Introduction
Esophageal cancer represents the sixth most frequent
cause of cancer-related mortality worldwide (1). Histologically, esophageal cancer is
classified primarily as either squamous cell carcinoma (SCC) or
adenocarcinoma. Esophageal SCC (ESCC) accounts for approximately
one-third of esophageal cancer cases in the United States and
>90% of esophageal cancer cases worldwide (2,3). The risk
factors for ESCC include tobacco smoking, heavy alcohol consumption
and a diet lacking fresh fruits and vegetables (2,3). To date,
the prognosis of esophageal SCC remains poor, despite improvements
in surgical techniques, perioperative management, chemotherapy
and/or radiotherapy (4–6). Thus, studies on early detection, novel
treatment options, prevention and predictive tumor markers for ESCC
treatment and prognosis are urgently required.
Epidermal growth factor receptor (EGFR) is a part of
an important transmembrane signal transduction pathway in human
epithelial cells and altered EGFR protein expression or gene
amplification occurs in a number of solid tumors, including
esophageal cancer (7–13). EGFR-mediated signaling is crucial for
cell proliferation, as well as for cancer progression, including
tumor angiogenesis, metastasis and cancer cell resistance to
apoptosis. EGFR overexpression is mostly due to EGFR gene
amplification or mutations, with the latter occurring frequently in
EGFR exons 18–21, which encode the tyrosine kinase section of the
EGFR protein. Previous studies have demonstrated an association
between EGFR alterations and aggressive biological behaviors (e.g.,
tumor cell dedifferentiation, advanced tumor stage or cancer
metastasis) of different human cancers of epithelial origin,
including head and neck, breast, colon, stomach and lung cancer
(7–19). It was also demonstrated that EGFR
overexpression and gene amplification are correlated with treatment
response or survival rates among patients with breast and lung
cancer (20–22). EGFR amplification and protein
overexpression have been reported in ESCC and premalignant lesions
(3,9,19) and the
overexpression of EGFR was found to be significantly associated
with the depth of invasion of the tumor (9). EGFR gene amplification may be a useful
biological marker for the prediction of lymph node metastasis and
poor prognosis for ESCC (19). In
addition, EGFR gene mutations are rare in ESCC, although they have
been reported (23).
The aim of the present study was to evaluate EGFR
overexpression using immunohistochemistry and EGFR gene
amplification using fluorescence in situ hybridization
(FISH) in ESCC tissue specimens, in order to determine the
associations between patient clinicopathological characteristics
and survival rates and the association between EGFR gene
amplification and overexpression in ESCC tissues.
Materials and methods
ESCC patients and tissue samples
In this retrospective study, a total of 56 tissue
specimens from patients with surgically resected ESCC were obtained
from Zhejiang Cancer Hospital (Hangzhou, China). The diagnosis of
these patients was based on the primary tumor-regional lymph
node-distant metastasis (TNM) staging system described in the
American Joint Committee on Cancer Staging manual, seventh edition
(24). The present study was approved
by the Ethics Review Committee of the Zhejiang Cancer Hospital. The
patients were followed up regularly following surgery for tumor
recurrence and metastasis, vital status, mortality and cause of
mortality. The last follow-up was conducted in June, 2012.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were
retrieved from the Department of Pathology (Zhejiang Cancer
Hospital) and cut into 4-mm sections for immunohistochemical
staining. The sections were first deparaffinized in xylene
(Chinasun Speciality Products Co., Ltd., Changshu, China) and
rehydrated in ethanol (Shanghai Ling Feng Chemical Reagent Co.,
Ltd., Changshu, China), subjected to antigen retrieval in 10 mM
citrate buffer (pH 9.0; Fuzhou Maixin Biotech Co., Ltd., Fuzhou,
China) using microwave irradiation and treated with 3% hydrogen
peroxide (Dako, Hamburg, Germany). The sections were subsequently
incubated with a prediluted primary rabbit monoclonal anti-EGFR
antibody (clone no. 5B7; cat no. 790–4347; Ventana Medical Systems,
Inc., Tucson, AZ, USA), a monoclonal mouse anti-human Ki67 antibody
(dilution, 1:400; clone no. MIB-1; cat. no. M7240; Dako) or a
monoclonal mouse anti-cyclin-D1 antibody (dilution, 1:100; clone
no. SP4; cat. no. RM-9104-S; Neomarker, Fremont, CA, USA) at 4°C
overnight. On the following day, the sections were further
incubated with an EnVision kit indirect peroxidase system (Dako)
and visualized using 3,3′-diaminobenzidine (Dako) as a chromogen.
