Introduction
Hypercalcemia is one of the most common
paraneoplastic syndromes, occurring in up to 30% of cancer patients
(5–10% with bone metastases) during the course of the disease
(1). By contrast, hypocalcemia is a
rare event, and is significantly more rare in neoplastic disease.
Several factors may be involved in the development of hypocalcemia
in cancer patients, including hypoalbuminemia, surgical and
infiltrative hypoparathyroidism, hypomagnesemia, vitamin D
deficiency, renal failure, massive cell lysis, drug effect, sepsis
and osteoblastic metastases (2).
Hypocalcemia derived from bone lesions has mainly
been reported in the advanced stages of prostate cancer, resulting
from the influx of calcium into bone following abnormal increases
in bone formation (3). In such
patients, hypocalcemia is typically mild and clinical signs are
rare (affecting ~0.9% of patients) (4); however, cases of severe hypocalcemia
have occasionally been reported (5–7). In breast
cancer patients, hypocalcemia is markedly more unusual than in
prostate cancer, and appears to be more frequently associated with
the abnormal endocrine activity induced by low parathyroid hormone
(PTH) levels (2,8–10).
The present report describes a case of severe
hypocalcemia and associated heart failure observed as a clinical
presentation of subsequently diagnosed bone-metastatic breast
cancer in a patient with normal PTH values.
Case report
A 67-year-old female was admitted to
Morgagni-Pierantoni Hospital (Forlì, Italy) in April 2013, with
symptoms of severe fatigue and dyspnea. Signs of heart failure were
identified, including lower leg edema and liver stasis, and a chest
X-ray revealed bilateral pleural effusion. Blood tests revealed
severe normochromic and normocytic anemia [hemaglobin (Hb) 6.1
mg/dl] and moderate thrombocytopenia (53,000
platelets/mm3). Elevated N-terminal of the prohormone
brain natriuretic peptide (NT-proBNP) levels were detected (4237
pg/ml; normal values, <900 pg/ml) and electrocardiography (ECG)
revealed normal left ventricular dimensions and a 59% ejection
fraction. Blood tests also indicated severe hypocalcemia [4.2 mg/dl
(1.05 mmol/l), corrected calcium 4.7 mg/dl (1.2 mmol/l)], albumin
34.1 g/l (range, 35–50 g/l) and normal PTH values (41 ng/l; range,
15–60 ng/l). Creatinine levels were 0.6 mg/dl, with an estimated
creatinine clearance of 95 ml/min, while 25-hydroxyvitamin D was
4.9 µg/l (range, 20–50 µg/l). No clinical signs of latent tetany
were detected.
Treatment for heart failure commenced with
administration of a β-blocker (bisoprolol, 1.25 mg daily; Bayer AG,
Leverkusen, Germany), an angiotensin-converting enzyme inhibitor
(ramipril, 2.5 mg daily; Sanofi S.A., Paris, France) and a diuretic
(furosemide, 25 mg daily; Sanofi S.A.). Calcium and vitamin D
supplements were also administered intravenously and orally.
Clinical examination identified a poorly defined
mass of ~6 cm in the right breast, with partial nipple retraction.
Mammography highlighted bilateral reticular enhancement of the
gland with skin thickening, and a biopsy revealed infiltrating
lobular carcinoma, with estrogen receptor (ER) 100%, progesterone
receptor (PR) 50%, proliferation index (Mib1) 5% and no
amplification of human epidermal growth factor receptor type 2
(HER2).
