Introduction
Despite the worldwide reduction in gastric cancer
mortality in the last decade, the disease remains a notable health
problem (1). Helicobacter
pylori (HP) infection results in gastric cancer through the
slow progression of the premalignant stages of intestinal
metaplasia (IM), atrophic gastritis and adenoma/dysplasia (2). In addition, a number of previous studies
have indicated the possibility that highly active inflammation
induced by HP is involved in cancer development (3,4). However,
the molecular mechanisms that underlie gastric cancer development
through the progression of HP-associated chronic gastritis have not
been well studied.
Gastric cancer results from the accumulation of
genetic and epigenetic alterations (5). In sporadic gastric cancer, the frequency
of p53 gene mutations is 25–50% (5,6) and the
frequency of DNA methylation of the DNA mismatch repair gene mutL
homolog 1 (MLH1) gene is 20–30% (5,7).
Hypermethylation of the MLH1 promoter region is the primary
cause of microsatellite instability in primary gastric cancer
(8). Previous studies have also
demonstrated that activation-induced cytidine deaminase (AID) is
important in HP-associated gastric cancer development (9). AID is an enzyme that edits DNA and RNA,
and was originally identified to induce somatic hypermutations and
class-switch recombination in immunoglobulin genes (10). Previous studies have indicated that
AID transgenic mice develop malignant T-cell lymphomas and lung
adenomas. These findings indicate that aberrant AID expression
results in tumor-associated gene mutations and may be a cause of
human malignancy (11).
Cag-pathogenicity island (cag-PAI)-positive HP infection leads to
the activation of nuclear factor (NF)-κB in the gastric epithelium.
As a result, AID is transcriptionally upregulated in gastric
epithelial cells, and may contribute to the accumulation of
p53 and other gene mutations, leading to gastric
carcinogenesis (12). However, the
histological association between AID expression, gastric tumors and
the background mucosa is not well understood.
Gastric cancer has been divided into two
histological types: Intestinal and diffuse [Lauren's classification
(13)] or differentiated and
undifferentiated [Nakamura et al (14)]. Previous studies using mucin-based
histochemical and immunohistochemical analyses indicated that
gastric and intestinal phenotypic cell markers are widely expressed
in gastric cancer, irrespective of the histological type (15–17).
In the present study, to improve the understanding
of the mechanisms underlying malignant transformation in gastric
cancer and its association with developmental processes, the
expression of AID, p53 and MLH1, and the phenotypic expression in
early gastric neoplasms were evaluated and compared with the
background mucosal condition.
Patients and methods
Patient and tissue samples
Gastric tumors were obtained from 151 patients (109
males and 42 females) who had undergone endoscopic or surgical
tumor resection at the Tottori University Hospital between 2003 and
2009, and who possessed histopathologically diagnosed tubular
adenomas (n=22; males, 14; females, 8; average age, 72.5 years),
intramucosal carcinomas (MCs; n=92; males, 67; females, 25; average
age, 70.2 years), and submucosal carcinomas (SMCs; n=37; males, 28;
females, 9; average age, 66.8 years), according to the Japanese
Classification of Gastric Carcinoma (Table I) (18).
All tubular adenomas corresponded to category 3, all MCs were
classified as category 4 and all SMCs as category 5 of the Vienna
classification (19). The mean age
was statistically younger in the SMC group compared with the
adenoma and MC groups. A younger age, larger tumor size and
depressed type tumors were more frequently observed in the patients
with SMCs compared with those with adenomas and MCs.
 | Table I.Clinicopathological characteristics
of early gastric neoplasias. |
Table I.
Clinicopathological characteristics
of early gastric neoplasias.
| Characteristic | Adenoma (n=22) | MC (n=92) | SMC (n=37) |
|---|
| Age, years (mean ±
SD) | 72.5±9.2 | 70.2±7.4 | 66.8±12.2 |
| Gender, n |
|
|
|
|
Male | 14 | 67 | 28 |
|
Female | 8 | 25 | 9 |
| Size, mm (mean ±
SD) | 11.6±7.2 | 10.0±4.5 | 25.3±14.9 |
| Site, n |
|
|
|
| Upper
third | 4 | 19 | 6 |
| Middle
third | 6 | 38 | 20 |
| Lower
third | 12 | 35 | 11 |
| Macroscopic type,
n |
|
|
|
|
Elevated | 11 | 31 | 4 |
| Flat or
depressed | 11 | 61 | 33 |
| Histology, n |
|
|
|
|
Differentiated |
| 91 | 23 |
|
Mixed | NA | 1 | 5 |
|
Undifferentiated |
| 0 | 9 |
All patients were classified as having HP infections
based on endoscopic atrophic changes or by testing seropositive for
HP-immunoglobulin G (IgG). It was further confirmed that these
patients had no HP eradication history. All specimens were assigned
a novel number without the inclusion of personal information in
order to maintain anonymity. This study was approved by the
Institutional Ethics Committee of Tottori University (no. 314) and
complies with the Declaration of Helsinki.
