|
| 0.212b |
| T1 | 10 | 9 |
| 9 | 10 |
|
| T2 | 10 | 21 |
| 12 | 19 |
|
| T3 | 4 | 17 |
| 6 | 15 |
|
| T4 | 1 | 4 |
| 0 | 5 |
|
| Nodal status |
|
| 0.026c |
|
| 0.021c |
| N0 | 17 | 18 |
| 18 | 17 |
|
| N1 | 6 | 26 |
| 8 | 24 |
|
| N2 | 2 | 7 |
| 1 | 8 |
|
| Metastatic
status |
|
| 0.073d |
|
| 0.057d |
| M0 | 25 | 45 |
| 27 | 43 |
|
| M1 | 0 | 6 |
| 0 | 6 |
|
| Histological
differentiation |
|
| 0.011e |
|
| 0.016e |
|
High | 21 | 25 |
| 22 | 24 |
|
|
Moderate | 4 | 22 |
| 5 | 21 |
|
|
Low | 0 | 4 |
| 0 | 4 |
|
Discussion
The optimal activation of antigen-specific
lymphocytes requires a combination of T-cell receptor and
costimulatory signals; however, this activation may be inhibited by
coinhibitory signals (3–5). Recent studies demonstrated that specific
B7 family ligands, including B7-H1, B7-DC, B7-H3 and B7-H4, were
highly expressed in a wide spectrum of human cancer types,
including esophageal, gastric, lung, ovarian, breast, renal and
pancreatic cancer. Their expression levels positively correlated
with a more advanced tumor stage and poor prognosis (23–30).
Furthermore, it was detected that B7 molecules contribute to an
immune-suppressive tumor microenvironment (31). However, the role of B7-H3 in adaptive
immune responses remains controversial. B7-H3 was initially
identified as a costimulatory molecule that engages with its
receptor on T cells to promote T-cell activation and the secretion
of interferon-γ (13,32). However, a number of recent studies
have demonstrated that B7-H3 exhibits an inhibitory effect during
autoimmunity by coinhibiting T-cell function (6,33).
OSCC is the most common type of cancer in the oral
cavity; however, the role of the B7 family in OSCC has yet to be
fully investigated. Tsushima et al (34) examined the expression of five B7
molecules, including B7-H1, B7-DC, B7 h, CD80 and CD86, in nine
human OSCC cell lines and four biopsied OSCC specimens. Various
levels of B7-H1 and B7-DC expression were detected in the majority
of the investigated OSCC cell lines; however, B7-H1 was detected in
all the cell lines, as well as the biopsied human OSCC specimens.
To the best of our knowledge, the present study investigated for
the first time the protein and gene expression levels of B7-H3 in
human OSCC. The results indicated that B7-H3 was overexpressed in
OSCC and was a critical determinant for predicting lymph node
metastasis and histological differentiation. Furthermore, the
results revealed an inhibitory role of B7-H3 in tumor immunity
against OSCC.
The interaction between B7-H3 and TLT-2 remains
controversial. Hashiguchi et al (13) have previously proposed TLT-2 as a
counter-receptor to B7-H3. Furthermore, the same authors considered
that the TLT-2/B7-H3 pathway costimulates T cell activation;
however, alternative studies identified no evidence of an
interaction between B7-H3 and TLT-2 (35). The data presented in the current study
demonstrated a similar expression pattern of TLT-2 and B7-H3 in
OSCC and healthy oral mucosa specimens. The distribution of B7-H3
and TLT-2 overexpression in OSCC indicated a correlation with
inflammation, although the mechanism of the association between
inflammation and the overexpression of B7-H3 or TLT-2 requires
further investigation. In addition, the present data strongly
indicated that TLT-2 and B7-H3 are critical determinants in the
prediction of lymph node metastasis in OSCC tumors. However, the
underlying mechanism through which these two molecules contribute
to lymph node metastasis in oral cancer requires further
investigation.
In conclusion, the present study identified B7-H3
and TLT-2 overexpression in OSCC specimens. Furthermore, the B7-H3
and TLT-2 expression levels were significantly associated with
lymph node metastasis and histological differentiation in the OSCC
samples. Therefore, B7-H3 along with TLT-2 may exhibit an
inhibitory role on the antitumoral immune response in OSCC. In
order to investigate the interactions between these two molecules
and individual antitumoral immune responses in OSCC patients,
prospective clinical studies with adequate sample sizes should be
conducted.
Acknowledgements
The present study was supported by the Shenzhen
Basic Research Foundation (grant no. JC201105201030A), Guangdong
Province Nature Science Foundation (grant no. S2012010010382) and
Shenzhen Science and Research Innovation Foundation (grant no.
JCY20130402114702120).
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
qRT-PCR
|
quantitative reverse
transcription-polymerase chain reaction
|
|
TLT-2
|
triggering receptor expressed on
myeloid cell-like transcript-2
|
References
|
1
|
Huang MN, Yu H and Moudgil KD: The
involvement of heat-shock proteins in the pathogenesis of
autoimmune arthritis: a critical appraisal. Semin Arthritis Rheum.
40:164–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
North RJ and Bursuker I: Generation and
decay of the immune response to a progressive fibrosarcoma. I.
Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+
effector T cells. J Exp Med. 159:1295–1311. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zang X and Allison JP: The B7 family and
cancer therapy: costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen YW, Tekle C and Fodstad O: The
immunoregulatory protein human B7H3 is a tumor-associated antigen
that regulates tumor cell migration and invasion. Curr Cancer Drug
Targets. 8:404–413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chavin G, Sheinin Y, Crispen PL, et al:
Expression of immunosuppresive B7-H3 ligand by hormone-treated
prostate cancer tumors and metastases. Clin Cancer Res.