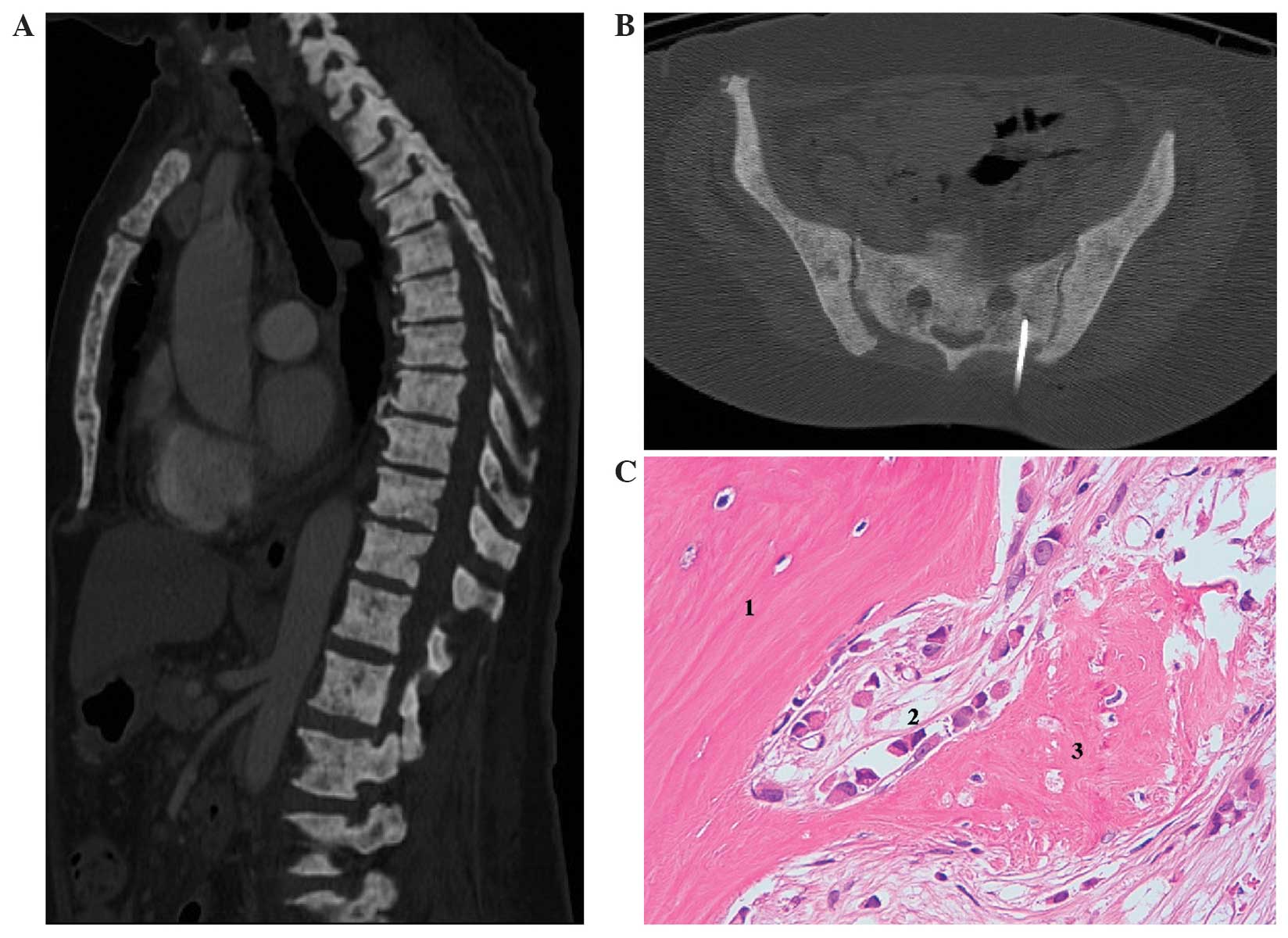
A computed tomography (CT) scan revealed moderate
pleural effusion and bilateral axillary adenopathies, mild ascites
associated with thickening of peritoneal fat and massive calcium
deposits in the bones (Fig. 1A). The
unusual bone images were initially interpreted as metabolic
syndrome secondary to low or absent PTH production. However, given
the patient's overall clinical situation, the possibility of bone
metastases was not ruled out. Due to the unusual radiological
features of the bone lesions, a bone biopsy was also performed
(Fig. 1B), revealing massive bone
marrow metastases as a result of lobular carcinoma of the breast
(ER, 100%; PR, 0%; Mib1, 1% and HER2, not amplified), with
associated bone-thickening (Fig.