The sections were then counterstained with hematoxylin and viewed
under a BX43 system microscope (Olympus Corporation, Tokyo, Japan)
for the evaluation of the percentage and intensity of nuclear and
non-nuclear staining in tumor cells, or background staining, by two
independent observers in a blinded manner.
The intensity of the immunohistochemical staining
was reviewed and scored using a four-tier system as follows: 0, no
discernible staining or background staining; 1+, definitive
cytoplasmic staining and/or equivocal discontinuous membrane
staining; 2+, unequivocal membrane staining with moderate
intensity; and 3+, strong and complete plasma membrane staining
(7,8,25). Scores
of 2+ and 3+ were classified as overexpression, whereas scores of 0
and 1 were classified as low expression (9).
FISH
FISH was used to assess EGFR gene
amplification in tissue microarray sections of all ESCC cases using
the Vysis EGFR/CEP7 FISH Probe kit (Abbott Laboratories, Abbott
Park, IL, USA) according to the manufacturer's instructions.
Chromosome 7 was considered to be amplified when the ratio of the
mean copy number of chromosome 7 centromeres (EGFR/CEP7 genes) was
>2.2, whereas a ≥two-fold increase in the EGFR signal
relative to the CEP7 signal was considered as EGFR
amplification.
Statistical analysis
All data were analyzed anonymously. The
χ2 or Fisher's exact test was used for independent data
to identify the associations between EGFR alterations and
clinicopathological factors. Overall survival (OS) was calculated
according to a Kaplan-Meier curve and the log-rank test was used to
evaluate the statistical significance of the differences. The
multivariate analysis was performed using the Cox proportional
hazard method. All the statistical analyses were performed using
SPSS v.16 software for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 in a two-tailed test was considered to indicate
statistically significant differences.
Results
Patient characteristics
The characteristics of the patients are summarized
in Table I. In brief, there were 50
male and 6 female patients, with a median age of 61 years (mean,
59.6±7.6 years; range, 42–76 years). Of the 56 tumors, 23 (41.1%)
were located in the lower and 33 (58.9%) in the upper and middle
esophagus. A total of 8 tumors (14.3%) were well-differentiated, 40
(71.4%) were moderately differentiated and 8 (14.3%) were poorly
differentiated SCCs, according to the World Health Organization
criteria. In addition, 31 patients had stage II and 25 patients had
