Introduction
Minimal change disease (MCD) is a well-described
glomerulopathy that accounts for 10–15% of primary nephrotic
syndrome cases in adults (1). It is
characterized by nephrotic-range proteinuria, edema,
hypoalbuminemia, and hyperlipidemia. Biopsy findings include an
absence of glomerular lesions on light microscopy and effacement of
foot processes on electron microscopy. Steroids are used for
first-line therapy of MCD. Immunomodulatory drugs such as
cyclosporine, tacrolimus and mycophenolate mofetil are used for
treatment of relapsed disease or in cases of steroid-resistance or
steroid-dependence (2,3). MCD is also the most common cause of
paraneoplastic glomerulonephritis in patients with thymoma, and
T-cell dysfunction is considered to play an important role in its
pathogenesis (4). However, the impact
of antitumor or immunosuppressive therapy on the immune system in
patients with thymoma-associated MCD is poorly understood. The
present study describes changes in immune cell subset composition
in response to tumor-directed therapy in a patient with relapsed,
thymoma-associated MCD, who achieved a complete radiological tumor
response and durable reduction in proteinuria.
Case report
A 63-year-old female with Masaoka stage IVA, World
Health Organization type B2 thymoma (5,6) was
referred for treatment of recurrent thymoma 3 years after the
initial diagnosis. Initial treatment consisted of surgical
resection. The patient had developed anasarca and acute kidney
injury 7 months before presentation with recurrent thymoma. A renal