Evaluation of the tumor-surrounding
mucosa
HP-associated gastritis adjacent to the tumor was
evaluated by using the updated Sydney system (USS) (20). Using this system, gastritis was scored
from 0 (absent) to 3 (marked). Glandular atrophy, IM and
mononuclear cell infiltration (chronic inflammation) were examined.
IM adjacent to the tumor was absent in 1 adenoma, 1 MC and 6 SMC
cases.
Immunohistochemical staining
Paraffin-embedded sections (4-µm thick) were
immunohistochemically stained with the indicated mouse monoclonal
antibodies against the following proteins: p53 (DO-7; Dako,
Glostrup, Denmark; dilution, 1:50), MutL homolog 1 (MLH1; G168-15;
BD Pharmingen, San Diego, CA, USA; dilution, 1:50), mucin 5AC,
oligomeric mucus/gel-forming (MUC5AC; 45M1; Leica Biosystems, Ltd.,
Newcastle, UK; dilution, 1:50), mucin 2, oligomeric mucus/gel-form
(MUC2; Ccp58; Leica Biosystems, Ltd.; dilution, 1:100) and cluster
of differentiation 10 (CD10; 56C6; Leica Biosystems, Ltd.;
dilution, 1:50) or with a rat monoclonal anti-AID antibody (EK2
5G9; Cell Signaling Technology, Inc., Danvers, CA, USA; dilution
1:400), using the avidin-biotin-peroxidase complex (ABC)
technique.
Immunohistochemical staining was performed in a
blinded manner with respect to the clinical information. The
sections were deparaffinized in xylene and rehydrated in ethanol.
The sections were then immersed in a citrate buffer (0.01 mol/l; pH
6.0) and heated in a microwave oven for 20–30 min to retrieve
antigens. Endogenous tissue peroxidase activity was blocked by
incubation with 3% H2O2. The sections were
subsequently incubated with primary antibody overnight at 4°C. As a
negative control, the primary antibody was replaced with normal
serum IgG at a similar dilution. Signal detection followed the
Vectastain Elite ABC kit instructions (Vector Laboratories, Inc.,
Burlingame, CA, USA) using diaminobenzidine as the chromogen. The
sections were counterstained with hematoxylin, and were
subsequently incubated with biotinylated anti-rat or anti-mouse IgG
and avidin-biotin-peroxidase (included with Vectastatin Elite ABC
Kit). The sections were subsequently visualized using
diaminobenzidine tetrahydrochloride. Two independent observers
evaluated protein expression using an optical microscope (BX50;
Olympus Corporation, Tokyo, Japan).
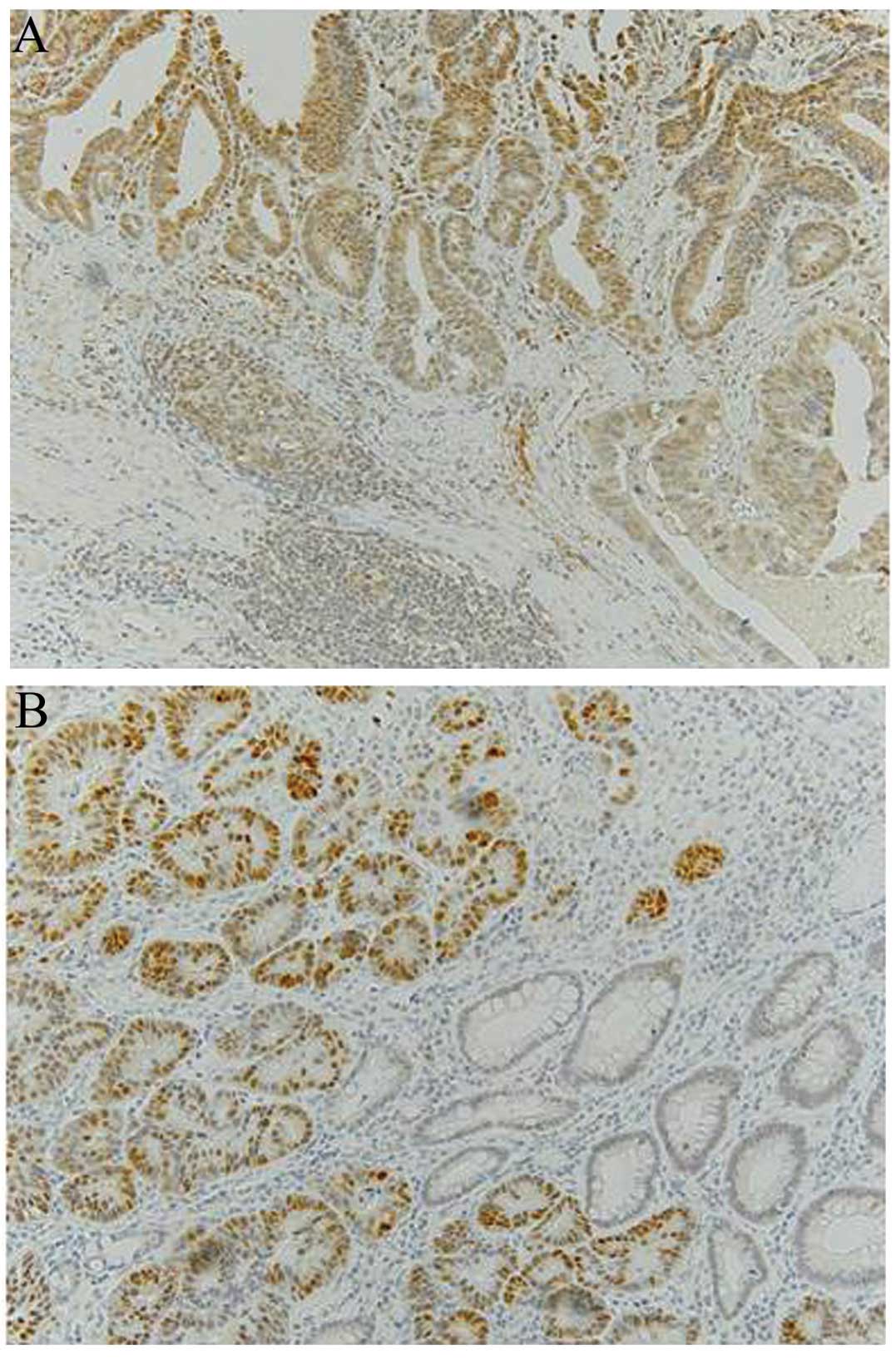
Assessment of AID immunostaining
The internal positive controls were lymphocytes of
germinal centers in lymphoid follicles (Fig. 1A). The follicles contain activated B
cells and all specimens stained markedly positive for AID. The
cytoplasm was scored as positive when >30% of the tumor cells