1C).
The patient was subsequently admitted to the
Department of Medical Oncology of the Istituto Scientifico
Romagnolo per lo Studio e la Cura dei Tumori (IRST) Istituto di
Ricovero e Cura a Carattere Scientifico (IRCCS). Despite the
administration of calcium (40 mEq/day) intravenously, in addition
to the 2.0 g administered orally during the previous month in
Morgagni-Pierantoni Hospital, the patient's serum calcium decreased
to 3.7 mg/dl (0.93 mmol/l), with a corrected calcium level of 4.1
mg/dl (1.03 mmol/l). An ECG indicated a prolonged QT-interval (489)
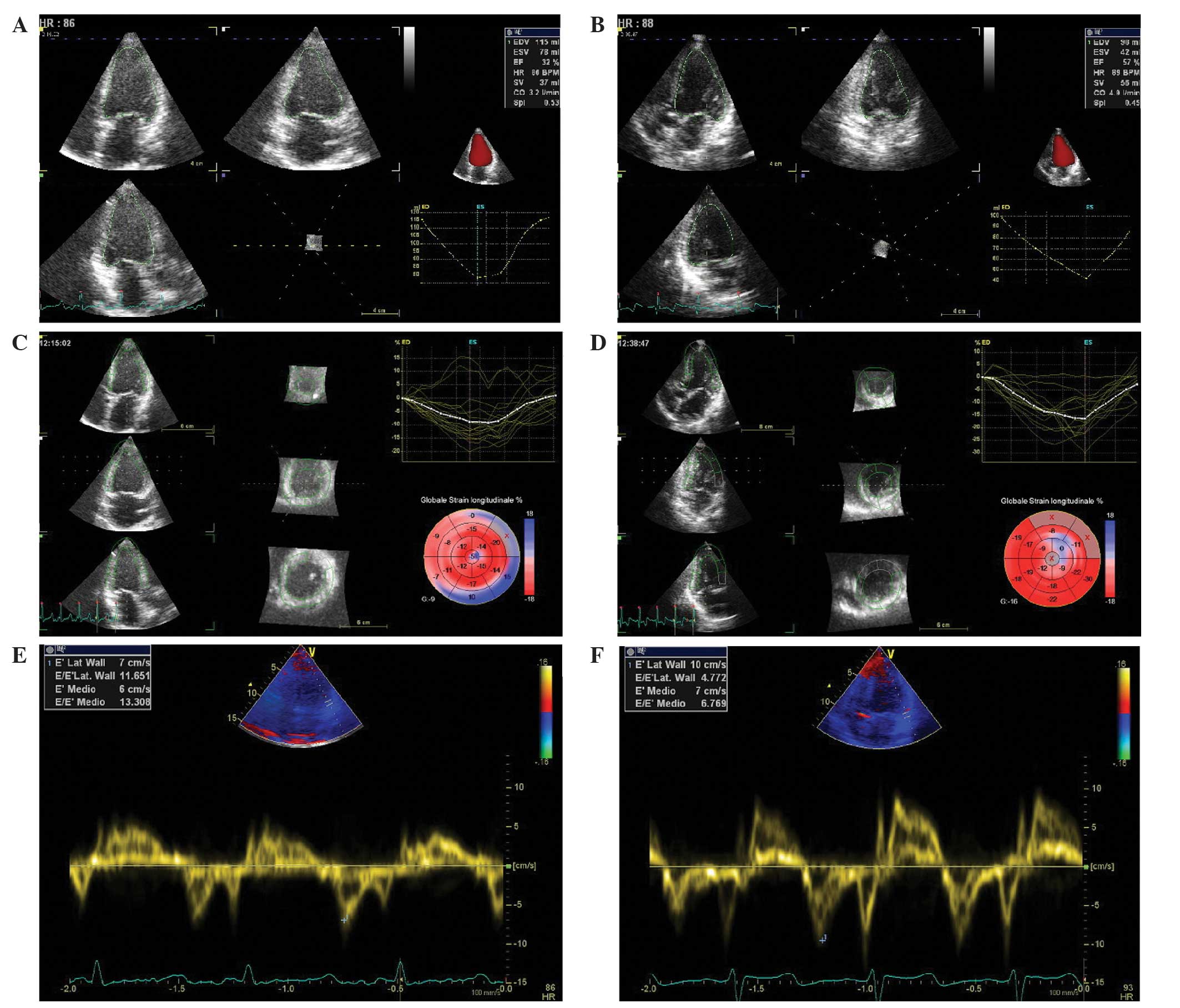
and three-dimensional (3D)-echocardiography revealed a dilated and
hypokinetic left ventricle, with a reduced 3D-left ventricular
ejection fraction (3D-LVEF) of 32% (Fig.