stage III disease. A total of 51 patients underwent esophagectomy
with a two-field technique and 5 patients with a three-field
technique. Of the 56 patients, 21 received postoperative
chemotherapy and/or radiotherapy, including 13 patients treated
with combined chemotherapy and radiotherapy and 8 patients treated
with chemotherapy alone.
 | Table I.Associations of EGFR overexpression
and gene amplification with the clinicopathological characteristics
of patients with esophageal squamous cell carcinoma. |
Table I.
Associations of EGFR overexpression
and gene amplification with the clinicopathological characteristics
of patients with esophageal squamous cell carcinoma.
|
|
| Patient no.
(%) |
|---|
|
|
|
|
|---|
|
Characteristics | Total no. | EGFR−
(n=26) | EGFR+
(n=30) | P-value | FISH−
(n=43) | FISH+
(n=13) | P-value |
|---|
| Age (years) |
|
|
| 0.757 |
|
| 0.480 |
|
<65 | 42 | 20 (47.6) | 22 (52.4) |
| 31 (73.8) | 11 (26.2) |
|
|
≥65 | 14 | 6
(42.9) | 8
(57.1) |
| 12 (85.7) | 2
(14.3) |
|
| Gender |
|
|
| 0.401 |
|
| 0.615 |
|
Male | 50 | 22 (44.0) | 28 (56.0) |
| 39 (78.0) | 11 (22.0) |
|
|
Female | 6 | 4
(66.7) | 2
(33.3) |
| 4
(66.7) | 2
(33.3) |
|
| Tumor
differentiation |
|
|
| 0.047 |
|
| 0.241 |
|
High | 8 | 6
(75.0) | 2
(25.0) |
|
8 (100.0) | 0
(0.0) |
|
|
Moderate | 40 | 18 (45.0) | 22 (55.0) |
| 29 (72.5) | 11 (27.5) |
|
|
Poor | 8 | 2
(25.0) | 6
(75.0) |
| 6
(75.0) | 2
(25.0) |
|
| Vascular
invasion |
|
|
| 0.693 |
|
| 0.553 |
|
Yes | 7 | 4
(57.1) | 3
(42.9) |
| 6
(85.7) | 1
(14.3) |
|
| No | 49 | 22 (44.9) | 27 (55.1) |
| 37 (75.5) | 12 (24.5) |
|
| Tumor location |
|
|
| 0.145 |
|
| 0.827 |
|
Upper/middle | 33 | 18 (54.5) | 15 (45.5) |
| 25 (75.8) | 8
(24.2) |
|
|
Lower | 23 | 8
(34.8) | 15 (65.2) |
| 18 (78.3) | 5
(21.7) |
|
| pT stage |
|
|
| 0.305 |
|
| 0.870 |
| T2 | 12 | 4
(33.3) | 8
(66.7) |
| 9
(75.0) | 3
(25.0) |
|
| T3 | 44 | 22 (50.0) | 22 (50.0) |
| 34 (77.3) | 10 (22.7) |
|
| pN stage |
|
|
| 0.972 |
|
| 0.002 |
| N0 | 27 | 14 (51.9) | 13 (48.1) |
| 26 (96.3) | 1
(3.7) |
|
| N1 | 21 | 8
(38.1) | 13 (61.9) |
| 13 (61.9) | 8
(38.1) |
|
| N2 | 6 | 2
(33.3) | 4
(66.7) |
| 3
(50.0) | 3
(50.0) |
|
| N3 | 2 |
2 (100.0) | 0
(0.0) |
| 1
(50.0) | 1
(50.0) |
|
| pTNM stage |
|
|
| 0.832 |
|
| 0.042 |
| II | 31 | 14 (45.2) | 17 (54.8) |
| 27 (87.1) | 4
(12.9) |
|
|
III | 25 | 12 (48.0) | 13 (52.0) |
| 16 (64.0) | 9
(36.0) |
|
Expression of EGFR protein in ESCC
tissues
The EGFR protein was expressed in 55 (98.2%) of the
