Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare
and primarily occur in patients <16 years of age (1). An IMT may also be defined as a plasma
cell granuloma, inflammatory myofibrohistiocytic proliferation,
fibroxanthoma, histiocytoma, fibrous histiocytoma, xanthomatous
pseudotumor, inflammatory pseudotumor, mast cell tumor or plasma
cell-histiocytoma (2). The most
common sites for IMTs include the lungs, mesenteries and omentum
(3), although they are also observed
in the head and neck region (4),
liver (5), spleen (6), thyroid (7), gastrointestinal tract (8) and genitourinary tract (9), among other systems (10). Although these types of tumors are
primarily benign, up to 25% of patients suffer from recurrences
(11). Recurrence rates have been
associated with body site, multifocality and whether the initial
tumor was completely resected. Rare malignant transformations have
been reported (12). To the best of
our knowledge, the current study is the first to report the
occurrence of IMT in the inguinal region. Furthermore, recurrence
and metastasis of IMT are comparatively rare. The present study
describes a 49-year-old male patient with IMT of the inguinal
region, which recurred 12 months following initial surgery. The
aims of this report were to describe a novel case of IMT in this
uncommon region and to emphasize that IMT may occasionally
demonstrate malignant biological behaviors, despite its
intermediate biological potential as a neoplasm that frequently
recurs, but rarely metastasizes.
Case report
A 49-year-old male patient with an unremarkable
medical history was admitted to Nanfang Hospital, Southern Medical
University (Guangdong, China) and presented with a low fever, night
sweats, anorexia, weight loss of 5 kg and 1 month of frequent
urination. A visible mass, measuring ~6×5 cm, was located in the
right inguinal region. The oval mass presented no adherence with
the surrounding tissue, and the position had no influence on the
size of the mass. The laboratory examination of the patients blood
revealed anemia (hemoglobin, 81 g/l), thrombocythemia (platelet
count, 517 g/l), hypoproteinemia (albumin, 29 g/l) and increased
inflammatory markers with an erythrocyte sedimentation rate and
C-reactive protein values of 130 mm/h and 113.6 mg/l, respectively.
All other laboratory results were within the normal range. Computed
tomography (CT) of the abdomen revealed an undefined lesion,
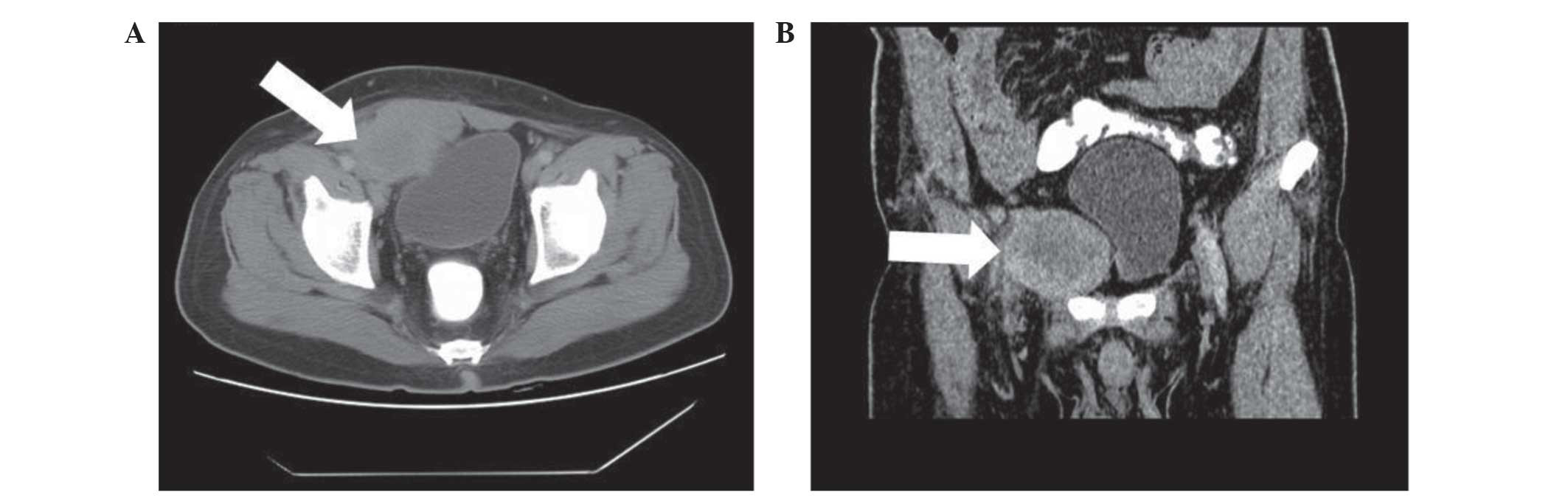
6.5×5.2 cm, occupying the soft tissue of the right inguinal region
(Fig. 1A). The mass was inhomogeneous
in density and was lower in density in the middle compared with the
periphery. A CT enhancement scan demonstrated moderate enhancement
of the solid portion of the lesion. The rectus abdominis muscles
and the bladder were compacted and difficult to differentiate from
the mass (Fig. 1B). The patient then
underwent a fine needle aspiration procedure. Histopathological
analysis of the lesion revealed a composition of spindle and
inflammatory cells, including plasma cells and lymphocytes. In
addition, immunohistochemical analysis established that the tumor
cells were positive for vimentin, actin, Ki-67, B cell lymphoma-2,
CD99, epithelial membrane antigen, CD34, S-100 and CD38; however,
tumor cells were negative for CD117, desmin, anaplastic lymphoma
kinase (ALK) and creatine kinase (Fig.
2). Following the initial surgery, the histopathological
characteristics of the mass were determined again and the results
were comparable to those of the initial specimen from the fine
needle aspiration (Fig. 3). Thus, the
patient was diagnosed with IMT and was advised to return for
regular follow-up appointments, at 3, 6, 12 and 24 months after
surgery.
