Introduction
Prostate cancer is the most common malignancy in
males in western countries, and the second most common cause of
cancer-related mortality (1). The
clinical symptoms of early prostate carcinoma are unspecific, and
the disease is therefore often diagnosed at a late stage. With the
extensive use of serum prostate-specific antigen detection and
biopsy of the prostate, the early diagnosis rate of prostate cancer
has improved (2,3).
A previous study found that iron metabolism plays a
significant role in cancer cell growth, angiogenesis and metastasis
(4). Hepcidin, predominantly
synthesized in the liver, is the principal regulator of systemic
iron homeostasis, and acts by inhibiting intestinal iron
absorption, iron recycling by macrophages, and iron mobilization
from hepatic stores (5). It has been
reported that hepcidin is closely associated with infection, tumor
and chronic inflammation (6).
Ferroportin protein is an important regulator of body iron
metabolism, and is a membrane transport protein that transfers
intracellular iron to the extracellular environment. Reduced
expression levels of ferroportin on the cell surface lead to an
increase in intracellular free iron, making the tumor cells more
aggressive. Changes in ferroportin protein expression caused by
abnormal iron metabolism often induce reactions in tumor invasion
and metastasis (7). Ferroportin has
been reported to be significantly correlated with prognosis in
breast cancer (8). However, thus far,
the role of ferroportin protein expression in prostate cancer
remains elusive.
The present study analyzed the expression levels of
ferroportin protein in different differentiation stages of prostate
cancer and prostate hyperplasia, as well as the differences in
prostate cancer and normal prostate cells.
Materials and methods
Subjects
The subjects of the present study were selected from
60 patients with prostate cancer and 30 patients with benign
prostatic hyperplasia (BPH) who visited the Third Affiliated
Hospital (Suzhou University, Changzhou, Jiangsu, China) between
January 2008 and December 2012. The study was approved by the
Ethics Committee/Institutional Review Board of the hospital, and
was performed in accordance with the Declaration of Helsinki.
Written informed consent was obtained from all patients. The age
range of the patients was 55–75 years, with a mean of 67 years.
Prostate cancer was pathologically diagnosed in the 60 cancer