56 tissue specimens, among which 30 cases (53.6%) were found to
overexpress the EGFR protein [10 cases (17.9%) were scored as 3+
and 20 cases (35.7%) as 2+]; the remaining 26 ESCC cases (46.4%)
exhibited low EGFR protein expression [25 cases (44.6%) were scored
as 1+ and 1 case (1.8%) as 0]. The EGFR protein was expressed
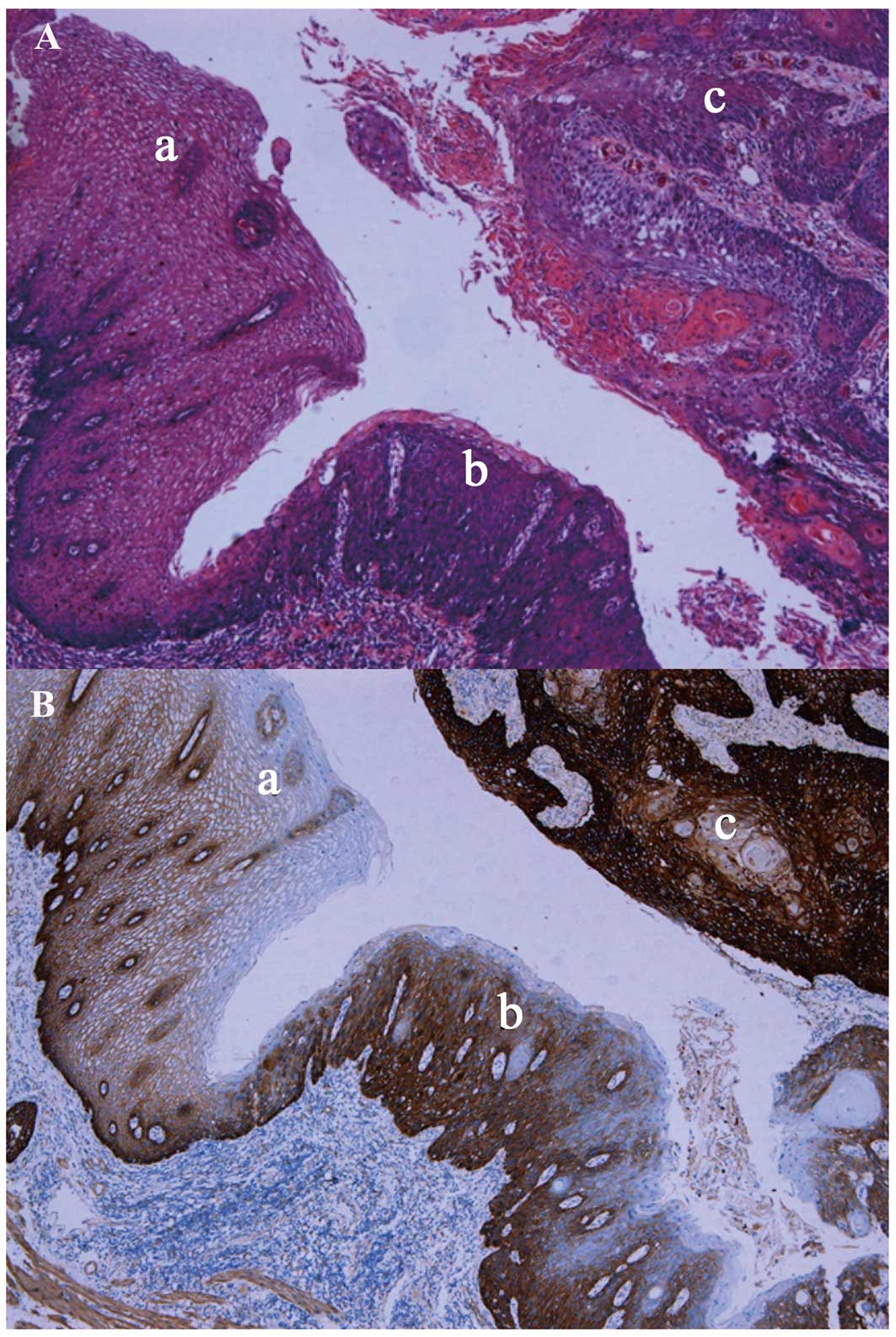
differentially in normal vs. abnormal tissues. For example, a
tissue with atypical epithelial hyperplasia had a score of 2+ for
EGFR staining, whereas normal esophageal tissue was negative for
EGFR immunostaining (Fig. 1).
Furthermore, the expression of the EGFR protein also differed
according to differentiation; i.e., poorly differentiated ESCC
tissues expressed high levels of the EGFR protein (score 3+), while
moderately differentiated ESCC tissues moderately expressed the
EGFR protein (score 2+). By contrast, well-differentiated ESCC
tissues expressed low levels of the EGFR protein (score 1+)
(P=0.047, Fig. 1). However, there
were no statistically significant correlations between EGFR
expression and age, gender, presence of vascular invasion, tumor
location, T stage, N stage, distant metastasis, or pathological TNM
stage (P>0.05, Table I).
EGFR amplification in ESCC
tissues
The FISH analysis demonstrated that the EGFR
gene was amplified in 13 (23.2%) of the 56 tumor samples. On
immunohistochemical staining, 11 of these samples exhibited a high
EGFR protein expression, while the remaining 2 samples exhibited
low EGFR protein expression. Clinically, 12 (92%) of these 13
patients received postoperative adjuvant chemotherapy. EGFR
gene amplification was associated with tumor lymph node metastasis
(P=0.002) and advanced pathological TNM stage (P=0.042). However,
no statistically significant correlations were identified between
EGFR gene amplification and other clinicopathological
parameters (Table I). EGFR
protein expression was statistically significantly associated with
EGFR gene amplification (P<0.05; Table II).
 | Table II.Association of EGFR protein
expression with gene amplification in esophageal squamous cell
carcinoma tissues. |
Table II.
Association of EGFR protein
expression with gene amplification in esophageal squamous cell
carcinoma tissues.
|
| EGFR
amplification |
|
|---|
|
|
|
|
|---|
| EGFR protein
expression level | – | + | P-value |
|---|
| 0 | 1 | 0 |
|
| 1+ | 25 | 2 |
|
| 2+ | 19 | 3 |
|
| 3+ | 11 | 8 | <0.05 |
Prognostic significance of EGFR
alterations
All the patients were followed up until June, 2012.