biopsy showed no global sclerosis on light microscopy and diffuse
foot process effacement on electron microscopy. These changes were
consistent with MCD. Oral prednisone was administered at a dose of
80 mg/day, which was decreased to 20 mg/day after 1 month due to
steroid-induced myopathy. Despite an initial reduction in
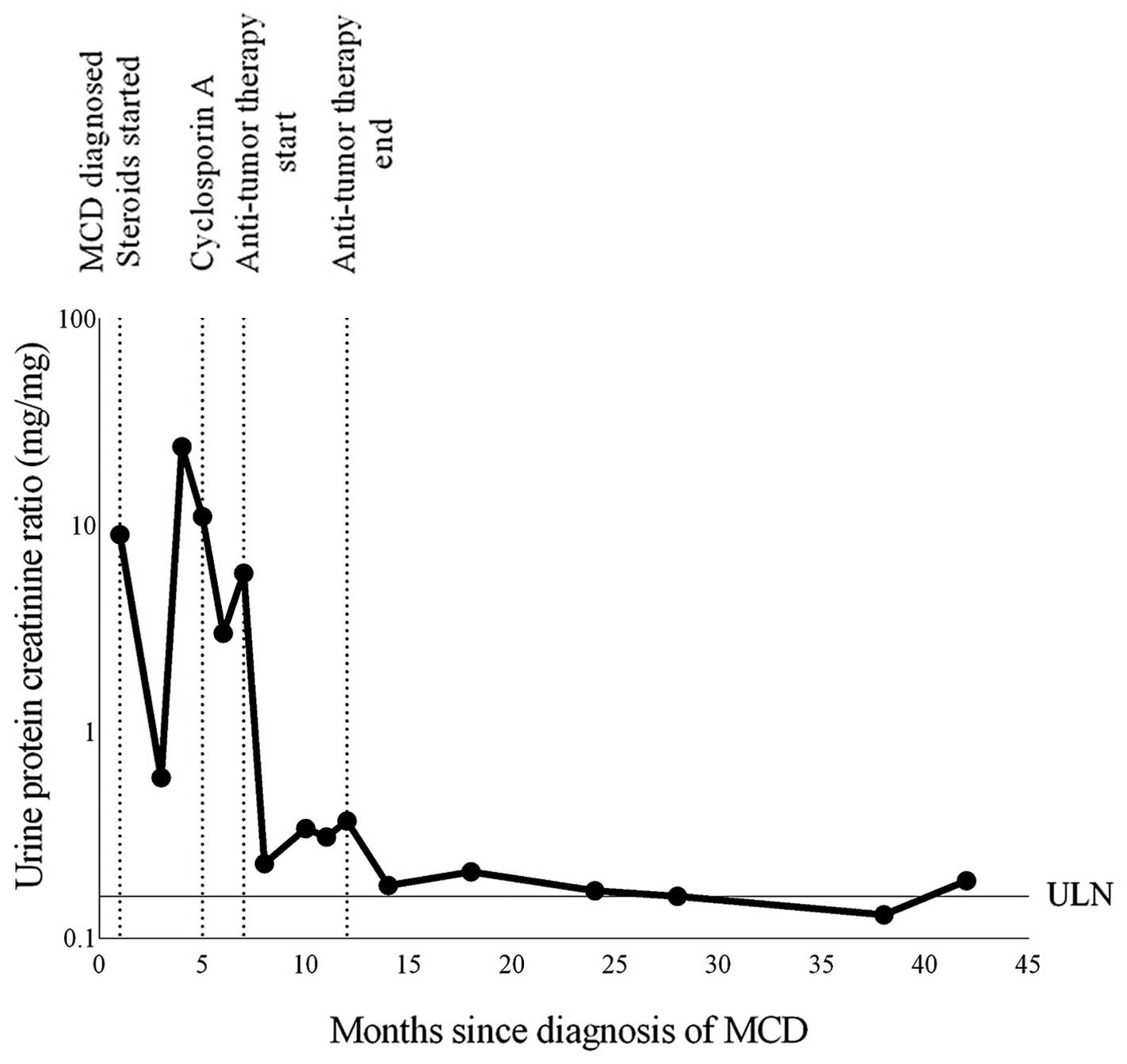
proteinuria, MCD relapse was observed within 4 months (Fig. 1). Cyclosporine was then administered;
however, the patient remained symptomatic with fatigue and dyspnea
on exertion. Upon presentation, medications included prednisone (5
mg/day) and cyclosporine (100 mg, orally, twice daily). Physical
examination revealed pitting pedal edema up to the knees.
Laboratory tests demonstrated hypoalbuminemia (serum albumin level,
2.3 g/dl; normal range, 3.5–5.2 g/dl), normal serum creatinine
levels (0.8 mg/dl), proteinuria (urine protein excretion, 2.7 g in
24 h; normal range, 30–150 mg in 24 h) and an elevated urine
protein-creatinine ratio of 5.9 mg/mg (normal range, 0.001–0.16
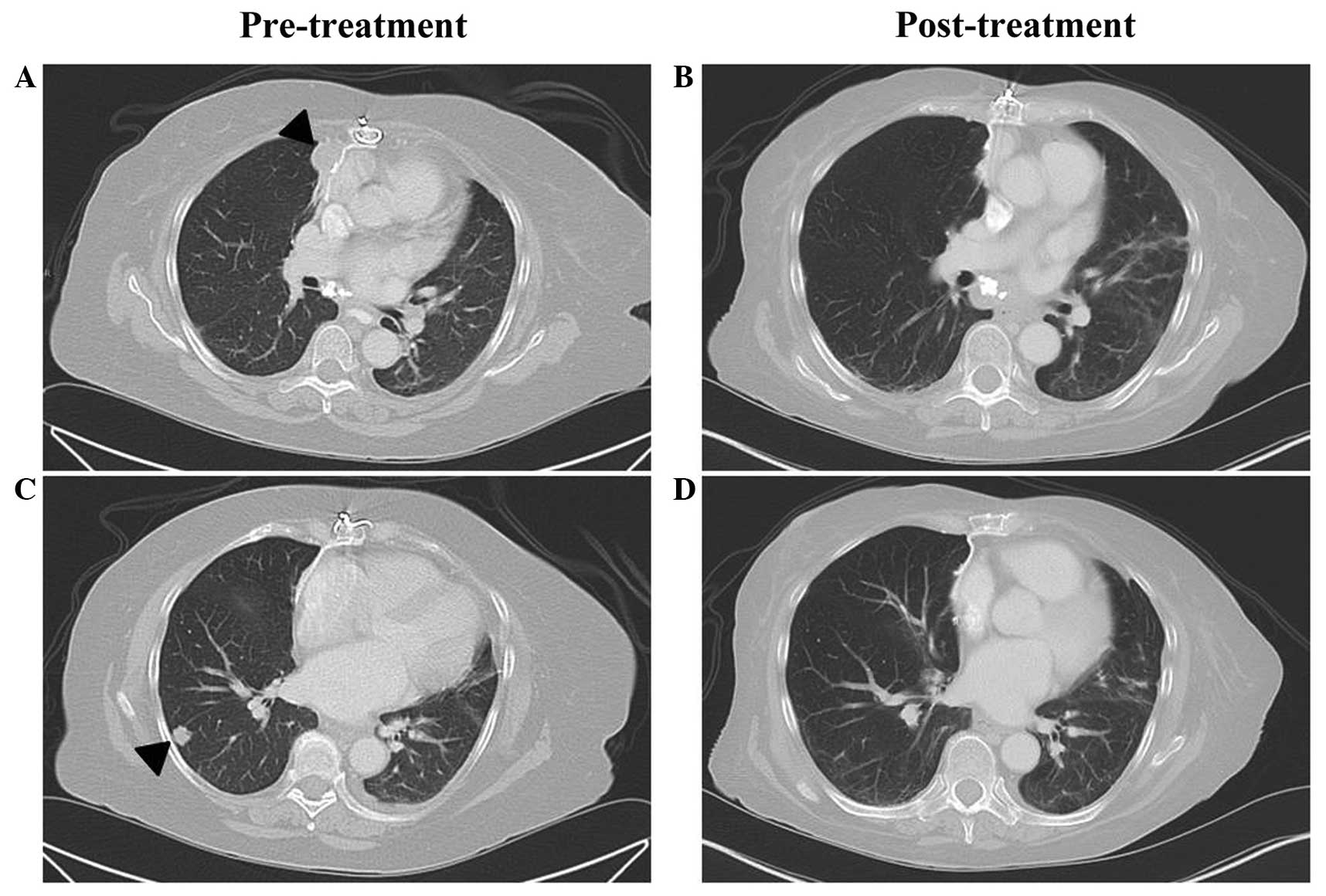
mg/mg). A computed tomography (CT) scan of the chest revealed a
right paracardiac mass and multiple pulmonary nodules (Fig. 2). After obtaining written informed
consent, the patient was enrolled in a phase I/II clinical trial
(no. NCT01100944) that was approved by the Institutional Review
Board of the National Cancer Institute (Bethesda, MD, USA). The
patient was treated with 6 cycles of the histone deacetylase
inhibitor, belinostat [250 mg/m2 administered as four