patients. According to the Gleason score (9), 20 cases presented with scores of <7,
15 cases with scores of 7 and 25 cases with scores of >7. For
the remaining 30 subjects, BPH was diagnosed by a transurethral
resection of the prostate pathology.
Ferroportin protein was measured by
immunohistochemistry
Surgical specimens were fixed in formalin and
embedded in paraffin blocks. Sections (4-µm thick) were incubated
for 1 h at 60°C, heated in an oven at 37°C for 15 min,
de-paraffinized and rehydrated using serial xylene and ethanol
(Sigma-Aldrich, St. Louis, MO, USA) incubations, then transferred
to sodium citrate buffer (pH 6.0; Sigma-Aldrich) for 15 min.
Following antigen retrieval, the sections were incubated in 3%
peroxide bicarbonate solution at room temperature for 10 min to
block endogenous peroxidase activity. Ferroportin protein
expression was detected using a horseradish peroxidase (HRP)
complex with a rabbit monoclonal anti-ferroportin primary antibody
(1:50; cat. no. ab85370; Abcam, Cambridge, MA, USA), visualized
using 3,3′-diaminobenzidine staining and observed by microscope
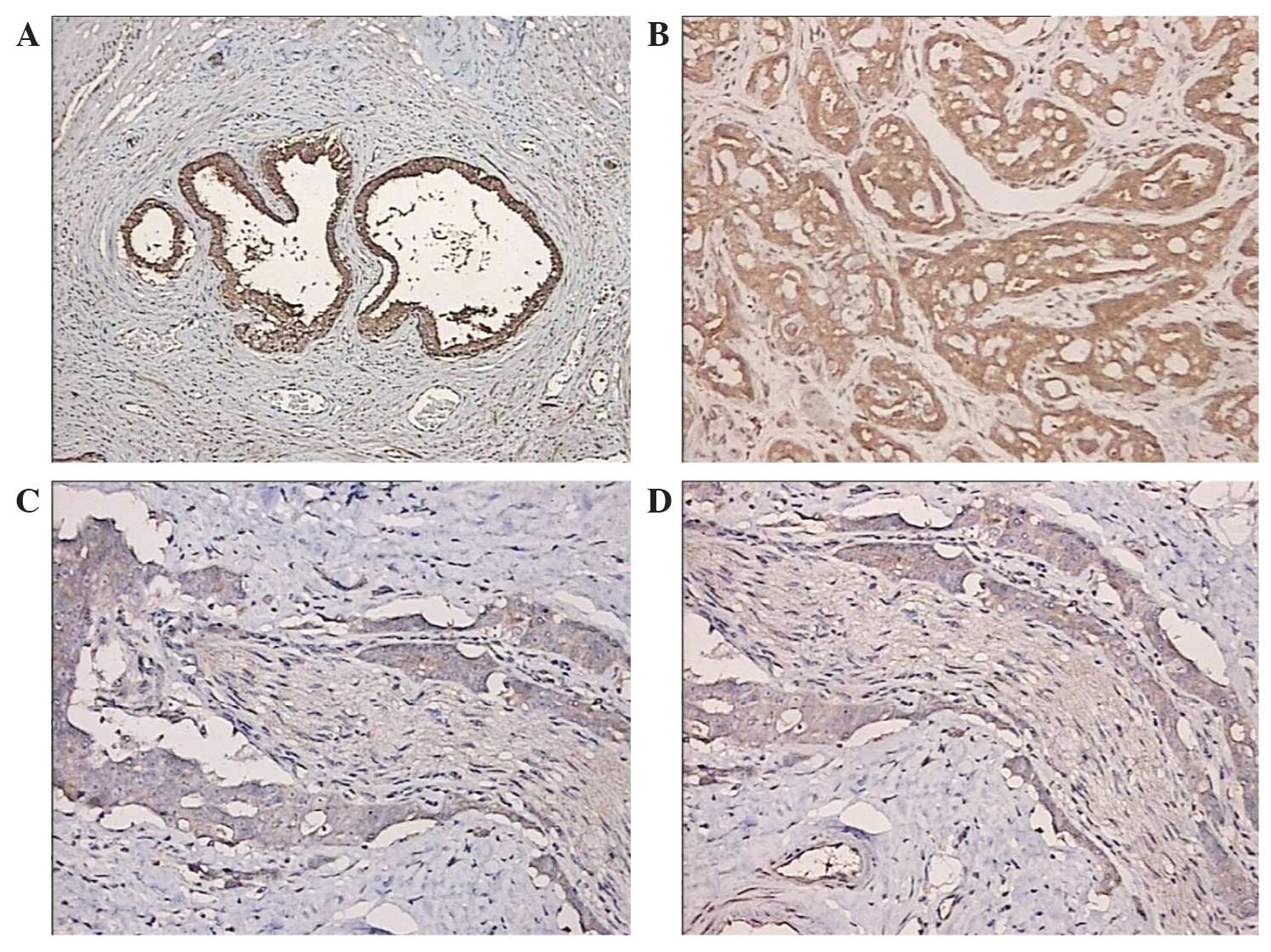
(Olympus BHS; Olympus Corporation, Tokyo, Japan) (Fig. 1).
Ferroportin gene expression measured
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
The prostate cancer PC3, DU145 and LNCAP cell lines,
and the normal prostate RWPE2 cell line were obtained from Shanghai
Institutes for Biological Sciences, Shanghai, China). The cells
were cultured in RMPI 1640 medium supplemented with 10% fetal
bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin
(Sigma-Aldrich) in an atmopshere of 5% CO2 at 37°C. RNA
was extracted with TRIzol (Invitrogen Life Technologies, Carlsbad,
CA, USA). RT was performed according to the manufacturer's
instructions by adding oligo(dT), 5X Reaction Buffer, RNase
Inhibitor, 10 mM dNTP mix and M-MuLV reverse transcriptase
sequentially (PCR supermix; catalog no. 10572-014; Invitrogen). The
Luminaris Color HiGreen qPCR Master Mix PCR kit was purchased from
Thermo Fisher Scientific, Inc., (Waltham, MA, USA) and the RT-PCR
reactions were performed at 94°C for 5 min, 94°C for 20 sec, 55°C
for 20 sec and 72°C 15 sec for 30 cycles, followed by 72°C for 5
min. The sequences of the primers are presented in Table I. PCR products were transferred to 2%
agarose gel and the band intensity was quantified using the Kodak
Gel Logic 200 imaging system (Kodak, Rochester, NY, USA).
 | Table I.Primers for ferroportin and GAPDH. |
Table I.