Among these patients, 26 succumbed to cancer-related ailments, with
a median OS of 24 months (range, 1–53 months). Clinicopathological
characteristics, including age, gender, pT stage, lymph node
metastasis, tumor differentiation, vascular invasion and EGFR
alterations were correlated with OS in patients with ESCC. The
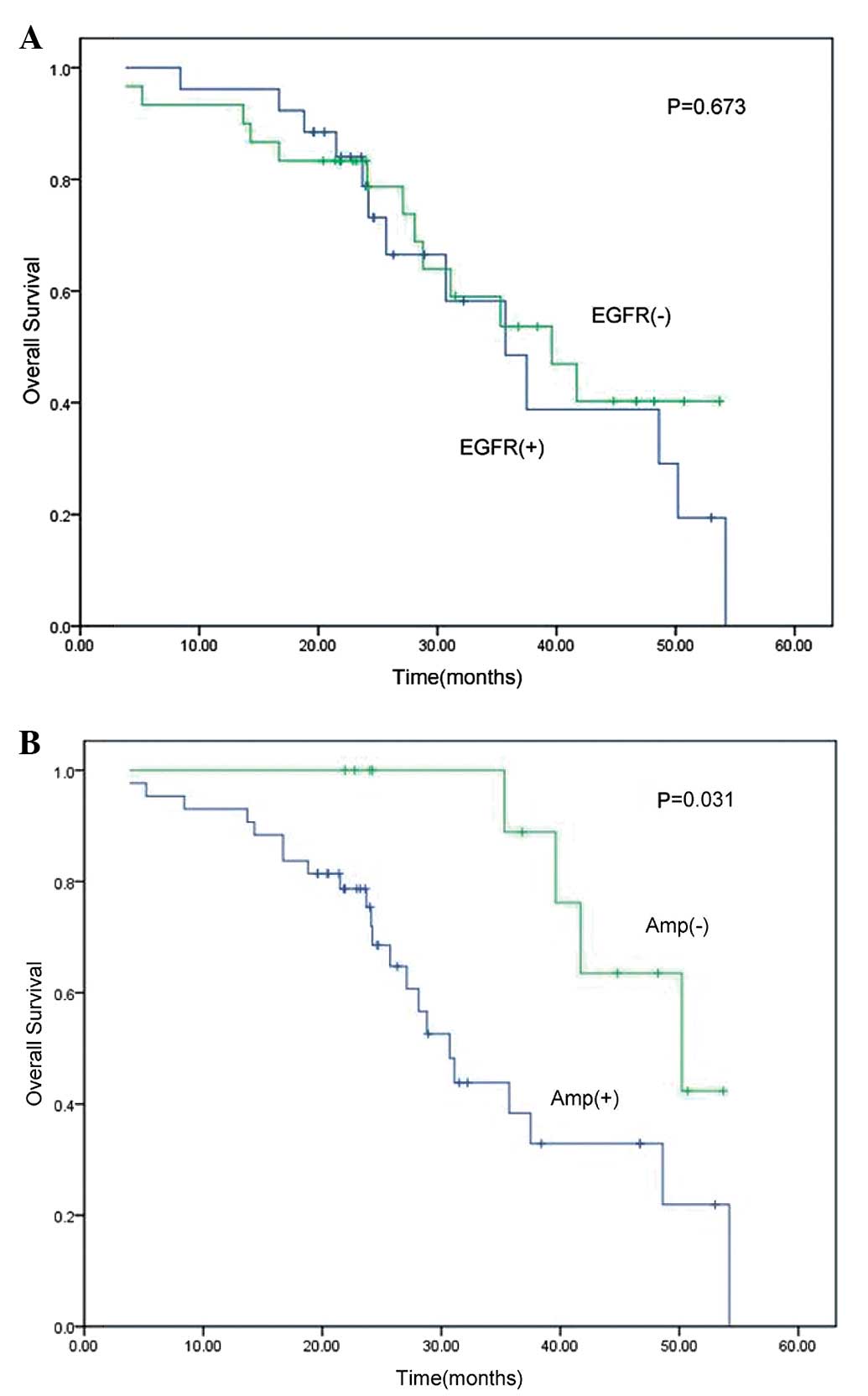
univariate analysis revealed no association between EGFR protein
expression and OS (P=0.673; Table
III, Fig. 2A), although
EGFR gene amplification was able to predict OS in these
patients (P=0.031; Table III,
Fig. 2B). However, on multivariate
analysis, there was only a marginal association between EGFR
gene amplification and OS (P=0.056), indicating that a larger
sample size is required.
 | Table III.Univariate and multivariate
regression analyses of overall survival in patients with esophageal
squamous cell carcinoma. |
Table III.