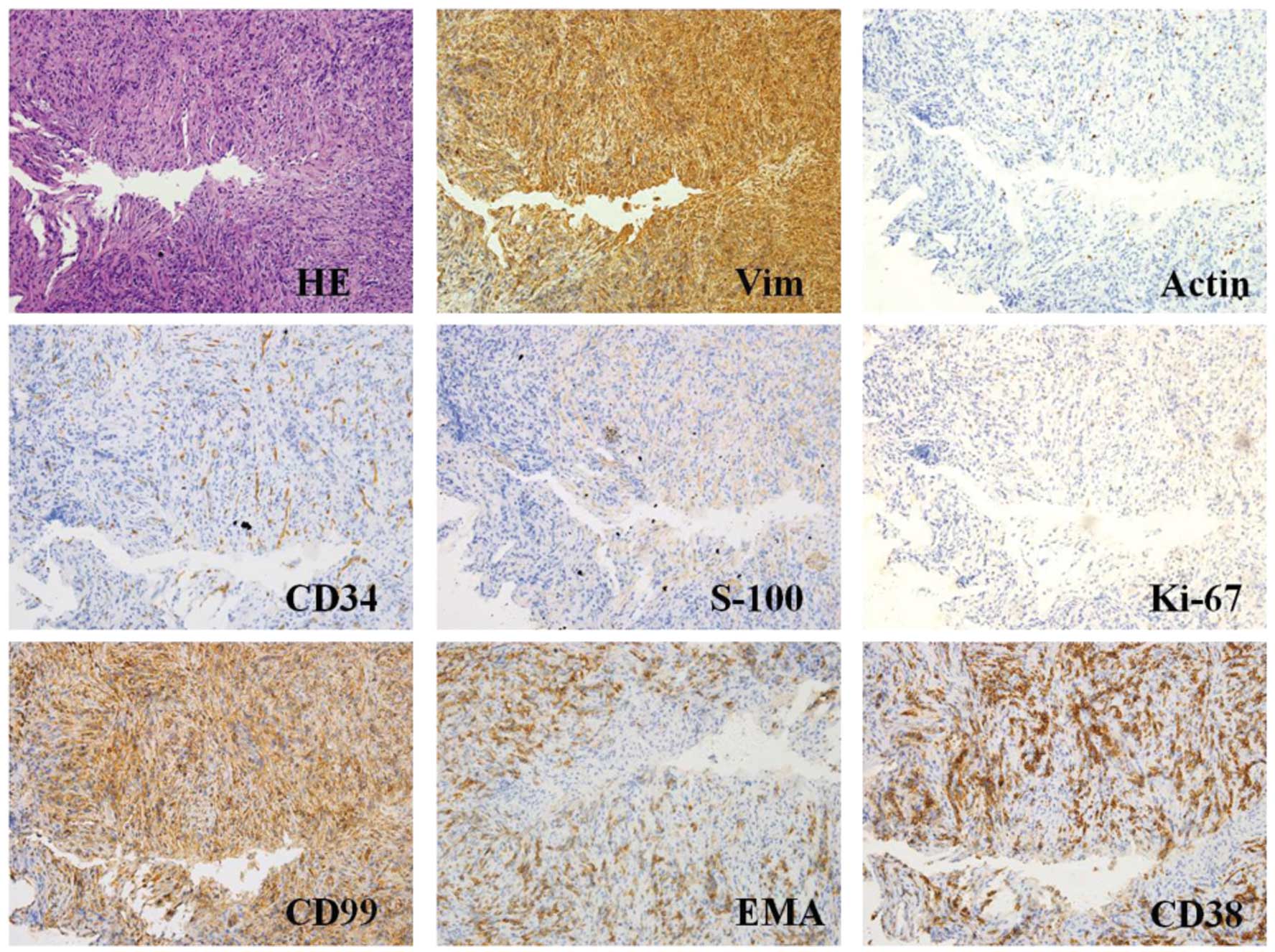 | Figure 2.HE staining and immunohistochemical
findings of the fine needle aspiration samples (magnification,
x400; microscope, Olympus IX71). Representative histological
cross-sections from the original tumor, obtained via fine needle
aspiration, demonstrated variable myofibroblasts, myxoid stroma and
mixed inflammation with lymphocytes, plasma cells and eosinophils.