stained as markedly as the germinal centers. Aberrant AID
expression refers to AID overexpression.
Assessment of p53 immunostaining
The tumors were scored as positive for p53 when a
distinct nuclear immunoreaction occurred in >25% of the tumor
cells (21), as presented in Fig. 1B. Positive staining for p53 is
considered aberrant p53 expression (overexpression).
Assessment of MLH1 immunostaining
MLH1 expression was classified as either normal or
reduced (aberrant). Tissue specimens with definite nuclear staining
in <30% of the tumor cells were categorized as having reduced
staining (22).
Assessment of MUC5AC, MUC2 and CD10
immunostaining, and classification of phenotypes
Staining of >10% of the adenoma and carcinoma
cells for MUC5AC, MUC2 or CD10 was classified as positive for that
marker, and staining of <10% was classified as negative. The
tumor phenotypes were classified into 3 categories according to the
combination of the expression of CD10, MUC2 and MUC5AC. The
intestinal phenotype (I-type) was positive for MUC2 and/or CD10,
but negative for MUC5AC. The gastric and intestinal mixed phenotype
(GI-type) was positive for MUC2 and/or CD10, and positive for
MUC5AC. The gastric phenotype (G-type) was positive for MUC5AC, but
negative for MUC2 and CD10. IM was also classified into GI- and
I-types using these markers (23).
Statistical analysis
All data were statistically analyzed using the
χ2 test with Yates' correction, Fisher's test and
Student's t-test using Stat View software, version 5.0 (SAS
Institute, Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Frequency of aberrant AID, p53 and
MLH1 expression
The protein expression of AID, p53 and MLH1 was
immunohistochemically determined in early gastric neoplasms
(Table II). Aberrant AID, p53 and
MLH1 expression (overexpression, overexpression and loss of
expression, respectively) was detected in 36.4% (8/22), 0% (0/22)
and 0% (0/22) of the adenomas, in 35.9% (33/92), 32.6% (30/92) and
16.3% (15/92) of the MCs, and in 56.8% (21/37), 62.2% (23/37) and
21.6% (8/37) of the SMCs, respectively. p53 overexpression and loss
of MLH1 expression was not detected in the adenomas. Overexpression
of AID and the p53 gene product was significantly more
frequent in the SMCs compared with the MCs (AID, P<0.05; p53,
P<0.01). There were no significant differences in the loss of
MLH1 between the MCs and SMCs. In addition, there were no
significant correlations between the expression levels of these
proteins and other clinicopathological data, including age, gender,
location or macroscopic type of tumor (data not shown).
 | Table II.Percentages of early gastric
neoplasias showing aberrant expression of AID, p53 and MLH1. |
Table II.