consecutive 12-h continuous intravenous infusions (CIVI), starting
on day 1 of a 21-day cycle], in combination with cisplatin [50
mg/m2 intravenous (i.v.) on day 2], doxorubicin (25
mg/m2 i.v. once daily on days 2 and 3) and
cyclophosphamide (500 mg/m2 i.v. on day 3) (7). Immune cell subsets, including regulatory
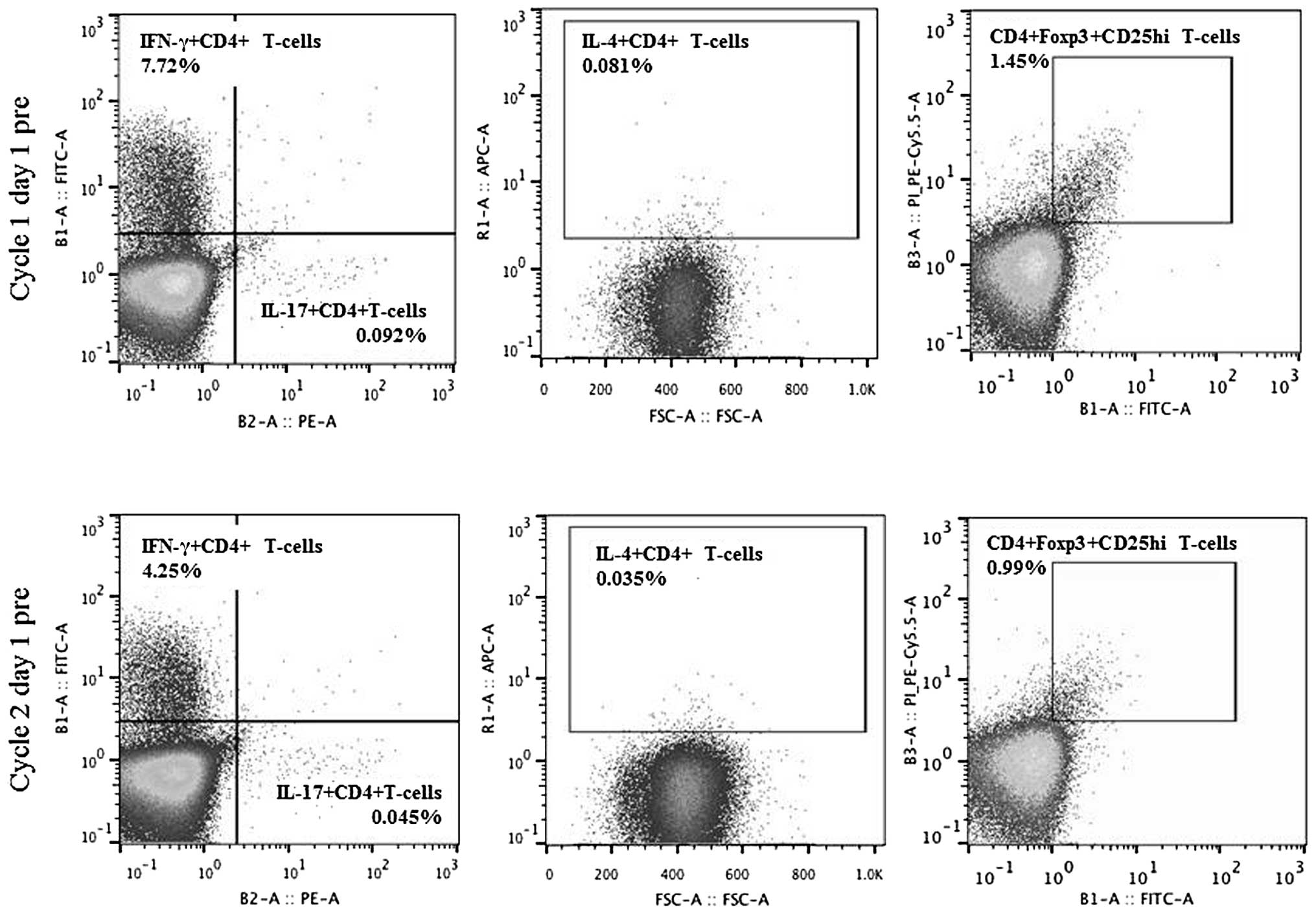
T (Treg) and T helper (Th) cells, were evaluated using
multiparameter flow cytometry on whole blood samples collected
prior to treatment (C1D1pre), on days 2 and 3 of cycle 1 (C1D2 and
C1D3, respectively) and prior to treatment on day 1 of cycle 2
(C2D1pre). The population of Th1, Th2, Th17 and Treg cells
decreased on C2D1pre when compared with the population on C1D1pre,
with fold-changes of 0.55, 0.43, 0.49 and 0.69, respectively
(Fig. 3). A reduction in the
Th17/Treg ratio was also observed, whereas the Th1/Th2 ratio
increased (C2D1pre fold-change, 0.71 and 1.29, respectively).
Proteinuria resolved following one cycle, and the urine
protein-creatinine ratio was 0.15 mg/mg. Cyclosporine and
prednisone were discontinued within 4 months and a complete
radiological response was observed within 6 months (Fig. 2). The reduction in proteinuria was
durable as demonstrated by a urine protein-creatinine ratio of 0.19
mg/mg 30 months after completion of the treatment (Fig. 1).
Discussion
To the best of our knowledge, the present study is
the first comprehensive analysis of changes in T-cell subsets in
response to tumor-directed treatment in thymoma-associated MCD. An