Tumor tissue stained positive for Vim, actin, CD34, S-100, Ki-67,
CD99, EMA, CD38 and B cell lymphoma-2, but negative for CD117,
desmin, anaplastic lymphoma kinase and creatine kinase. HE,
hematoxylin and eosin; Vim, vimentin; EMA, epithelial membrane
antigen. |
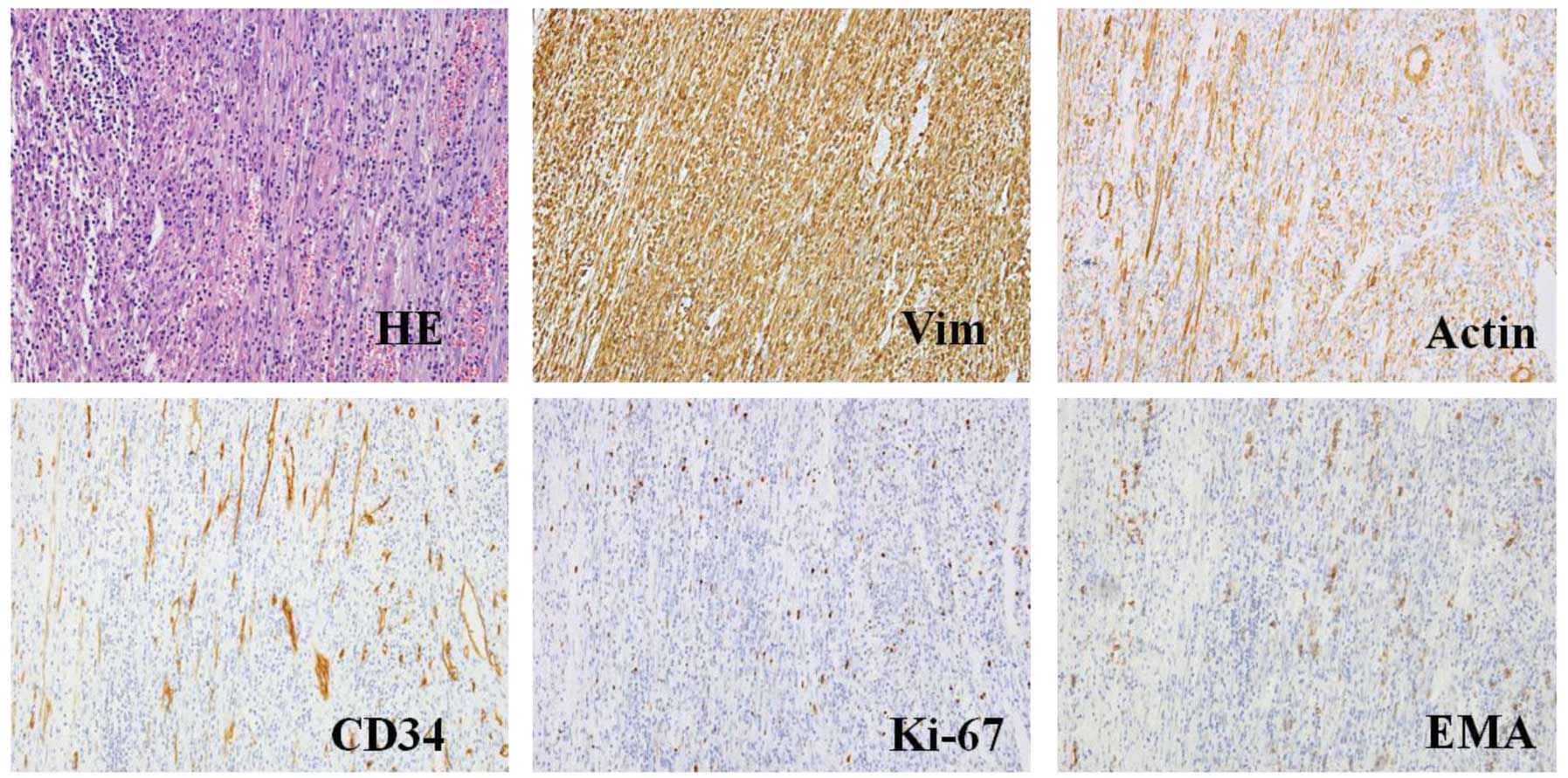 | Figure 3.HE staining and representative
histological cross-sections from the original tumor following
surgery (magnification, x400; microscope, Olympus IX71). The tumor
stained positive for Vim, actin, Ki-67, EMA, CD34, S-100, but
negative for CD117, desmin, anaplastic lymphoma kinase, β-catenin,
myogenin, creatine kinase and p53. HE, hematoxylin and eosin; Vim,
vimentin; EMA, epithelial membrane antigen. |
The patient developed a local recurrence 12 months
following the initial surgery with no clinical symptoms and
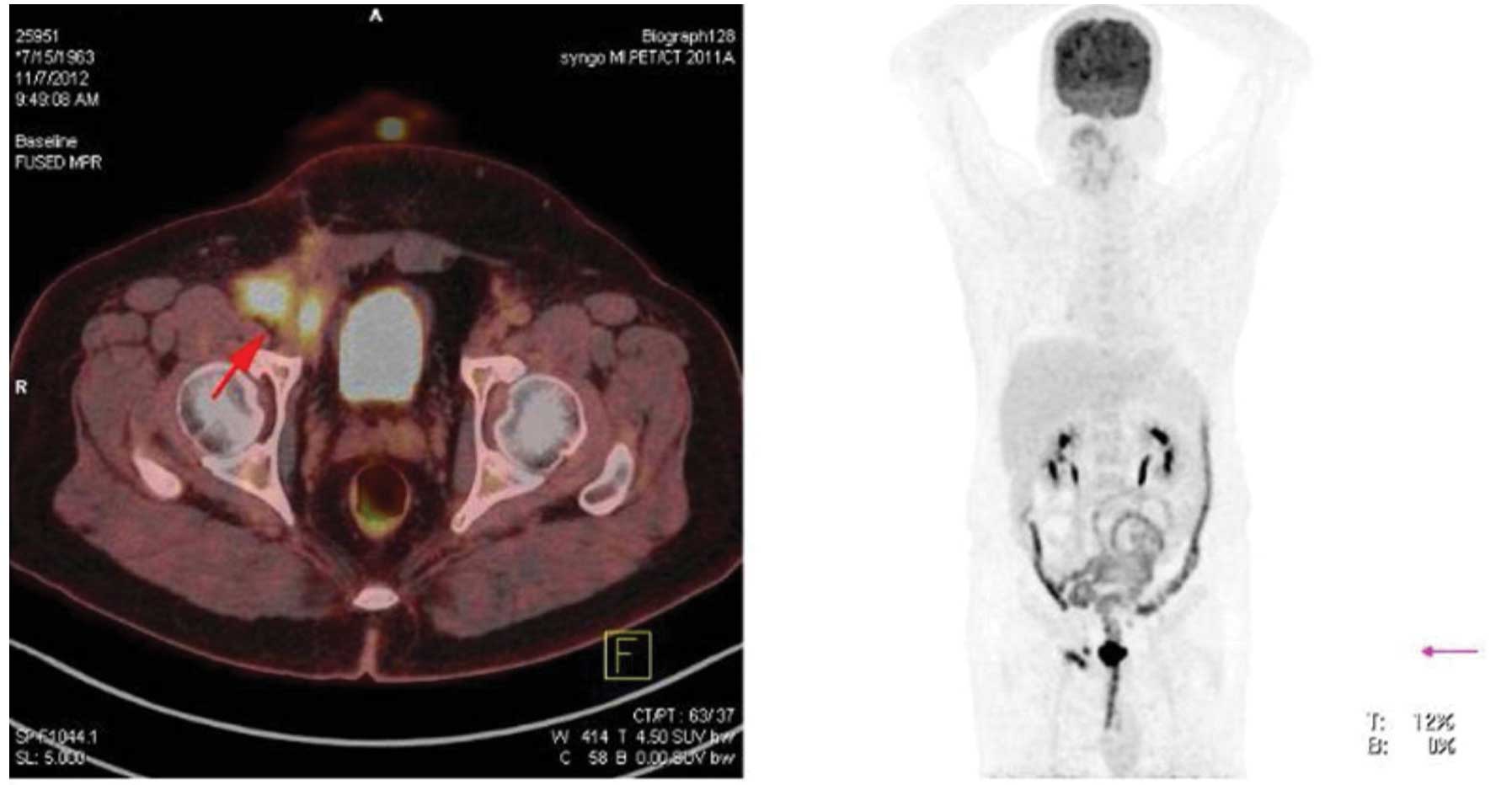
negative laboratory results. Positron emission tomography-CT
revealed multiple integration lesions in the right inguinal region
and iliac fossa; the largest measured ~3.3×2.0×2.0 cm and was
invading into the right abdominal wall (Fig. 4). Thus, a second surgery was
performed. Of note, the histopathological characteristics of the
recurrent lesions were comparable to those of the initial specimen
(Fig. 5). Following the second
surgery, the patient received fractionated radiotherapy (FRT; 46
Gy/23 fractions/30 days; the patient received radiotherapy, 5
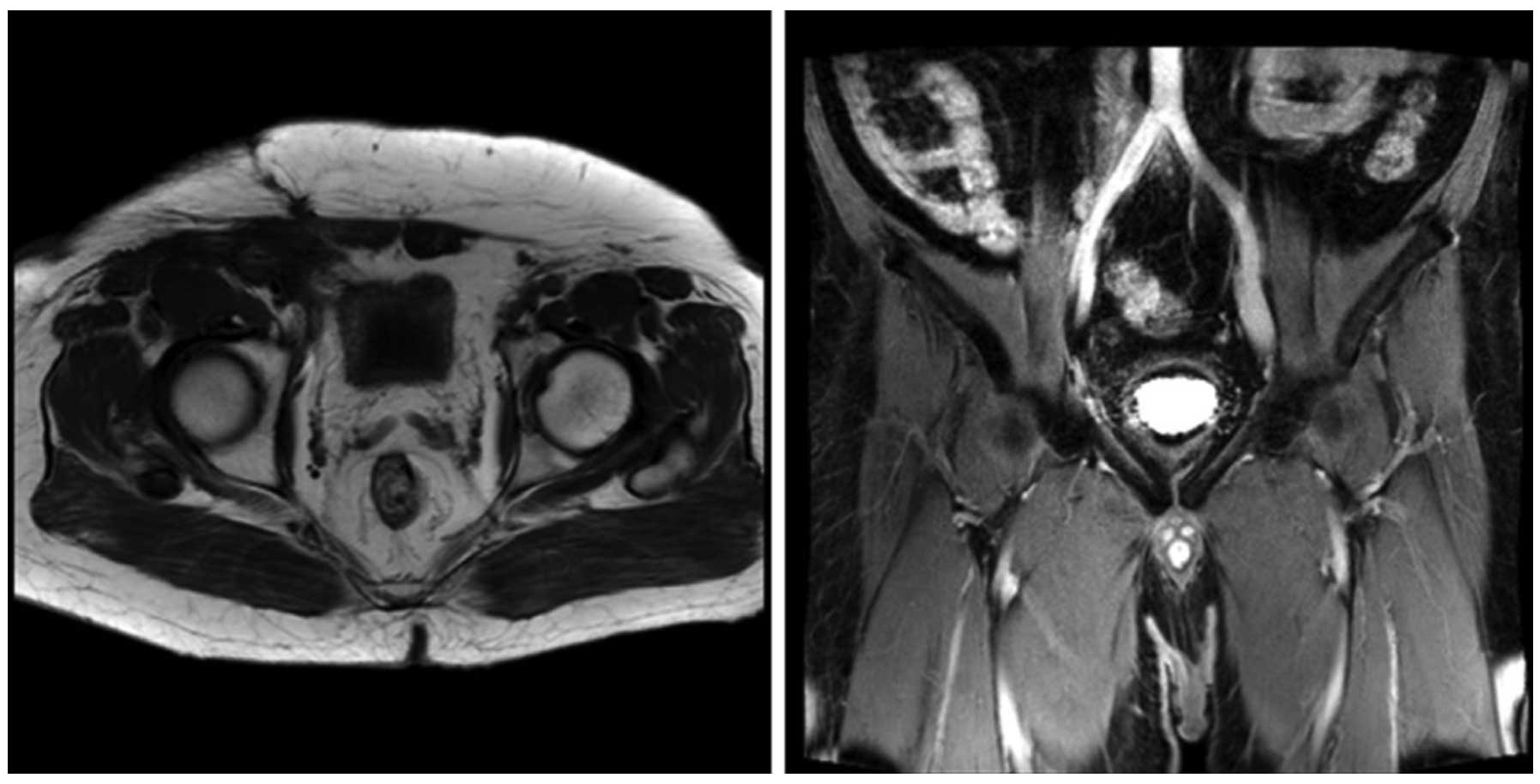
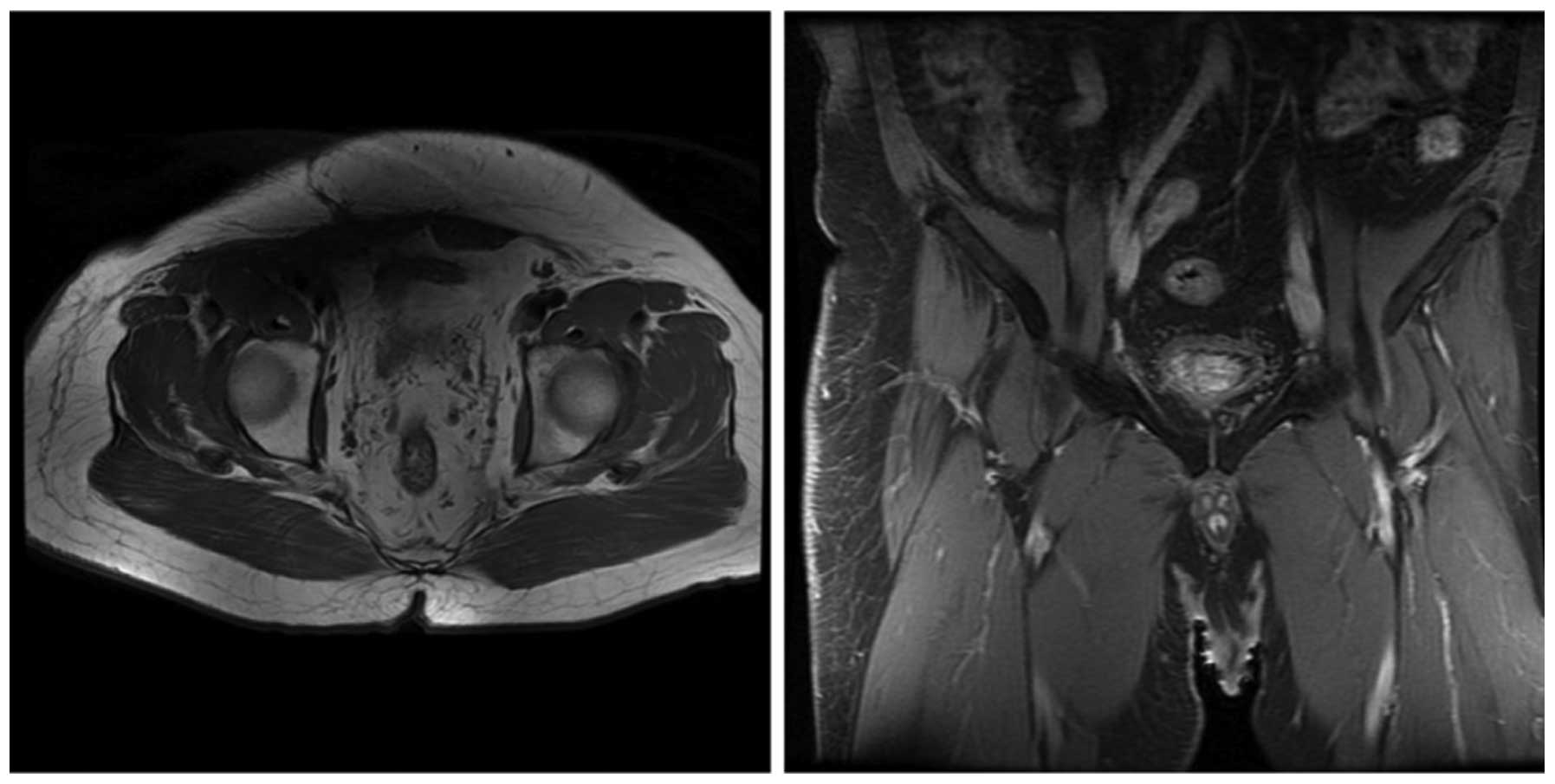