2A), pulmonary hypertension (44 mmHg) and reduced global
longitudinal speckle tracking strain (GLS, −9%) (Fig. 2B and C). 3D-tissue Doppler imaging
also revealed an abnormal degree of ventricular filling pressure,
estimated by the transmitral flow velocity to mitral annular
velocity ratio (E/E', 13.3; normal values, <8). The NT-proBNP
value had also increased to 15388 pg/ml.
The PTH was 51 ng/ml (range, 15–65 ng/ml) and
calcitonin <2.00 ng/l, with a 25-hydroxyvitamin D value of 5.2
µg/ml. Serum phosphate was 5.3 mg/dl (range, 2.70–4.50 mg/dl),
magnesium 1.96 mg/dl (range, 1.60–2.60 mg/dl), sodium 136 mMol/l
(range, 136–145 mMol/l) and potassium 4.6 mMol/l (range, 3.5–5.1
mMol/l). Electrolyte values in a 24-h urine test were unremarkable
(calciuria, <1 mg/dl). Following approximately one week in our
department, during which the patient was treated with calcium
supplements (60 mEq/day administered intravenously and 1.5 g
administered orally), serum calcium was 5.8 mg/dl (1.45 mmol/l),
corrected calcium 3.0 mg/dl (0.75 mmol/l) and serum phosphate 5.3
mg/dl (range, 2.7–4.5 mg/dl). Treatment with 0.5 mcg calcitriol
twice a day was continued.
The patient subsequently began a course of oral
aromatase inhibitor (letrozole; 2.5 mg daily) and weekly paclitaxel
(taxane; 90 mg/m2 on days 1, 8 and 15 of a 28-day cycle)
treatment.