Percentages of early gastric
neoplasias showing aberrant expression of AID, p53 and MLH1.
|
| Aberrant
expression, n/total n (%) |
|---|
|
|
|
|---|
| Protein | Adenoma (n=22) | MC (n=92) | SMC (n=37) |
|---|
| AID | 8/22
(36.4) | 33/92
(35.9)a | 21/37
(56.8)a |
| p53 | 0/22 (0.0) | 30/92
(32.6)b | 23/37
(62.2)b |
| MLH1 | 0/22 (0.0) | 15/92 (16.3) | 8/37
(21.6) |
Association between AID, p53 and MLH1
expression
Aberrant AID expression was significantly associated
with p53 overexpression in the SMCs (P<0.01), but not in the MCs
(Table III). p53 overexpression was
significantly and inversely associated with the loss of MLH1
expression, particularly in the MCs (P<0.05). The percentage of
SMCs that demonstrated aberrant AID and p53 expression (45.9%) was
significantly increased compared with that of the MCs (10.9%)
(P<0.0001). When the expression of aberrant AID and p53 was used
for the diagnosis of submucosal invasion, the sensitivity was
45.9%, the specificity was 89.1%, the positive predictive value was
63.0% and the negative predictive value was 80.4% (Table IV).
 | Table III.Association between AID and p53
expression in early gastric cancers. |
Table III.
Association between AID and p53
expression in early gastric cancers.
|
| AID expression |
|---|
|
|
|
|---|
|
| MC (n=92) |
| SMC (n=37) |
|
|---|
|
|
|
|
|
|
|---|
| p53 expression | Positive, n | Negative, n | P-value | Positive, n | Negative, n | P-value |
|---|
| Positive | 10 | 20 |
| 17 | 6 |
|
| Negative | 23 | 39 | P=0.724 | 4 | 10 | P=0.0069 |
 | Table IV.Association between AID and p53
expression and submucosal invasion in early gastric cancers. |
Table IV.
Association between AID and p53
expression and submucosal invasion in early gastric cancers.
| Aberrant
expression | MCs + SMCs | MC (n=92) | SMC (n=37) | P-value |
|---|
| AID(+)/p53(+),
n | 27 | 10 | 17 |
|
| Other, n | 102 | 82 | 20 | P<0.0001 |
Association between AID, p53 and MLH1
expression levels, and the background mucosa
Next, the gastritis in the background mucosa of the
tumors was analyzed by evaluating the glandular atrophy, IM and
chronic inflammation (mononuclear cell infiltration). Table V shows the mean USS scores for these
parameters. The mean scores of gastritis surrounding the adenomas,
MCs and SMCs were as follows: Glandular atrophy, 2.22, 1.83 and
1.89; chronic inflammation, 1.77, 1.89 and 2.24; and IM, 2.31, 1.90
and 1.78, respectively. A significant increase was noted in the
mean scores of glandular atrophy and IM surrounding the adenomas
compared with those surrounding the MCs and SMCs (P<0.05), and
the mean score of chronic inflammation surrounding the SMCs was
significantly increased compared with those surrounding the
adenomas and MCs (P<0.01). The mean score of chronic
inflammation in the AID-positive neoplasms was significantly
increased compared with that in the AID-negative neoplasms,