days/week, at 2 Gy/fraction, for a total of 30 days). At 3 and 6
months following radiotherapy, magnetic resonance imaging was
performed and the scans did not indicate tumor recurrence or
metastasis (Figs. 6 and 7, respectively).
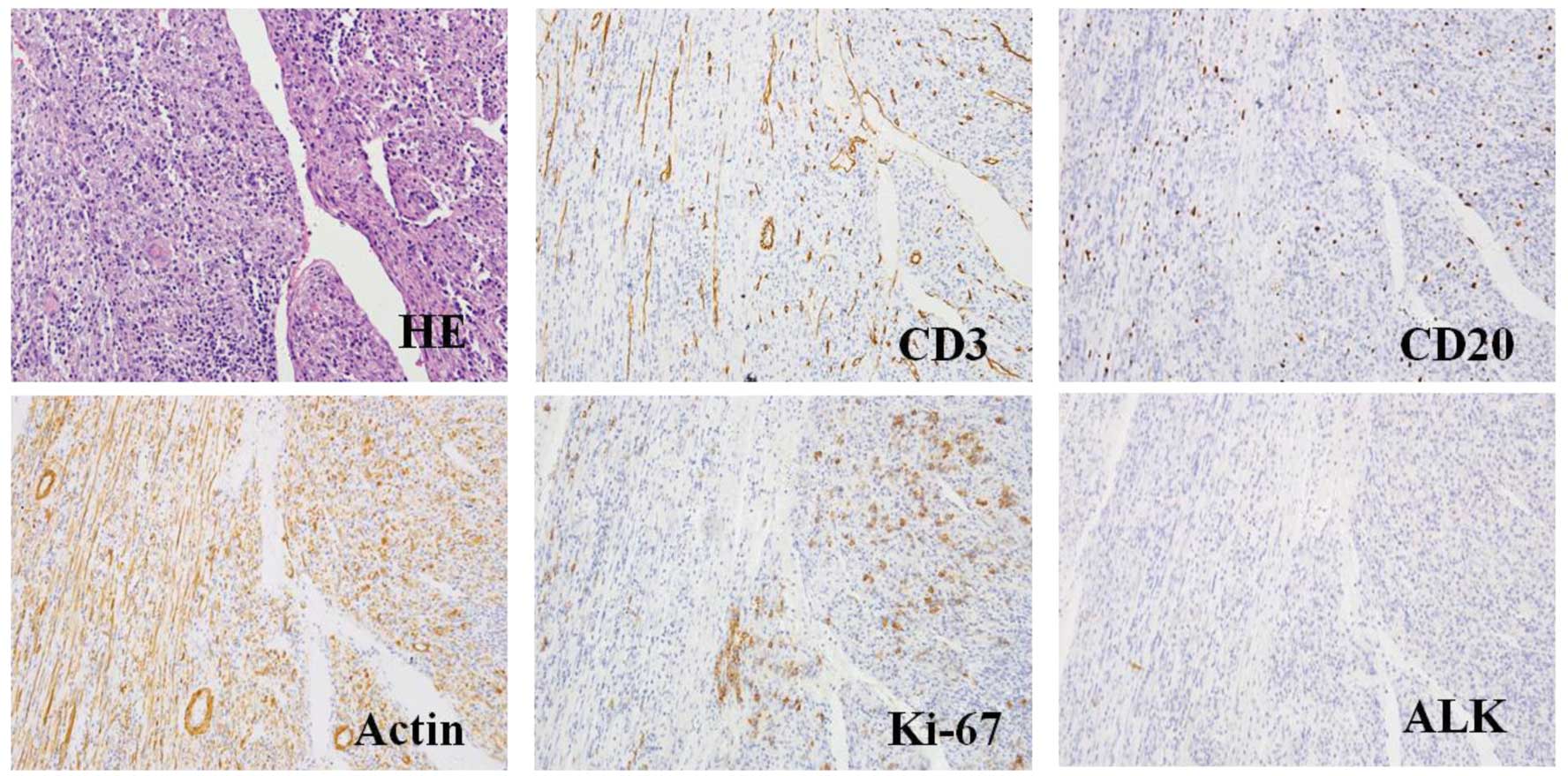 | Figure 5.HE staining and representative
histological cross-sections from the recurrent tumor
(magnification, x400; microscope, Olympus IX71). Representative
histological cross-sections from the recurrent tumors revealed
variable myofibroblasts, myxoid stroma and mixed inflammation with
lymphocytes, plasma cells and eosinophils. The tumor stained
positive for actin, Ki-67, desmin, CD3 and CD20, but negative for
ALK and p53. HE, hematoxylin and eosin; ALK, anaplastic lymphoma
kinase. |
Written informed consent was obtained from the
patient prior to publication of the study and the study was
approved by the ethics committee of Nanfang Hospital.
Discussion
The first case of IMT was described in the lungs in
1939 (13). IMT, also referred to as
plasma cell granulomas, plasma cell pseudotumors, inflammatory
myofibrohistiocytic proliferations, omental mesenteric myxoid
hamartomas and inflammatory pseudotumors, is a rare type of
low-grade malignant mesenchymal tumor. In 2002, the World Health
Organization defined IMT as a distinctive lesion consisting of
myofibroblastic spindle cells with an inflammatory infiltrate of
plasma cells, lymphocytes and eosinophils (14).
As reported in the literature, IMT predominantly
occurs in the soft tissue and viscera of children and young adults