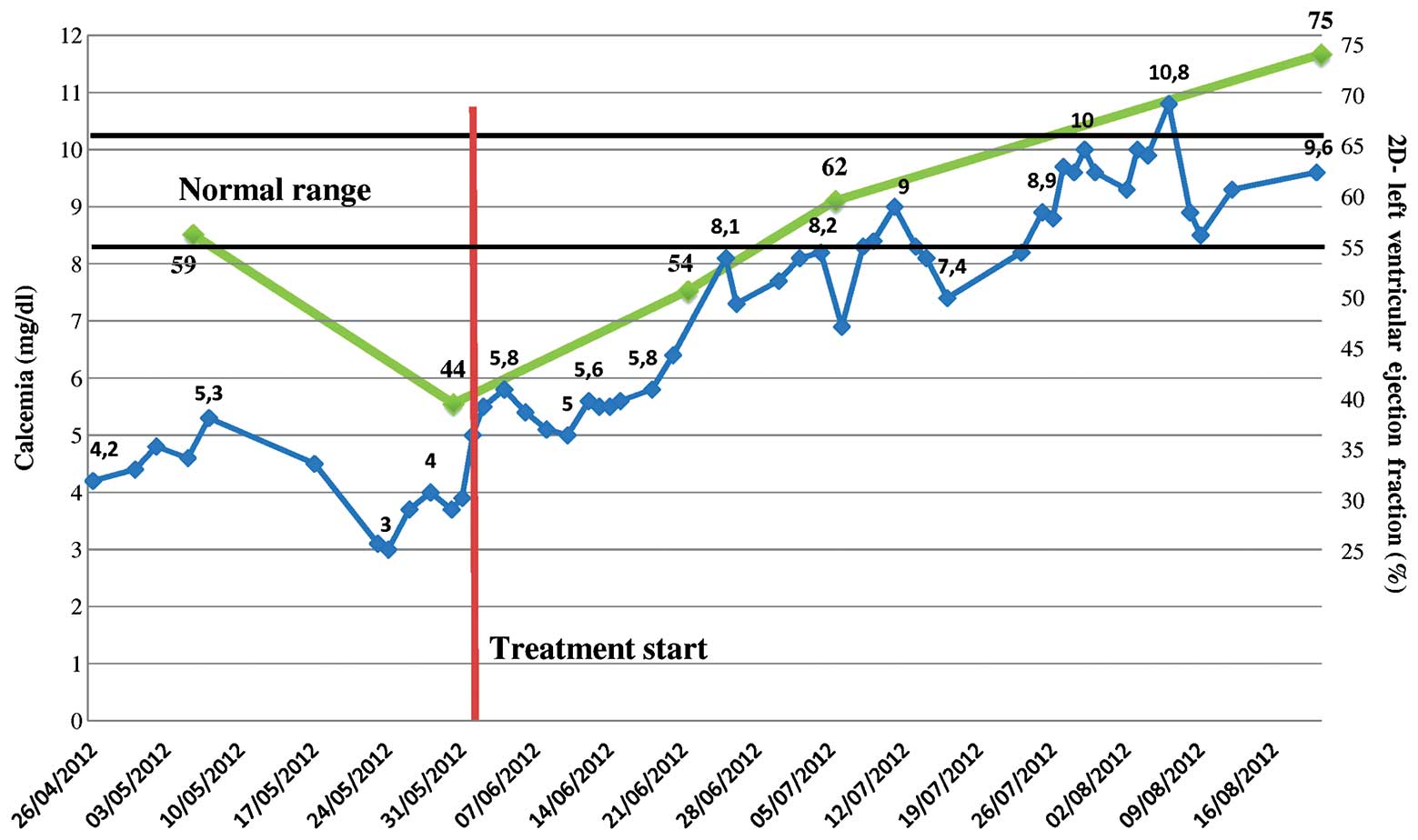
Following 3 weeks of treatment with paclitaxel and
letrozole, calcium values had almost returned to normal (8.1
mg/dl), NT-proBNP had decreased to 1927 pg/ml, the QT-interval had
fallen to 467 and a 3D-echocardiography indicated improved systolic
function (3D-LVEF, 57%) with a reduction in pulmonary arterial
pressure (30 mmHg). GLS had increased from −9 to −16% and E/E' had
normalized (6.7) (Fig. 2D–F). As
treatment continued, the calcium levels and LVEF continued to
markedly improve (Fig. 3).
The patient subsequently developed respiratory
symptoms diagnosed as acute respiratory distress syndrome (ARDS),
likely a result of an atypical reaction to paclitaxel. Taxane
treatment was thus suspended and treatment proceeded with
letrozole. Following resolution of the ARDS, the patient's clinical
condition continued to improve, with a concomitant clinical
reduction in the size of the right breast mass. Intravenous calcium
supplements were gradually discontinued and treatment with
zoledronic acid commenced, without complications.
To date, the patient remains asymptomatic and the
most recent CT scan indicated an improvement on the previous
radiological findings. Treatment with letrozole and oral calcitriol
and calcium continues.
Written informed consent for the publication of this
case report was obtained from the patient.