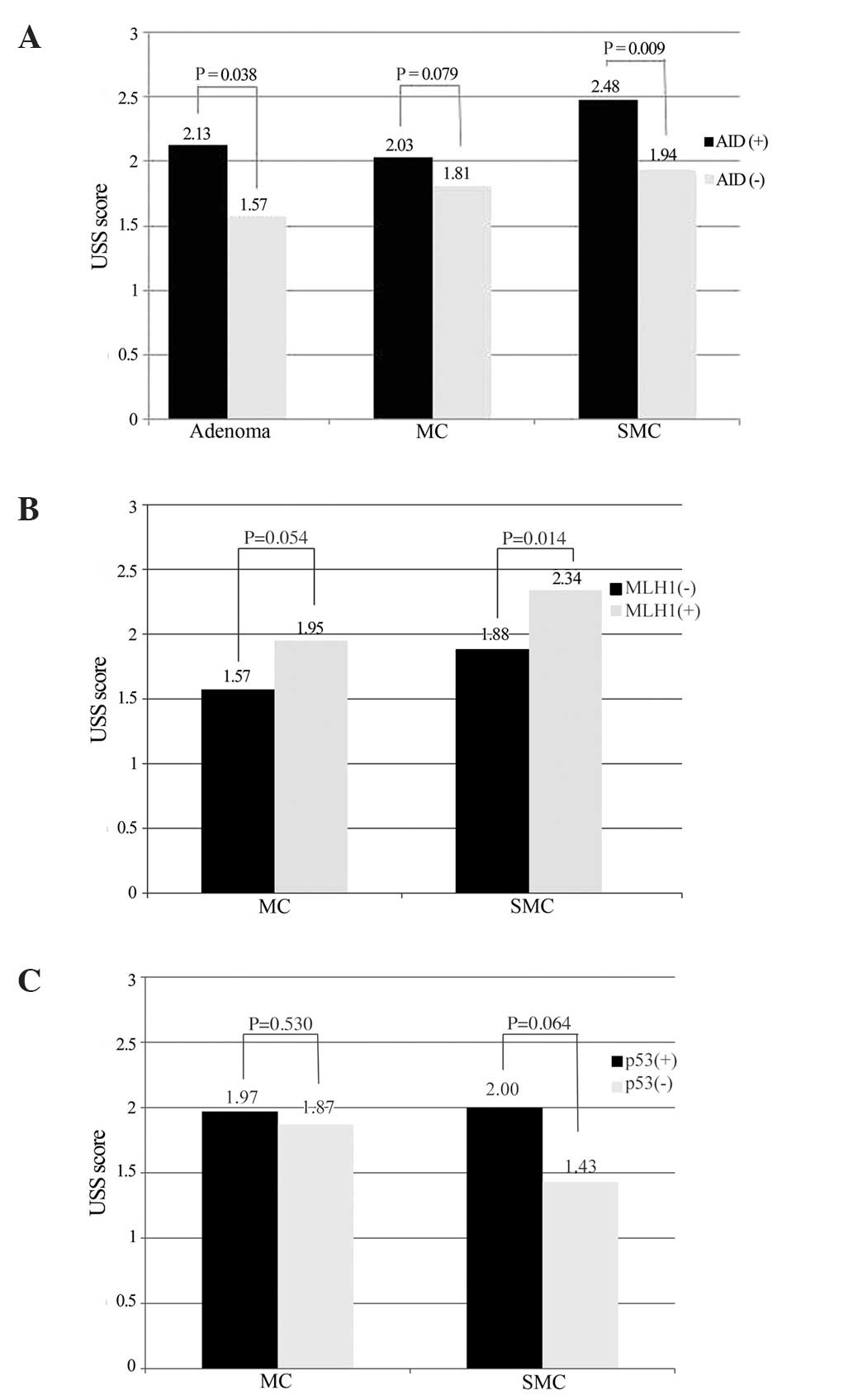
particularly in the SMC cases (P<0.05) (Fig. 2A). In addition, the mean score of
chronic inflammation in the MLH1-negative neoplasms was reduced
compared with the MLH1-positive neoplasms (P<0.05; Fig. 2B). The mean score of IM adjacent to
the p53-positive SMCs was increased compared with that adjacent to
the p53-negative SMCs (Fig. 2C).
 | Table V.Updated Sydney system score of the
background mucosa of early gastric neoplasias. |
Table V.
Updated Sydney system score of the
background mucosa of early gastric neoplasias.
| Parameter | Adenoma (n=22) | MC (n=92) | SMC (n=37) |
|---|
| Atrophy |
2.22a | 1.83 | 1.89 |
| IM |
2.31b | 1.90 | 1.78 |
| Chronic
inflammation | 1.77 | 1.89 |
2.24c |
Phenotype of tumors and their
surrounding IM
The gastric/intestinal phenotypes of the tumors and
their surrounding IM were analyzed (Table VI). A high percentage of MCs (34.8%)
and SMCs (24.3%) were of the G-type, and a high percentage of
adenomas (54.5%) were of the I-type (P<0.05). Using the same
phenotypic markers, the tumor-surrounding IM was divided into two
major types: A GI-type and a solely I-type. The phenotype of the IM
surrounding SMCs was largely classified as the I-type (45.2%), and
the phenotypes of the IMs surrounding the adenomas and the majority
of the MCs were classified as the GI-type (71.4 and 72.5%,
respectively; P=0.063). The combined data indicated that the
background IM phenotype was similar for the adenomas and MCs, but
differed for the SMCs. In addition, the cellular phenotype in
tumors and the background mucosa were not correlated with
tumor-associated protein expression or with the mean scores of the
USS classification (data not shown).
 | Table VI.Phenotype of early gastric neoplasias
and their surrounding IM. |
Table VI.
Phenotype of early gastric neoplasias
and their surrounding IM.
| Parameter | Adenoma (n=22) | MC (n=92) | SMC (n=37) |
|---|
| Phenotype of tumor,
n (%) |
|
|
|
|
G-type | 4
(18.2) | 32 (34.8) | 9
(24.3) |
|
GI-type | 6
(27.3) | 33 (35.9) | 16 (43.2) |
|
I-type | 12 (54.5) | 27 (29.3) | 12 (32.4) |
| Phenotype of
surrounding IM, n (%)a |
|
|
|
|
GI-type | 15 (71.4) | 66 (72.5) | 17 (54.8) |
|
I-type | 6
(28.6) | 25 (27.5) | 14 (45.2) |
Discussion
The present study examined AID, p53 and MLH1
expression, and the cellular phenotype of early gastric neoplastic
samples, and then these results were compared with patient
clinicopathological characteristics and the condition of the mucosa
surrounding or adjacent to the tumor. The expression levels of p53
and MLH1, and a gastric phenotype were significantly associated
with carcinogenesis. In addition, the features of the background
mucosa of the adenomas and MCs were similar. Notably, chronic
inflammation and AID and p53 expression were significantly
associated with submucosal invasion.