(15); in addition, IMT is most
commonly localized to the lung (16),
although it may also occur in other regions of the body, including
the lymph nodes, soft tissue, viscera, gastrointestinal tract,
omentum majus and central nervous system. Among these
extra-pulmonary IMTs, 43% arise in the mesenteries (17) or omentum (18). IMTs are exceptionally rare in the
inguinal region and to the best of our knowledge no similar cases
have been previously reported.
The etiology of IMT remains to be defined; however,
trauma to the affected region secondary to inflammation has been
proposed as a cause of IMT. A previous study demonstrated the
occurrence of ectopic chromosomal rearrangements in chromosomes 2
(long arm) and 9 (short arm); in addition, this previous study
confirmed the monoclonal identity of IMT through genetic and
molecular techniques (19).
Furthermore, it has been reported that ~50% of IMTs exhibit clonal
cytogenetic aberrations, which results in the genetic activation
ALK-receptor tyrosine kinase at 2p23 (20). This therefore suggested that IMT is a
true neoplasm, as opposed to an inflammatory pseudotumor, as it was
previously considered. In the present case report, the aggressive
features of IMT, including local recurrence, metastasis and
malignant transformation, indicated that IMT development may be a
neoplastic process.
Due to the inconsistency of the pathological
diagnoses of IMTs and the limited number of patients typically
diagnosed with IMTs, the treatment of choice for IMT patients