Discussion
Severe hypocalcemia presents mainly with
paresthesia, neuromuscular irritability and tetany. Other less
frequent symptoms include cardiovascular complications, often
characterized by ECG alterations (prolongation of the QT interval,
which may induce torsades de pointes) and heart failure,
particularly if calcium plasma levels are notably low (11).
The most frequent causes of symptomatic hypocalcemia
in patients with malignancies are bisphosphonate therapy and tumor
lysis syndrome (12); however these
conditions were not applicable to the patient in the current study.
Ectopic secretion of calcitonin, a paraneoplastic syndrome
typically associated with lung cancer (13), was also excluded as normal serum
levels of this hormone were present. Oncogenic osteomalacia is
another rare paraneoplastic syndrome characterized by
hypophosphatemia, low 1,25-dihydroxyvitamin D serum levels and
typical radiological features (14).
This syndrome is thought to be induced by humoral factors produced
mainly by mesenchymal tumors that inhibit renal tubular resorption
of phosphate and 1α-hydroxylation of 25-hydroxyvitamin D (15). The patient did not exhibit
hypophosphatemia or hyperphosphaturia, thereby excluding this
syndrome as a potential cause of the observed symptoms.
To the best of our knowledge, this is the first
described case of paraneoplastic hypocalcemia-induced heart failure
with normal PTH values (2,16). Frequently reported in advanced
prostate cancer, hypocalcemia is suggested to be a consequence of
osteoblastic metastases inducing increased calcium uptake in the
bones, which may result in secondary hyperparathyroidism (12,17). This
is due to calcium-sensing receptors in parathyroid cells that
detect very small changes in the plasma calcium-ion concentration,
triggering the release of PTH when serum calcium is decreased
(18). In the kidney, PTH increases
tubular calcium resorption and induces the hydroxylation of
25-hydroxyvitamin D to the active metabolite of vitamin D,
1,25-dihydroxyvitamin D. Within the bone, resorption is enhanced,
facilitating greater entry of calcium to the plasma (19). In patients with chronic kidney
disease, low serum calcium induced by renal calcium loss results in
secondary hyperparathyroidism (20,21).
Therefore, chronic hypocalcemia should result in a concomitant
increase in PTH secretion.
Hypocalcemia-induced heart failure has previously
been associated with idiopathic or postsurgical hypoparathyroidism,
vitamin D deficiency and celiac disease (22–24). In
the cardiac muscle, calcium functions as a direct central mediator
of electrical activation and ion channel gating, has a crucial role
in the mediation of excitation-contraction coupling and is also
necessary for epinephrine-induced glycogenolysis (25).
Notably, there was a complete restoral of heart
function and pulmonary artery pressure in the patient following
normalization of serum calcium concentration. However, the precise
mechanisms underlying the development of heart failure in the event
of extracellular calcium depletion remains to be elucidated
(26). In the present study, although
cardiac depression was identified, the patient demonstrated no
weakness or abnormal muscle tone.
It was previously demonstrated that hypocalcemia is
the only independent predictive factor for left ventricular
diastolic dysfunction in patients with chronic kidney disease
(27). Accumulating evidence has
suggested that PTH stimulates aldosterone secretion by enhancing
calcium concentration within the cells. Elevated aldosterone levels
in primary and secondary hyperaldosteronism are accompanied by
enhanced urinary and fecal loss of magnesium and calcium. The
resulting decrease in serum calcium concentration further
stimulates production of PTH, which induces amplification of
adrenal aldosterone synthesis. Excess PTH subsequently induces
calcium overload and oxidative stress in cardiomyocytes and
exacerbates the reduction in intra-mitochondrial adenosine
triphosphate levels, resulting in necrotic cell death and
myocardial fibrosis (28,29).