Previous studies have demonstrated important roles
for AID, a cellular genome mutator, in HP-associated gastric cancer
development (9). Infection with HP
triggers the aberrant expression of AID within the gastric
epithelium, which results in the accumulation of altered
nucleotides in the p53 gene (12,24,25). The
percentage of adenomas (36.4%), MCs (35.9%) and SMCs (56.8%) that
demonstrated aberrant AID expression in the present study was
slightly increased compared with the percentage of GCs that
demonstrated aberrant AID expression in two previous studies (26.9
and 22.5%) (25,26). Notably, in the present study, the
percentage of adenomas and MCs that demonstrated aberrant AID
expression was the same, whereas the percentage of SMCs
demonstrating aberrant AID expression was significantly increased
compared with that of MCs. The variability in the findings may be a
result of the differences in the stage of carcinoma progression and
in the degree of tumor differentiation.
Kim et al (25)
observed a significant association between the aberrant expression
of AID and the nuclear overexpression of p53 in a range of gastric
cancer types. However, our previous study did not demonstrate an
association between aberrant AID expression and p53 overexpression
in early differentiated gastric cancer (26). Similarly, Goto et al (27) observed no correlation between AID and
p53 expression in early differentiated and poorly-differentiated
gastric cancer. In the present study, aberrant AID expression was
significantly associated with p53 overexpression in the SMCs
(P<0.01), but not in the MCs or adenomas. Therefore, mutations
in p53 as a result of aberrant AID expression may increase
with tumor progression. However, the p53 protein may also become
altered through cigarette smoking, as occurs in lung and esophageal
carcinogenesis (28,29). AID is a well-described molecule that
is involved in DNA mutations in the human genome. The typical
pattern of nucleotide alteration induced by its enzymatic activity
is cytosine/guanine (C/G) to thymine/adenine (T/A) transition
(9). Tobacco smoking also induces G/C
to T/A transversion (30). Further
investigation is required to clarify the correlation between the
expression of p53 and aberrant AID expression.
The expression of AID in gastric epithelial cells
may be altered by the direct action of HP macromolecules through
the type IV secretion system encoded by cag-PAI (31). Additionally, in human gastric
epithelial cells, AID expression is induced by HP-associated
inflammatory mediators, including tumor necrosis factor α and
interleukin-1β via activation of NF-κB (12). Furthermore, the expression of AID in
tumors, including colon cancer, cholangiocarcinoma and
hepatocellular carcinoma, is also mediated by stimulation of
proinflammatory cytokines via NF-κB (32–34).
Aberrant AID expression correlates with chronic active
inflammation, glandular atrophy and IM in non-neoplastic gastric
mucosa (26). The present study
demonstrated that aberrant AID expression in tumors correlated with
mononuclear cell activity in the mucosa surrounding the tumor,
particularly in SMC cases, which supports the aforementioned
mechanism of AID expression and is consistent with previous studies
(26,27). Nagata et al (35) demonstrated that the enhanced
expression of AID in human gastric mucosa infected by HP is reduced
following HP eradication. In addition, Kang et al (36) reported that rebamipide suppressed
HP-induced AID expression and also suppressed invasion of gastric
cancer cells through the downregulation of phospholipase D1 (PLD1),
via inhibition of the HP cagA-NFκB-PLD1 signaling pathway.
Therefore, HP eradication and a drug that results in AID
suppression via inhibition of HP-mediated PLD1 may prevent
submucosal invasion in gastric cancer.
Submucosal invasion in gastric cancer has also been
associated with aberrant AID and p53 expression. Sugai et al
(37) reported that gastric cancer
with p53 overexpression is associated with a high risk of
submucosal invasion. These data indicate that AID is important in
the progression of gastritis to gastric cancer, particularly in
SMC. The results of the present study indicated that tumor
progression may be associated with p53 mutations that arise
due to aberrant AID expression. Also, combined aberrant AID
expression and p53 overexpression may provide valuable information
for the prediction of aggressive tumor behavior.
In the present study, the percentage of the MCs and
SMCs that demonstrated loss of MLH1 expression was 16.3 and 21.6%,
respectively, and this loss of MLH1 expression was inversely
associated with p53 overexpression in the MCs. These findings are
consistent with previous studies (5).
Aberrant DNA methylation of promoter CpG islands appears to be the
predominant mechanism associated with the loss of hMLH1
function in chronic inflammation-associated sporadic gastric
carcinomas (8). In addition, previous
studies have demonstrated that AID is crucial in inducing
p53 mutations and DNA demethylation (38). This mechanism may explain this
association. In addition, in the present study there was no
difference in MLH1 expression between MCs and SMCs, indicating that
loss of MLH1 expression appears to be independent of the degree of
invasion, which is consistent with previous study findings
(37).