Univariate and multivariate
regression analyses of overall survival in patients with esophageal
squamous cell carcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95%CI |
P-valuea | HR | 95%CI |
P-valuea |
|---|
| Ageb | 1.358 | 0.533–3.462 | 0.520 | 1.301 | 0.607–3.011 | 0.490 |
| Gender
c | 1.574 | 0.461–5.380 | 0.460 | 1.427 | 0.433–4.557 | 0.570 |
|
Locationd | 0.927 | 0.408–2.106 | 0.850 | 1.020 | 0.481–2.230 | 0.880 |
| Differentiation
e | 0.670 | 0.305–1.474 | 0.320 | 0.737 | 0.371–1.487 | 0.430 |
| Vascular
invasionf | 1.970 | 0.652–5.953 | 0.220 | 1.332 | 0.587–4.026 | 0.610 |
| pT
statusg | 1.715 | 0.586–5.016 | 0.320 | 1.492 | 0.673–4.542 | 0.430 |
| pN
statush | 1.013 | 0.456–2.252 | 0.970 | 1.162 | 0.531–2.103 | 0.760 |
| pTNM
stagei | 1.075 | 0.489–2.362 | 0.850 | 0.983 | 0.565–1.967 | 0.960 |
| EGFR
expressionj | 0.793 | 0.233–2.341 | 0.673 | 0.710 | 0.167–2.015 | 0.640 |
| EGFR gene
amplificationk | 0.392 | 0.146–0.896 | 0.031 | 0.392 | 0.197–1.062 | 0.056 |
| Postoperative
treatmentl | 1.320 | 0.967–1.802 | 0.080 | 1.296 | 0.985–1.874 | 0.062 |
Discussion
EGFR and its signaling play important roles in tumor
cell proliferation, migration, apoptosis resistance and
angiogenesis. Thus, the role of EGFR overexpression and gene
amplification in cancer development, progression and aggressiveness
has been extensively investigated (10–12). Upon
binding of the EGFR extracellular domain to several different
ligands (including EGF and transforming growth factor-α), the EGFR
protein forms a dimerized receptor to activate the EGFR
intracellular tyrosine kinase domain, triggering a cascade of
phosphorylation events in the cytoplasm, which result in the
activation of target gene transcription and expression and a
subsequent change in cell behavior. Mitogen-activated protein
kinases (MAPKs), activator protein 1 and protein kinase B are all
downstream cascade genes in the EGFR signaling pathway (26). For example, EGFR activates MAPKs and
MAPK/extracellular signal-regulated kinase (ERK) kinase through Ras
and then activates ERK1/2, which, in turn, translocates into the
nucleus and promotes the expression of genes, including c-Jun,
c-Fos and cyclooxygenase-2, thus promoting cell growth and
angiogenesis (27). It was previously
demonstrated that EGFR overexpression occurs in ≤65% of ESCC cases
(13,28), whereas EGFR gene amplification occurs
in 2–19% of ESCC cases (14,15,29,30). The
present study demonstrated overexpression of the EGFR protein in
53.6% of the ESCC cases and gene amplification in 23.2% of the
cases. These data suggest that overexpression of the EGFR protein
and increased EGFR gene copy number are frequent events in ESCC;
thus, targeted therapy using anti-EGFR inhibitors may be effective
in treating ESCC.
Previous studies also demonstrated that EGFR
overexpression/gene amplification may be indicative of unfavorable
parameters for ESCC (16,17). For example, Delektorskaya et al
(18) reported that overexpression of
the EGFR protein is significantly correlated with tumor
intravascular invasion and depth of invasion, whereas EGFR gene
amplification is associated with tumor dedifferentiation. Kitagawa
et al (19) demonstrated a
significant correlation between EGFR gene amplification and ESCC
lymph node metastasis. The present study revealed that EGFR protein
expression was associated with ESCC dedifferentiation, whereas EGFR
gene amplification was associated with advanced stage and lymph
node metastasis. These data are consistent with the results of
previous studies (14–19). Furthermore, Nicholson et al
(31) demonstrated that EGFR
overexpression is correlated with prognosis in esophageal, head and
neck, ovarian, cervical and bladder cancer. In these types of
cancer, increased EGFR expression was found to be associated with
reduced recurrence-free survival or OS rates. Other previous
studies also demonstrated that EGFR overexpression/gene
amplification is associated with poor postoperative prognosis,
reduced OS and an increased risk of local recurrence in patients
with ESCC (15,32–34).
However, our data did not demonstrate that EGFR overexpression is
of prognostic value for ESCC; however, EGFR gene amplification may
predict a poorer prognosis. The reason for this discrepancy is
unknown, although it may be due to the antibody used to detect EGFR
protein expression or the quality of the tissue specimens. In
addition, the present data revealed that patients in whom the EGFR
protein was overexpressed exhibited a higher EGFR gene
amplification rate compared with those with low EGFR protein
expression. Further studies, including larger sample sizes, are
required to confirm our data.
The present findings suggest that the frequent
alterations of EGFR in patients with ESCC may indicate that EGFR is
a candidate for targeted therapy using anti-EGFR inhibitors, such
as nimotuzumab, cetuximab and gefinitib. Indeed, a previous in
vitro study demonstrated that nimotuzumab, an anti-EGFR
monoclonal antibody, promotes the radiosensitivity of
EGFR-overexpressing ESCC cells (35).
A phase II clinical trial demonstrated that cetuximab is an
effective and safe adjuvant to chemotherapy and radiotherapy for
esophageal cancer patients with a clinical complete response rate
of 70% (40/57) (36). Another phase
II study, which used gefinitib as second-line treatment for
advanced esophageal cancer, reported a significantly higher disease
control rate (overall response and stable disease) in patients with
EGFR-overexpressing ESCC (37).