By contrast, the patient in the present study
exhibited normal PTH values, which perturbed diagnosis of the
underlying cause of the severe hypocalcemia. The normalization of
serum calcium levels following antineoplastic treatment, associated
with a clinical response, confirmed the paraneoplastic nature of
the condition, which was likely induced by hungry osteoblastic bone
metastases. However, it may also be hypothesized that the tumor
cells released PTH-related peptide (PTH-rP), which functions as a
decoy hormone for parathyroid glands; not activating osteoclasts
and bone resorption, but inhibiting cardiac function (30). PTH receptors have also been identified
in cardiomyocytes (28). Whether the
mechanism was via normalization of serum calcium levels or
reduction in PTH-rP release, the clinical response resulted in
improved heart function.
In conclusion, the results of the present study
indicated that the patient likely experienced an effect known as
ʻhungry-bone syndromeʼ, which induced extreme, prolonged
hypocalcemia and eventually resulted in heart failure (31). The response to antineoplastic
treatment resulted in decreased tumor load, confirmed by lower
tumor marker values and a reduction in the size of the breast mass,
which consequently resulted in decreased calcium utilization by the
metastatic process and an improvement in heart function.
Acknowledgements
The authors wish to thank Grainne Tierney of the
IRST IRCCS for editing the manuscript.
References
|
1
|
Ibrahim T, Farolfi A, Mercatali L, Ricci M
and Amadori D: Metastatic bone disease in the era of bone-targeted
therapy: Clinical impact. Tumori. 99:1–9. 2013.PubMed/NCBI
|
|
2
|
Bergkamp FJ, van Berkel AM, van der Linden
PW and Gorgels JP: Unexpected prolonged extreme hypocalcaemia and
an inadequate PTH response in a patient with metastatic breast
carcinoma. Neth J Med. 61:371–375. 2003.PubMed/NCBI
|
|
3
|
Fokkema MI, de Heide LJ, van Schelven WD
and Hamdy NA: Severe hypocalcaemia associated with extensive
osteoblastic metastases in a patient with prostate cancer. Neth J
Med. 63:34–37. 2005.PubMed/NCBI
|
|
4
|
Berruti A, Dogliotti L, Bitossi R, Fasolis
G, Gorzegno G, Bellina M, Torta M, Porpiglia F, Fontana D and
Angeli A: Incidence of skeletal complications in patients with bone
metastatic prostate cancer and hormone refractory disease:
Predictive role of bone resorption and formation markers evaluated
at baseline. J Urol. 164:1248–1253. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kukreja SC, Shanmugam A and Lad TE:
Hypocalcaemia in patients with prostate cancer. Calcif Tissue Int.
43:340–345. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szentirmai M, Constantinou C, Rainey JM
and Loewenstein JE: Hypocalcaemia due to avid calcium uptake by
osteoblastic metastases of prostate cancer. West J Med.
163:577–578. 1995.PubMed/NCBI
|
|
7
|
Smallridge RC, Wray HL and Schaaf M:
Hypocalcaemia with osteoblastic metastases in a patient with
prostatic carcinoma. A cause of secondary hyperparathyroidism. Am J
Med. 71:184–188. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bouvier DP: Hypocalcaemia and an
inappropriate endocrine response in osteoblastic metastatic breast
cancer. South Med J. 82:1574–1576. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mariette X, Khalifa P, Boissonnas A,
Sereni D and Cremer G: Hypocalcaemia due to parathyroid metastases.
Eur J Med. 2:242–244. 1993.PubMed/NCBI
|
|
10
|
Hermus A, Beex L, van Liessum P, Pieters
G, Smedts F, Smals A and Kloppenborg P: Hypocalcaemia due to
osteoblastic metastases and diminished parathyroid reserve in a
patient with advanced breast cancer. Klin Wochenschr. 66:643–646.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hurley K and Baggs D: Hypocalcaemic
cardiac failure in the emergency department. J Emerg Med.