Marked differences between the biological behavior
of gastric phenotype carcinomas and intestinal phenotype carcinomas
have previously been reported (39).
In the present study, the percentage of the MCs (34.8%) and SMCs
(24.3%) that were of the gastric phenotype was significantly
increased compared with that of the adenomas (18.2%), and the
percentage of the adenomas that were of the intestinal phenotype
(54.5%) was increased compared with that of the MCs and SMCs (29.3
and 32.4%). Based on these data, the gastric phenotype may be
important for malignant transformation. This result is consistent
with previous studies (40). The
current study further demonstrated that the phenotype of the
background IM of the SMCs was largely classified as the intestinal
type (45.2%), whereas the majority of the phenotypes of the
background IMs of the adenomas and MCs were classified as the mixed
type (71.4 and 72.5% respectively). The combined data indicated
that the background IM phenotype of the adenomas and MCs was
similar, but that the phenotype of the SMCs was different. Previous
observations in animal models indicated that the phenotype of IM
changed from the GI mixed-type to type I during tumor progression
(41). This may indicate a phenotypic
shift in expression from the gastric to intestinal phenotype during
tumor progression.
In conclusion, p53 and MLH1 expression, and a
gastric phenotype may be important for carcinogenesis, and adenomas
and MCs may arise from a similar background mucosa. Furthermore,
chronic inflammation and AID and p53 expression may affect
submucosal invasion. These findings may provide valuable
information for the prediction of aggressive tumor behavior and may
aid in the decision between endoscopic therapies and surgical
resection. However, additional extensive future studies are
required to confirm the results of the present study.
Acknowledgements
This study has been presented as a 2013 United
European Gastroenterology Week oral presentation.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American cancer
society award lecture on cancer epidemiology and prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
3
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sipponen P and Kimura K: Intestinal
metaplasia, atrophic gastritis and stomach cancer: Trends over
time. Eur J Gastroenterol Hepatol. 6:(Suppl 1). S79–S83.
1994.PubMed/NCBI
|
|
5
|
Tamura G: Alterations of tumor suppressor
and tumor-related genes in the development and progression of
gastric cancer. World J Gastroenterol. 12:192–198. 2006.PubMed/NCBI
|
|
6
|
Yamazaki K, Tajima Y, Makino R, et al:
Tumor differentiation phenotype in gastric differentiated-type
tumors and its relation to tumor invasion and genetic alterations.
World J Gastroenterol. 12:3803–3809. 2006.PubMed/NCBI
|
|
7
|
Hong SH, Kim HG, Chung WB, et al: DNA
hypermethylation of tumor-related genes in gastric carcinoma. J
Korean Med Sci. 20:236–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fleisher AS, Esteller M, Wang S, et al:
Hypermethylation of the hMLH1 gene promoter in human gastric
cancers with microsatellite instability. Cancer Res. 59:1090–1095.
1999.PubMed/NCBI
|
|
9
|
Chiba T, Marusawa H and Ushijima T:
Inflammation-associated cancer development in digestive organs:
Mechanisms and roles for genetic and epigenetic modulation.
Gastroenterology. 143:550–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muramatsu M, Kinoshita K, Fagarasan S,
Yamada S, Shinkai Y and Honjo T: Class switch recombination and
hypermutation require activation-induced cytidine deaminase (AID),
a potential RNA editing enzyme. Cell. 102:553–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okazaki IM, Hiai H, Kakazu N, et al:
Constitutive expression of AID leads to tumorigenesis. J Exp Med.
197:1173–1181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto Y, Marusawa H, Kinoshita K, et
al: Helicobacter pylori infection triggers aberrant expression of
activation-induced cytidine deaminase in gastric epithelium. Nat
Med. 13:470–476. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. Acta Pathol Microbiol Scand. 64:31–49. 1965.PubMed/NCBI
|
|
14
|
Nakamura K, Sugano H and Takagi K:
Carcinoma of the stomach in incipient phase: Its histogenesis and
histological appearance. Gan. 59:251–258. 1968.PubMed/NCBI
|
|
15
|
Egashira Y, Shimoda T and Ikegami M: Mucin
histochemical analysis of minute gastric differentiated
adenocarcinoma. Pathol Int. 49:55–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshino T, Shimoda T, Saito A, et al:
Macroscopic features of differentiated adenocarcinoma with gastric
or intestinal phenotype expression in early gastric cancer. Stomach
Intestine. 34:513–525. 1999.
|
|
17
|
Koseki K, Takizawa T, Koike M, Ito M,
Nihei Z and Sugihara K: Distinction of differentiated type early
gastric carcinoma with gastric type mucin expression. Cancer.