However, there are currently no established eligibility criteria
for targeted therapy in patients with ESCC; for example, it is not
clear whether EGFR overexpression or gene amplification should be
used as an indicator. Thus, there is a requirement for predictive
biomarkers that identify the ESCC patients most likely to respond
to EGFR-targeted therapy. Evaluation of EGFR overexpression
detected by immunohistochemistry and EGFR gene amplification
detected by FISH may aid the selection of patients and prediction
of sensitivity to adjuvant EGFR-targeted therapy for ESCC.
There were certain limitations to this study,
including the small sample size and fact that only patients with
ESCC were recruited. The results of the present study demonstrated
that EGFR overexpression and increased EGFR gene copy number are
common events in ESCC and contribute to ESCC malignant biological
behaviors, including tumor dedifferentiation and lymph node
metastasis. Therefore, EGFR gene amplification may be useful in
predicting the OS of patients with ESCC.
Acknowledgements
The authors would like to thank Mr. Li-Ming Sheng
for his assistance in data collection and analysis. This study was
supported in part by the China Wu Jieping Medical Foundation-EGFR
targeted therapy basic research projects (no. 08-ZH-006). This
study was presented on May 30th to June 3th at the 50th annual
meeting of the American Society of Clinical Oncology in Chicago,
IL, USA (Abstract ID: e15043).
Glossary
Abbreviations
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
EGFR
|
epidermal growth factor receptor
|
|
FISH
|
fluorescence in situ
hybridization
|
|
OS
|
overall survival
|
References
|
1
|
Homs MY, Voest EE and Siersema PD:
Emerging drugs for esophageal cancer. Expert Opin Emerg Drugs.
14:329–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoner GD and Gupta A: Etiology and
chemoprevention of esophageal squamous cell carcinoma.
Carcinogenesis. 22:1737–1746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mandard AM, Hainaut P and Hollstein M:
Genetic steps in the development of squamous cell carcinoma of the
esophagus. Mutat Res. 462:335–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neuner G, Patel A and Suntharalingam M:
Chemoradiotherapy for esophageal cancer. Gastrointest Cancer Res.
3:57–65. 2009.PubMed/NCBI
|
|
5
|
Campbell NP and Villaflor VM: Neoadjuvant
treatment of esophageal cancer. World J Gastroenterol.
16:3793–3803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shah MA and Kelsen DP: Combined modality
therapy of esophageal cancer: changes in the standard of care? Ann
Surg Oncol. 11:641–643. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takehana T, Kunitomo K, Suzuki S, et al:
Expression of epidermal growth factor receptor in gastric
carcinomas. Clin Gastroenterol Hepatol. 1:438–445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ooi A, Takehana T, Li X, et al: Protein
overexpression and gene amplification of HER-2 and EGFR in
colorectal cancers: an immunohistochemical and fluorescent in situ
hybridization study. Mod Pathol. 17:895–904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanawa M, Suzuki S, Dobashi Y, et al: EGFR
protein overexpression and gene amplification in squamous cell
carcinomas of the esophagus. Int J Cancer. 118:1173–1180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Normanno N, De Luca A, Bianco C, et al:
Epidermal growth factor receptor (EGFR) signaling in cancer. Gene.
366:2–16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shepard HM, Brdlik CM and Schreiber H:
Signal integration: a framework for understanding the efficacy of
therapeutics targeting the human EGFR family. J Clin Invest.
118:3574–3581. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Sheng L and Mao W: Role of epidermal
growth factor receptor tyrosine kinase inhibitors in the treatment
of esophageal carcinoma and the suggested mechanisms of action.
Oncol Lett. 5:19–24. 2013.PubMed/NCBI
|
|
13
|
Suo Z, Su W, Holm R and Nesland JM: Lack
of expression of c-erbB-2 oncoprotein in human esophageal squamous
cell carcinomas. Anticancer Res. 15:2797–2798. 1995.PubMed/NCBI
|
|
14
|
Sunpaweravong P, Sunpaweravong S,
Puttawibul P, et al: Epidermal growth factor receptor and cyclin D1
are independently amplified and overexpressed in esophageal
squamous cell carcinoma. J Cancer Res Clin Oncol. 131:111–119.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sato-Kuwabara Y, Neves JI, Fregnani JH,
Sallum RA and Soares FA: Evaluation of gene amplification and
protein expression of HER-2/neu in esophageal squamous cell
carcinoma using fluorescence in situ hybridization (FISH) and
immunohistochemistry. BMC Cancer. 9:62009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akamatsu M, Matsumoto T, Oka K, et al:
c-erbB-2 oncoprotein expression related to chemoradioresistance in
esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys.