improvement in proteinuria coincided with a reduction in the
Th17/Treg ratio and an increase in the Th1/Th2 ratio.
A high Th17/Treg ratio has been previously observed
in association with increased proteinuria and decreased serum
albumin levels in primary MCD (8).
Corticosteroid therapy has been demonstrated to result in a
reduction in proteinuria and normalization of the Th17/Treg ratio
due to a decrease in the Th17 cell population and an increase in
the Treg cell population (8). In the
current study, a reduction in the Th17/Treg ratio and a significant
decline in proteinuria were observed following antitumor treatment,
thus suggesting that a high Th17/Treg state may also play a role in
the development of thymoma-associated MCD. However, in contrast to
primary MCD, a decline in Treg cells accompanied by a sharper
decline in Th17 cells, resulting in a reduction of the Th17/Treg
ratio, was observed. These changes were also accompanied by a
reduction in the tumor size. The population of Treg cells is
frequently increased in the presence of a tumor and these cells
play a critical role in the suppression of antitumor immune
responses (9). In idiopathic
nephrotic syndrome, induction of Treg cells is considered to
represent a potential novel therapeutic strategy (10); however, in the present study, we
hypothesize that a reduction in Treg cells by effective antitumor
therapy plays an important role in the restoration of antitumor
immune responses and results in an indirect improvement in
thymoma-associated MCD.