remains controversial. IMTs are commonly located in the peritoneum,
liver, spleen, breast, spinal cord, brain or respiratory system
(6,21–25). They
are more frequently observed in the lower lobe of the right lung
and form a solitary, oval-shaped and well-defined mass that is
located peripherally (26–28).
At present, due to the occurrence of rare IMT cases
with a more aggressive clinical picture, including local
recurrence, malignant transformation or metastasis, IMTs are
classed as low-grade mesenchymal malignancies. Surgical resection
should be considered as the first therapeutic option when feasible;
however, radiotherapy (29),
anticancer chemotherapy (30),
steroids (31) or non-steroidal
anti-inflammatory drugs (32) have
previously been used for the treatment of anatomically and
functionally inoperable patients as well as in patients with
disease recurrence. The results of these treatments have been
variable, ranging from ineffective to complete regression.
A limited number of studies have investigated the
use of radiation therapy for IMT. Seider et al (33) reported a case in which progression of
IMT was observed at a 1 month following initial resection of the
tumor. Further surgery to eradicate the tumor completely would have
been extensive and disfiguring; therefore, the patient was
administered 40 Gy FRT in 20 fractions and a 27 months follow-up
demonstrated local control of the IMT (33).
Certain studies have reported 66–100% complete
remission rates in orbital inflammatory pseudotumor patients
following radiotherapy (29,34–36).