89:724–732. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japanese Gastric Cancer Association, .
Japanese Classification of Gastric Carcinoma. 13th. Kanehara &
Co., Ltd.; Tokyo: 1998, (In Japanese).
|
|
19
|
Schlemper RJ, Riddell RH, Kato Y, et al:
The Vienna classification of gastrointestinal epithelial neoplasia.
Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis The updated Sydney
System. International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andachi H, Yashima K, Koda M, et al:
Reduced Fhit expression is associated with mismatch repair
deficiency in human advanced colorectal carcinoma. Br J Cancer.
87:441–445. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baek MJ, Kang H, Kim SE, et al: Expression
of hMLH1 is inactivated in the gastric adenomas with enhanced
microsatellite instability. Br J Cancer. 85:1147–1152. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niwa T, Ikehara Y, Nakanishi H, et al:
Mixed gastric- and intestinal-type metaplasia is formed by cells
with dual intestinal and gastric differentiation. J Histochem
Cytochem. 53:75–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morisawa T, Marusawa H, Ueda Y, et al:
Organ-specific profiles of genetic changes in cancers caused by
activation-induced cytidine deaminase expression. Int J Cancer.
123:2735–2740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim CJ, Song JH, Cho YG, et al:
Activation-induced cytidine deaminase expression in gastric cancer.
Tumour Biol. 28:333–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takeda Y, Yashima K, Hayashi A, et al:
Expression of AID, P53 and Mlh1 proteins in endoscopically resected
differentiated-type early gastric cancer. World J Gastrointest
Oncol. 4:131–137. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goto A, Hirahashi M, Osada M, et al:
Aberrant activation-induced cytidine deaminase expression is
associated with mucosal intestinalization in the early stage of
gastric cancer. Virchows Arch. 458:717–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pfeifer GP, Denissenko MF, Olivier M,
Tretyakova N, Hecht SS and Hainaut P: Tobacco smoke carcinogens,
DNA damage and p53 mutations in smoking-associated cancers.
Oncogene. 21:7435–7451. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saeki H, Ohno S, Miyazaki M, et al: p53
protein accumulation in multiple oesophageal squamous cell
carcinoma: Relationship to risk factors. Oncology. 62:175–179.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kozack R, Seo KY, Jelinsky SA and Loechler
EL: Toward an understanding of the role of DNA adduct conformation
in defining mutagenic mechanism based on studies of the major
adduct (formed at N(2)-dG) of the potent environmental carcinogen,
benzo[a]pyrene. Mutat Res. 450:41–59. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Viala J, Chaput C, Boneca IG, et al: Nod1
responds to peptidoglycan delivered by the Helicobacter pylori cag
pathogenicity island. Nat Immunol. 5:1166–1174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Endo Y, Marusawa H, Kou T, et al:
Activation-induced cytidine deaminase links between inflammation
and the development of colitis-associated colorectal cancers.
Gastroenterology. 135:889–898. 898.e1–898e.3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Endo Y, Marusawa H, Kinoshita K, et al:
Expression of activation-induced cytidine deaminase in human
hepatocytes via NF-kappaB signaling. Oncogene. 26:5587–5595. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komori J, Marusawa H, Machimoto T, et al:
Activation-induced cytidine deaminase links bile duct inflammation
to human cholangiocarcinoma. Hepatology. 47:888–896. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagata N, Akiyama J, Marusawa H, et al:
Enhanced expression of activation-induced cytidine deaminase in
human gastric mucosa infected by Helicobacter pylori and its
decrease following eradication. J Gastroenterol. 49:427–435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kang DW, Hwang WC, Park MH, et al:
Rebamipide abolishes Helicobacter pylori CagA-induced phospholipase
D1 expression via inhibition of NFκB and suppresses invasion of
gastric cancer cells. Oncogene. 32:3531–3542. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sugai T, Habano W, Endoh M, et al:
Molecular analysis of gastric differentiated-type intramucosal and
submucosal cancers. Int J Cancer. 127:2500–2509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fritz EL and Papavasiliou FN: Cytidine
deaminases: AIDing DNA demethylation? Genes Dev. 24:2107–2114.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tajima Y, Shimoda T, Nakanishi Y, et al:
Gastric and intestinal phenotypic marker expression in gastric
carcinomas and its prognostic significance: Immunohistochemical
analysis of 136 lesions. Oncology. 61:212–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsukashita S, Kushima R, Bamba M, Sugihara
H and Hattori T: MUC gene expression and histogenesis of
adenocarcinoma of the stomach. Int J Cancer. 94:166–170. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yuasa H, Inada K, Watanabe H and Tatematsu
M: A phenotypic shift from gastric-intestinal to solely intestinal
cell types in intestinal metaplasia in rat stomach following
treatment with X-rays. J Toxicol Pathol. 15:85–93. 2002. View Article : Google Scholar
|