Previous studies using the Buffalo/Mna rat model of
spontaneous thymoma and nephrotic syndrome have demonstrated that
polarization of the immune response toward a Th2 profile is
associated with the development of glomerulonephritis (11,12). To
the best of our knowledge, the current study is the first to
demonstrate a shift away from Th2 cells and an increase in the
Th1/Th2 ratio with an associated reduction in proteinuria in a
patient with thymoma-associated MCD. These observations are
suggestive of an improvement in underlying T-cell dysfunction
following administration of systemic antitumor therapy. Although
these results require further validation, they may help in
understanding the pathophysiologic mechanisms underlying
thymoma-associated glomerulonephritis, and provide a rationale for
rapid initiation of tumor-directed therapy. The benefits of this
approach in controlling thymoma-associated paraneoplastic syndromes
have been described previously (13).
In conclusion, the present study describes a case of
relapsed, thymoma-associated MCD with a durable reduction in
proteinuria following successful treatment of thymoma, accompanied
by changes in immune cell subsets in peripheral blood. Early
administration of antitumor therapy should be considered in such
cases.
Acknowledgements
The authors would like to acknowledge the Intramural
Research Program of the NIH, the National Cancer Institute and the
Center for Cancer Research for their support of this study.
References
|
1
|
Nachman PH, Jennette JC and Falk R:
Primary glomerular diseaseThe Kidney. Taal MW, Chertow GM, Marsden
PA, Skorecki K, Yu ASL and Brenner BM: Elsevier; Philadelphia, PA:
pp. 1100–1191. 2012
|
|
2
|
Hogan J and Radhakrishnan J: The treatment
of minimal change disease in adults. J Am Soc Nephrol. 24:702–711.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chugh SS, Clement LC and Macé C: New
insights into human minimal change disease: Lessons from animal
models. Am J Kidney Dis. 59:284–292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lien YH and Lai LW: Pathogenesis,
diagnosis and management of paraneoplastic glomerulonephritis. Nat
Rev Nephrol. 7:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koga K, Matsuno Y, Noguchi M, Mukai K,
Asamura H, Goya T and Shimosato Y: A review of 79 thymomas:
Modification of the staging system and reappraisal of conventional
division into invasive and non-invasive thymoma. Pathol Int.
44:359–367. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Tumors of the thymusWorld Health Organization
Classification of Tumors: Pathology and Genetics of Tumors of the
Lung, Pleura, Thymus and Heart. IARC Press; Lyon, France: pp.
146–248. 2004
|
|
7
|
Thomas A, Rajan A, Szabo E, Tomita Y, et
al: A phase I/II trial of belinostat in combination with cisplatin,
doxorubicin, and cyclophosphamide in thymic epithelial tumors: A
clinical and translational study. Clin Cancer Res. 20:5392–5402.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li
H, Chen LM, Li MX, Li XM and Li XW: Th17/Treg imbalance in adult
patients with minimal change nephrotic syndrome. Clin Immunol.
139:314–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Curr Opin Immunol. 27:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Le Berre L, Bruneau S, Naulet J, Renaudin
K, Buzelin F, Usal C, Smit H, Condamine T, Soulillou JP and Dantal
J: Induction of T regulatory cells attenuates idiopathic nephrotic
syndrome. J Am Soc Nephrol. 20:57–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lien YH and Lai LW: Pathogenesis,
diagnosis and management of paraneoplastic glomerulonephritis. Nat
Rev Nephrol. 7:85–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Berre L, Hervé C, Buzelin F, Usal C,
Soulillou JP and Dantal J: Renal macrophage activation and Th2
polarization precedes the development of nephrotic syndrome in
Buffalo/Mna rats. Kidney Int. 68:2079–2090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rajan A, Kotlyar D and Giaccone G: Acute
autoimmune hepatitis, myositis, and myasthenic crisis in a patient
with thymoma. J Thorac Oncol. 8:e87–e88. 2013. View Article : Google Scholar : PubMed/NCBI
|