Sasagawa et al (37) observed
local control following 20 Gy FRT treatment and other studies have
also demonstrated clinical responses following FRT (38,39).
Ong et al (40)
classified head and neck IMT patients into categories according to
risk of relapse (high, moderate or low), which were dependent on
the diameter of their tumors, the composition of the pseudocapsule
and immunohistochemistry, among other prognostic factors. This
previous study suggested that high and moderate-risk groups
required post-operative radiotherapy. Adjunct radiation therapy of
60–64 Gy was performed for the moderate-risk group and 66–70 Gy was
used for the high-risk group. In the low-risk group, post-operative
radiotherapy of 50–54 Gy was recommended if the lesion had a
diameter of >5 cm with conditioned ALK and Ki-67 overexpression.
For other cases of the low-risk group, post-operative radiotherapy
was not required.
The prognosis of IMT is usually good; however, in
rare cases, this type of tumor may exhibit local invasion.
Recurrence has been associated with the tumors location, resection
ability and multinodularity. The metastatic rate of IMTs has been
reported as 5% (41) and metastasis
is predominantly observed in children with intra-abdominal
tumors.
The malignant potential of IMT is incompletely
characterized. IMT has been previously confused with malignant
conditions based on commonalities in the pathological examination,
radiological appearance and clinical presentation. However, an
increasing number of studies have reported the malignant potential
of IMTs. For instance, Anderson et al (42) reported the case of a 15-year-old boy
that was diagnosed with IMT of the heart and experienced recurrence
6 months after the initial surgery. In addition, Navinan et
al (43) reported the case of a
33-year-old South Asian male who was diagnosed with inoperable IMT
of the paranasal sinuses and orbit. As curative excision of the
tumour was not feasible, medical management was offered. Despite
early features of remission to glucocorticoids, tapering resulted
in recurrence.
In conclusion, IMTs, in particular those with
inguinal region involvement, are rare in adults. The most relevant
therapy for this type of tumor is open surgical resection. Regular
follow-up is recommended to monitor patients for recurrence. In
addition, radiotherapy should be considered in patients whose
surgical resection was incomplete, in those with postoperative
recurrences and in those whose tumors are non-resectable due to
associated medical conditions.
Glossary
Abbreviations
Abbreviations:
|
IMT
|
inflammatory myofibroblastic
tumors
|
|
FRT
|
fractionated radiotherapy
|
References
|
1
|
Cassivi SD and Wylam ME: Pulmonary
inflammatory myofibroblastic tumor associated with histoplasmosis.
Interact Cardiovasc Thorac Surg. 5:514–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoon SH, Kim KJ, Chung SK, et al:
Inflammatory myofibroblastic tumor in the intradural extramedullary
space of the lumbar spine with spondylolisthesis: Case report and
review of the literature. Eur Spine J. 19 (Suppl 2):S153–S157.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coffin CM and Fletcher JA: Inflammatory
myofibroblastic tumourWHO Pathology & Genetics of Tumours of
Soft Tissue and Bone. Fletcher CDM, Unni KK and Mertens F: IARC
Press; Lyon: pp. 90–93. 2002
|
|
4
|
Gao F, Zhong R, Li GH and Zhang WD:
Computed tomography and magnetic resonance imaging findings of
inflammatory myofibroblastic tumors of the head and neck. Acta
Radiol. 55:434–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagarajan S, Jayabose S, McBride W, et al:
Inflammatory myofibroblastic tumor of the liver in children. J
Pediatr Gastroenterol Nutr. 57:277–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawaguchi T, Mochizuki K, Kizu T, et al:
Inflammatory pseudotumor of the liver and spleen diagnosed by
percutaneous needle biopsy. World J Gastroenterol. 18:90–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kojima M, Suzuki M, Shimizu K and Masawa
N: Inflammatory pseudotumor of the thyroid gland showing prominent
fibrohistiocytic proliferation. A case report. Endocr Pathol.
20:186–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arslan D, Gündüz S, Tural D, et al:
Inflammatory myofibroblastic tumor: a rarely seen submucosal lesion
of the stomach. Case Rep Oncol Med. 2013:3281082013.PubMed/NCBI
|
|
9
|
Kim HW, Choi YH, Kang SM, et al: Malignant
inflammatory myofibroblastic tumor of the bladder with rapid
progression. Korean J Urol. 53:657–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duan W, Xu Y, Dong Y, Cao L, Tong J and
Zhou X: Ectopic expression of miR-34a enhances radiosensitivity of
non-small cell lung cancer cells, partly by suppressing the LyGDI
signaling pathway. J Radiat Res. 54:611–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lawrence B, Perez-Atayde A, Hibbard MK, et
al: TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic
tumors. Am J Pathol. 157:377–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oztuna F, Pehlivanlar M, Abul Y, Tekinbas
C, Ozoran Y and Ozlu T: Adult inflammatory myofibroblastic tumor of
the trachea: Case report and literature review. Respir Care.
58:e72–e76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brunn H: Two interesting benign lung
tumors of contradictory histopathology: remarks on the necessity
for maintaining the chest tumor registry. J Thorac Cardiovasc Surg.
9:119–131. 1939.
|
|
14
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El-Desoky T, Nasef N, Osman E, Osman A,
Zaki A and Zalata K: Endobronchial inflammatory pseudotumor: A rare
cause of a pneumothorax in children. J Bronchol Interv Pulmonol.