15:2174–2180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crispen PL, Sheinin Y, Roth TJ, et al:
Tumor cell and tumor vasculature expression of B7-H3 predict
survival in clear cell renal cell carcinoma. Clin Cancer Res.
14:5150–5157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roth TJ, Sheinin Y, Lohse CM, et al: B7-H3
ligand expression by prostate cancer: a novel marker of prognosis
and potential target for therapy. Cancer Res. 67:7893–7900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamato I, Sho M, Nomi T, et al: Clinical
importance of B7-H3 expression in human pancreatic cancer. Br J
Cancer. 101:1709–1716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun J, Chen LJ, Zhang GB, et al: Clinical
significance and regulation of the costimulatory molecule B7-H3 in
human colorectal carcinoma. Cancer Immunol Immunother.
59:1163–1171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zang X, Sullivan PS, Soslow RA, Waitz R,
Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, et al:
Tumor associated endothelial expression of B7-H3 predicts survival
in ovarian carcinomas. Mod Pathol. 23:1104–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B
and Zhang X: Diagnosis value of serum B7-H3 expression in non-small
cell lung cancer. Lung Cancer. 66:245–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashiguchi M, Kobori H, Ritprajak P, et
al: Triggering receptor expressed on myeloid cell-like transcript 2
(TLT-2) is a counter-receptor for B7-H3 and enhances T cell
responses. Proc Natl Acad Sci USA. 105:10495–10500. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elango KJ, Anandkrishnan N, Suresh A, Iyer
SK, Ramaiyer SK and Kuriakose MA: Mouth self-examination to improve
oral cancer awareness and early detection in a high-risk
population. Oral Oncol. 47:620–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silverman S Jr: Demographics and
occurrence of oral and pharyngeal cancers. The outcomes, the
trends, the challenge. J Am Dent Assoc. 132 (Suppl):7S–11S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Regezi JA, Sciubba JJ and Jordan RCK: Oral
Pathology: Clinical Pathologic Correlations. 5th. Elsevier
Saunders; St. Louis, MO: 2007
|
|
17
|
Greenlee RT, Murray T, Bolden S and Wingo
PA: Cancer statistics, 2000. CA Cancer J Clin. 50:7–33. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carvalho AL, Magrin J and Kowalski LP:
Sites of recurrence in oral and oropharyngeal cancers according to
the treatment approach. Oral Dis. 9:112–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Modjtahedi H: Molecular therapy of head
and neck cancer. Cancer Metastasis Rev. 24:129–146. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Roy PG: Helsinki and the Declaration of
Helsinki. World Med J. 50:9–11. 2004.
|
|
21
|
O'Sullivan B and Shah J: New TNM staging
criteria for head and neck tumors. Semin Surg Oncol. 21:30–42.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shuyi Y, Feng W, Jing T, et al: Human
beta-defensin-3 (hBD-3) upregulated by LPS via epidermal growth
factor receptor (EGFR) signaling pathways to enhance lymphatic
invasion of oral squamous cell carcinoma. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 112:616–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohigashi Y, Sho M, Yamada Y, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun J, Xu K, Wu C, et al: PD-L1 expression
analysis in gastric carcinoma tissue and blocking of
tumor-associated PD-L1 signaling by two functional monoclonal
antibodies. Tissue Antigens. 69:19–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Konishi J, Yamazaki K, Azuma M, et al:
B7-H1 expression on non-small cell lung cancer cells and its
relationship with tumor-infiltrating lymphocytes and their PD-1
expression. Clin Cancer Res. 10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamanishi J, Mandai M, Iwasaki M, et al:
Programmed cell death 1 ligand 1 and tumor-infiltrating
CD8+ T lymphocytes are prognostic factors of human
ovarian cancer. Proc Natl Acad Sci USA. 104:3360–3365. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghebeh H, Mohammed S, Al-Omair A, et al:
The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in
breast cancer patients with infiltrating ductal carcinoma:
correlation with important high-risk prognostic factors. Neoplasia.
8:190–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krambeck AE, Thompson RH, Dong H, et al:
B7-H4 expression in renal cell carcinoma and tumor vasculature:
associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tringler B, Zhuo S, Pilkington G, et al:
B7-h4 is highly expressed in ductal and lobular breast cancer. Clin
Cancer Res. 11:1842–1848. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nomi T, Sho M, Akahori T, et al: Clinical
significance and therapeutic potential of the programmed death-1
ligand/programmed death-1 pathway in human pancreatic cancer. Clin
Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chapoval AI, Ni J, Lau JS, et al: B7-H3: a
costimulatory molecule for T cell activation and IFN-gamma
production. Nat Immunol. 2:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Prasad DV, Nguyen T, Li Z, Yang Y, Duong
J, Wang Y and Dong C: Murine B7-H3 is a negative regulator of T
cells. J Immunol. 173:2500–2506. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsushima F, Tanaka K, Otsuki N, Youngnak
P, Iwai H, Omura K and Azuma M: Predominant expression of B7-H1 and
its immunoregulatory roles in oral squamous cell carcinoma. Oral
Oncol. 42:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leitner J, Klauser C, Pickl WF, et al:
B7-H3 is a potent inhibitor of human T-cell activation: No evidence
for B7-H3 and TREML2 interaction. Eur J Immunol. 39:1754–1764.
2009. View Article : Google Scholar : PubMed/NCBI
|