Primers for ferroportin and GAPDH.
| Genes | 5′-Primers | 3′-Primers | Products, bp |
|---|
| Ferroportin |
AGCCTGCCACCACCAACCCGTAGA |
TGGCTCCCAGGACCAGAAC | 225 |
| GAPDH |
CAAGGTCATCCATCCATGACAACTTTG |
GTCCACCACCCTGTTGCTGTAG | 496 |
Ferroportin protein expression
measured by western blotting
Cells grown in a 25-cm2 flask were rinsed
with PBS prior to harvesting. Total proteins were extracted with a
cell lysis buffer (Pierce Biotechnology, Inc., Rockford, IL, USA)
containing phenylmethylsulfonyl fluoride and incubated on ice for
10 min. The lysate was separated by centrifugation at 100,000 × g
for 30 min. The amount of protein was measured by bicinchoninic
acid assay (Pierce Biotechnology, Inc.). For 12% SDS-PAGE, a total
of 20 µg of protein was loaded per well. Polyvinylidene difluoride
(PVDF) membranes (Roche Diagnostics, Indianapolis, IN, USA) were
used for the transfer process. For western blotting, PVDF membranes
were incubated in 5% skimmed milk blocking buffer for 1 h, followed
by washing 3 times with Tris-buffered saline plus 0.1% Tween 20.
Ferroportin was detected using a rabbit monoclonal
ferroportin-specific antibody (1:500; cat. no. ab85370) and
horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary
antibodies (1:2,000; cat. no. ab6728; Abcam). Mouse monoclonal
GAPDH (1:2,000; cat. no. ab130099; Abcam) was used as a loading
control. The protein bands were developed using the
Electrochemiluminescence Plus kit (GE Healthcare, Bio-Sciences,
Pittsburgh, PA, USA), and band intensity was quantified using
ImageJ (http://imagej.nih.gov/ij/).
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed by
homogeneity of variance tests and are expressed as the mean ±
standard deviation. Differences were analyzed by t-test. Pearson
correlation was used to analyze the association between all studied
parameters. For heterogeneity of variance, non-parametric tests