20:256–260. 2013. View Article : Google Scholar
|
|
16
|
Toma CL, Belaconi IN, Dumitrache-Rujinski
S, et al: A rare case of lung tumor - pulmonary inflammatory
pseudotumor. Pneumologia. 62:30–32. 2013.PubMed/NCBI
|
|
17
|
Shatzel J, Wooten K, Ankola A, Cheney RT,
Morrison CD and Skitzki JJ: Inflammatory myofibroblastic tumor of
the mesentery: A clinical dilemma. Int J Clin Oncol. 17:380–384.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta CR, Mohta A, Khurana N and Paik S:
Inflammatory pseudotumor of the omentum: An uncommon pediatric
tumor. Ind J Pathol Microbiol. 52:219–221. 2009. View Article : Google Scholar
|
|
19
|
Cole B, Zhou H, McAllister N, Afify Z and
Coffin CM: Inflammatory myofibroblastic tumor with thrombocytosis
and a unique chromosomal translocation with ALK rearrangement. Arch
Pathol Lab Med. 130:1042–1045. 2006.PubMed/NCBI
|
|
20
|
O'Malley DP, Poulos C, Czader M, Sanger WG
and Orazi A: Intraocular inflammatory myofibroblastic tumor with
ALK overexpression. Arch Pathol Lab Med. 128:e5–e7. 2004.PubMed/NCBI
|
|
21
|
al-Sarraj S, Wasserberg J, Bartlett R and
Bridges LR: Inflammatory pseudotumour of the central nervous
system: Clinicopathological study of one case and review of the
literature. Br J Neurosurg. 9:57–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coffin CM, Humphrey PA and Dehner LP:
Extrapulmonary inflammatory myofibroblastic tumor: A clinical and
pathological survey. Semin Diagn Pathol. 15:85–101. 1998.PubMed/NCBI
|
|
23
|
Neuhauser TS, Derringer GA, Thompson LD,
et al: Splenic inflammatory myofibroblastic tumor (inflammatory
pseudotumor): A clinicopathologic and immunophenotypic study of 12
cases. Arch Pathol Lab Med. 125:379–385. 2001.PubMed/NCBI
|
|
24
|
Pettinato G, Manivel JC, Insabato L, De
Chiara A and Petrella G: Plasma cell granuloma (inflammatory
pseudotumor) of the breast. Am J Clin Pathol. 90:627–632.
1988.PubMed/NCBI
|
|
25
|
Zemmoura I, Hamlat A and Morandi X:
Intradural extramedullary spinal inflammatory myofibroblastic
tumor: Case report and literature review. Eur Spine J. 20 (Suppl
2):S330–S335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hedlund GL, Navoy JF, Galliani CA and
Johnson WH Jr: Aggressive manifestations of inflammatory pulmonary
pseudotumor in children. Pediatr Radiol. 29:112–116. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobashi Y, Fukuda M, Nakata M, Irei T and
Oka M: Inflammatory pseudotumor of the lung: Clinicopathological
analysis in seven adult patients. Int J Clin Oncol. 11:461–466.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laufer L, Cohen Z, Mares AJ, Maor E and
Hirsch M: Pulmonary plasma-cell granuloma. Pediatr Radiol.
20:289–290. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maire JP, Eimer S, San Galli F, et al:
Inflammatory myofibroblastic tumour of the skull base. Case Rep
Otolaryngol. 2013:1036462013.PubMed/NCBI
|
|
30
|
Tian H, Liu T, Wang C, Tang L, Chen Z and
Xing G: Inflammatory pseudotumor of the temporal bone: Three cases
and a review of the literature. Case Rep Med.
2013:4804762013.PubMed/NCBI
|
|
31
|
Lee DK, Cho YS, Hong SH, Chung WH and Ahn
YC: Inflammatory pseudotumor involving the skull base: Response to
steroid and radiation therapy. Otolaryngol Head Neck Surg.
135:144–148. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moon CH, Yoon JH, Kang GW, et al: A case
of recurrent pulmonary inflammatory myofibroblastic tumor with
aggressive metastasis after complete resection. Tuberc Respir Dis.
75:165–169. 2013. View Article : Google Scholar
|
|
33
|
Seider MJ, Cleary KR, van Tassel P, et al:
Plasma cell granuloma of the nasal cavity treated by radiation
therapy. Cancer. 67:929–932. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ampil FL and Bahrassa FS: Primary orbital
lymphoma-pseudotumor (pseudolymphoma): Case reports and review of
radiotherapy literature. J Surg Oncol. 30:91–95. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Jesús O, Inserni JA, Gonzalez A and
Colón LE: Idiopathic orbital inflammation with intracranial
extension. Case report. J Neurosurg. 85:510–513. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Noble SC, Chandler WF and Lloyd RV:
Intracranial extension of orbital pseudotumor: A case report.
Neurosurgery. 18:798–801. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasagawa Y, Akai T, Itou S and Iizuka H:
Multiple intraosseous inflammatory myofibroblastic tumors
presenting with an aggressive clinical course: Case report.
Neurosurgery. 69:E1010–E1015. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frohman LP, Kupersmith MJ, Lang J, et al:
Intracranial extension and bone destruction in orbital pseudotumor.
Arch Ophthalmol. 104:380–384. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kaye AH, Hahn JF, Craciun A, Hanson M,
Berlin AJ and Tubbs RR: Intracranial extension of inflammatory
pseudotumor of the orbit. Case report. J Neurosurg. 60:625–629.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ong HS, Ji T, Zhang CP, et al: Head and
neck inflammatory myofibroblastic tumor (IMT): Evaluation of
clinicopathologic and prognostic features. Oral Oncol. 48:141–148.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao HD, Wu T, Wang JQ, et al: Primary
inflammatory myofibroblastic tumor of the breast with rapid
recurrence and metastasis: A case report. Oncol Lett. 5:97–100.
2013.PubMed/NCBI
|
|
42
|
Andersen ND, DiBernardo LR, Linardic CM,
Camitta MG and Lodge AJ: Recurrent inflammatory myofibroblastic
tumor of the heart. Circulation. 125:2379–2381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Navinan MR, Liyanage I, Herath S, et al:
Inoperable inflammatory myofibroblastic tumour of the para-nasal
sinuses and orbit with recurrence responding to methotrexate and
prednisolone: A case report. BMC Res Notes. 8:272015. View Article : Google Scholar : PubMed/NCBI
|