57:1323–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gotoh M, Takiuchi H, Kawabe S, et al:
Epidermal growth factor receptor is a possible predictor of
sensitivity to chemoradiotherapy in the primary lesion of
esophageal squamous cell carcinoma. Jpn J Clin Oncol. 37:652–657.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delektorskaya VV, Chemeris GY, Zavalishina
LE, et al: Squamous cell carcinoma of the esophagus: evaluation of
the status of epidermal growth factor receptors (EGFR and HER-2) by
immunohistochemistry and in situ hybridization. Bull Exp Biol Med.
149:615–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitagawa Y, Ueda M, Ando N, Ozawa S,
Shimizu N and Kitajima M: Further evidence for prognostic
significance of epidermal growth factor receptor gene amplification
in patients with esophageal squamous cell carcinoma. Clin Cancer
Res. 2:909–914. 1996.PubMed/NCBI
|
|
20
|
Park HS, Jang MH, Kim EJ, et al: High EGFR
gene copy number predicts poor outcome in triple-negative breast
cancer. Mod Pathol. 27:1212–1222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwangbo W, Lee JH, Ahn S, et al: EGFR gene
amplification and protein expression in invasive ductal carcinoma
of the breast. Korean J Pathol. 47:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cappuzzo F, Hirsch FR, Rossi E, et al:
Epidermal growth factor receptor gene and protein and gefitinib
sensitivity in non-small-cell lung cancer. J Natl Cancer Inst.
97:643–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sudo T, Mimori K, Nagahara H, et al:
Identification of EGFR mutations in esophageal cancer. Eur J Surg
Oncol. 33:44–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: the 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki S, Dobashi Y, Sakurai H, Nishikawa
K, Hanawa M and Ooi A: Protein overexpression and gene
amplification of epidermal growth factor receptor in nonsmall cell
lung carcinomas. An immunohistochemical and fluorescence in situ
hybridization study. Cancer. 103:1265–1273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oda K, Matsuoka Y, Funahashi A and Kitano
H: A comprehensive pathway map of epidermal growth factor receptor
signaling. Mol Syst Biol. 1:2005.0010–2005. PubMed/NCBI
|
|
27
|
Song S, Lippman SM, Zou Y, Ye X, Ajani JA
and Xu XC: Induction of cyclooxygenase-2 by benzo[a]pyrene diol
epoxide through inhibition of retinoic acid receptor-beta 2
expression. Oncogene. 24:8268–8276. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abedi-Ardekani B, Dar NA, Mir MM, et al:
Epidermal growth factor receptor (EGFR) mutations and expression in
squamous cell carcinoma of the esophagus in central Asia. BMC
Cancer. 12:6022012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mimura K, Kono K, Hanawa M, et al:
Frequencies of HER-2/neu expression and gene amplification in
patients with oesophageal squamous cell carcinoma. Br J Cancer.
92:1253–1260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reichelt U, Duesedau P, Tsourlakis MCh, et
al: Frequent homogeneous HER-2 amplification in primary and
metastatic adenocarcinoma of the esophagus. Mod Pathol. 20:120–129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):9–15. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dreilich M, Wanders A, Brattström D, et
al: HER-2 overexpression (3+) in patients with squamous cell
esophageal carcinoma correlates with poorer survival. Dis
Esophagus. 19:224–231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gibault L, Metges JP, Conan-Charlet V, et
al: Diffuse EGFR staining is associated with reduced overall
survival in locally advanced oesophageal squamous cell cancer. Br J
Cancer. 93:107–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Q, Zhu H, Xiao Z, et al: Expression
of epidermal growth factor receptor is an independent prognostic
factor for esophageal squamous cell carcinoma. World J Surg Oncol.
11:2782013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, He LR, Xi M, et al: Nimotuzumab
promotes radiosensitivity of EGFR-overexpression esophageal
squamous cell carcinoma cells by upregulating IGFBP-3. J Transl
Med. 10:2492012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Safran H, Suntharalingam M, Dipetrillo T,
et al: Cetuximab with concurrent chemoradiation for esophagogastric
cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys.
70:391–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Janmaat ML, Gallegos-Ruiz MI, Rodriguez
JA, et al: Predictive factors for outcome in a phase II study of
gefitinib in second-line treatment of advanced esophageal cancer
patients. J Clin Oncol. 24:1612–1619. 2006. View Article : Google Scholar : PubMed/NCBI
|