28:155–159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yener S, Demir O, Ozdogan O, Akinci B and
Yesil S: Severe hypocalcaemia because of osteoblastic prostate
carcinoma metastases. Int J Clin Pract. 62:1630–1631. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coners K, Woods SE and Webb M: Dual
paraneoplastic syndromes in a patient with small cell lung cancer:
A case report. J Med Case Rep. 5:3182011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yeung SJ, McCutcheon IE, Schultz P and
Gagel RF: Use of long-term intravenous phosphate infusion in the
palliative treatment of tumor-induced osteomalacia. J Clin
Endocrinol Metab. 85:549–555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Battoo AJ, Salih S, Unnikrishnan AG, Jojo
A, Bahadur S, Iyer S and Kuriakose MA: Oncogenic osteomalacia from
nasal cavity giant cell tumor. Head Neck. 34:454–457. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grieve RJ, Dixon PF, Roberts H and Hunter
RD: Hypocalcaemia - an unusual complication of successful
chemotherapy for metastatic breast cancer. Clin Oncol. 9:337–342.
1983.PubMed/NCBI
|
|
17
|
Talapatra I and Tymms DJ: Bone metastases
as a cause of hypocalcaemia. Int J Clin Pract. 59:1366–1367. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boden SD and Kaplan FS: Calcium
homeostasis. Orthop Clin North Am. 21:31–42. 1990.PubMed/NCBI
|
|
19
|
Brown EM: Mechanisms underlying the
regulation of parathyroid hormone secretion in vivo and in vitro.
Curr Opin Nephrol Hypertens. 2:541–551. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fraser WD: Hyperparathyroidism. Lancet.
374:145–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Promberger R, Ott J, Kober F, Karik M,
Freissmuth M and Hermann M: Normal parathyroid hormone levels do
not exclude permanent hypoparathyroidism after thyroidectomy.
Thyroid. 21:145–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tziomalos K, Kakavas N, Kountana E,
Harsoulis F and Basayannis E: Reversible dilated hypocalcaemic
cardiomyopathy in a patient with primary hypoparathyroidism. Clin
Endocrinol (Oxf). 64:717–718. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gulati S, Bajpai A, Juneja R, Kabra M,
Bagga A and Kalra V: Hypocalcemic heart failure masquerading as
dilated cardiomyopathy. Indian J Pediatr. 68:287–290. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mavroudis K, Aloumanis K, Stamatis P,
Antonakoudis G, Kifnidis K and Antonakoudis C: Irreversible
end-stage heart failure in a young patient due to severe chronic
hypocalcemia associated with primary hypoparathyroidism and celiac
disease. Clin Cardiol. 33:E72–E75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bers D and Guo T: Calcium signaling in
cardiac ventricular myocytes. Ann NY Acad Sci. 1047:86–98. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solzbach U, Kitterer HR and Haas H:
Reversible congestive heart failure in severe hypocalcemia. Herz.
35:507–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gromadziński L, Januszko-Giergielewicz B
and Pruszczyk P: Hypocalcemia is related to left ventricular
diastolic dysfunction in patients with chronic kidney disease. J
Cardiol. 63:198–204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomaschitz A, Ritz E, Pieske B,
Fahrleitner-Pammer A, Kienreich K, Horina JH, et al: Aldosterone
and parathyroid hormone: A precarious couple for cardiovascular
disease. Cardiovasc Res. 94:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomaschitz A, Ritz E, Pieske B, Rus-Machan
J, Kienreich K, Verheyen N, et al: Aldosterone and parathyroid
hormone interactions as mediators of metabolic and cardiovascular
disease. Metabolism. 63:20–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wannamethee SG, Welsh P, Papacosta O,
Lennon L, Whincup PH and Sattar N: Elevated parathyroid hormone,
but not vitamin D deficiency, is associated with increased risk of
heart failure in older men with and without cardiovascular disease.
Circ Heart Fail. 7:732–739. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Riveros HA, Almodóvar LO, Danés CF and
Domingo JP: Hungry bone syndrome: Persistent hypocalcemia related
to osteoblastic bone metastases of prostate cancer. J Palliat Med.
16:1496–1497. 2013. View Article : Google Scholar : PubMed/NCBI
|