were used, whereas the Mann-Whitney U test was used for pairwise
comparisons. P<0.05 was used to indicate a statistically
significant difference.
Results
Expression levels of ferroportin in
prostate cancer
The expression levels of ferroportin were examined
by immunohistochemical analysis in the prostate cancer and
prostatic hyperplasia tissue samples (Fig. 1). Reduced ferroportin expression
levels were found in the prostate cancer tissue samples. With the
decline in prostate cancer cell differentiation and increased
degree of malignancy, ferroportin expression was reduced and
exhibited less immunostaining in the cytoplasm. Moreover, the
prostatic hyperplasia tissue samples exhibited increased
ferroportin immunostaining suggesting enhanced expression
levels.
mRNA expression of ferroportin in
cancer cell lines
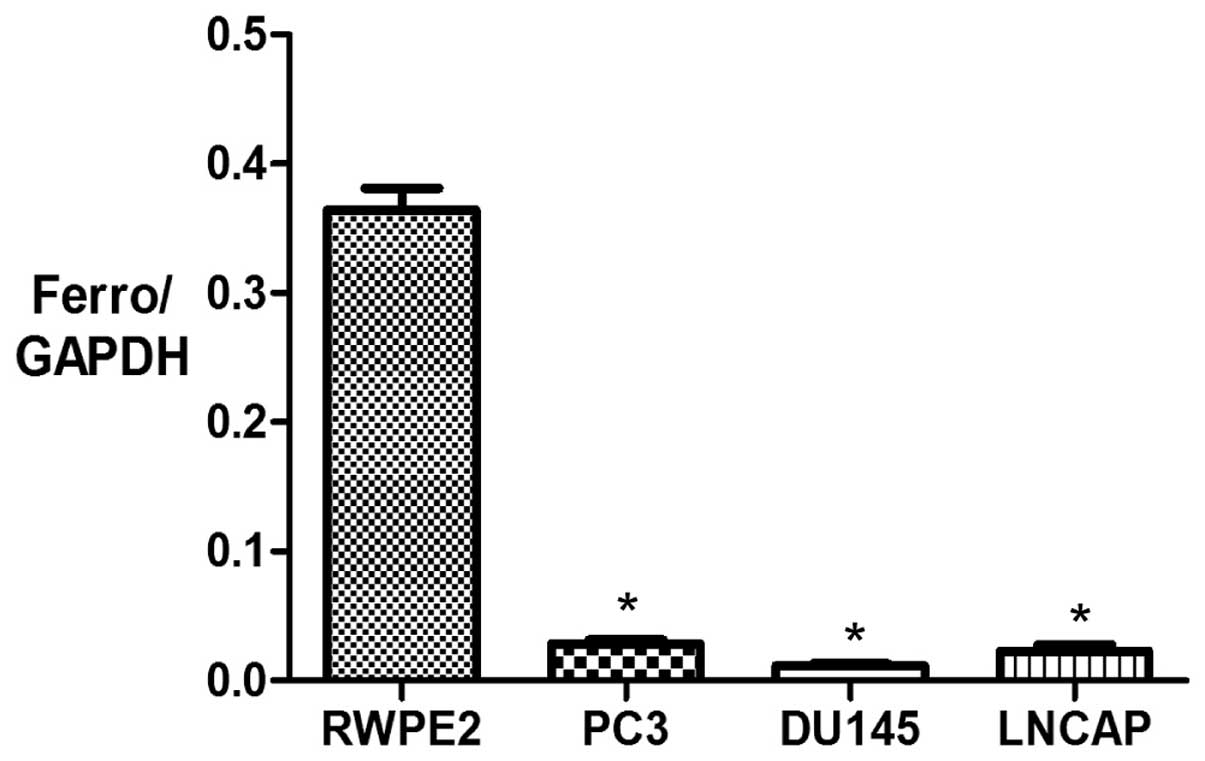
RT-qPCR was used to measure mRNA expression levels
of ferroportin in the prostate cancer PC3, DU145 and LNCAP cell
lines compared with the normal prostate RWPE2 cell line. GAPDH was
used as an internal standard to normalize the data. The mRNA
expression levels of ferroportin were significantly lower in the
prostate cancer PC3, DU145 and LNCAP cell lines (0.028±0.004,
0.011±0.002 and 0.022±0.008, respectively) compared with the normal
prostate RWPE2 cell line (0.36±0.03) (P<0.05; Fig. 2).
Expression of ferroportin in prostate
cancer cell lines
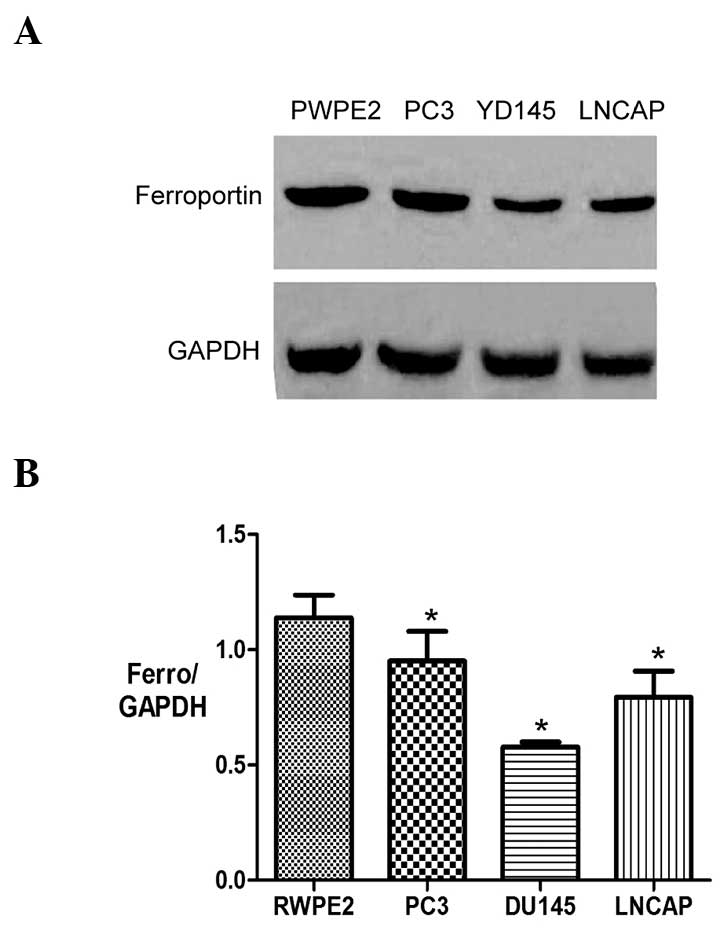
Western blotting was employed to measure the protein
expression levels of ferroportin in the prostate cancer PC3, DU145
and LNCAP cell lines and the normal prostate RWPE2 cell line.
Consistent with the findings of the mRNA expression levels, the
protein expression levels of ferroportin were significantly lower
in the prostate cancer PC3, DU145 and LNCAP cell lines (0.95±0.22,
0.57±0.04 and 0.79±0.19, respectively) compared with the normal
prostate RWPE2 cell line (1.13±0.17) (P<0.05) (Fig. 3).
Discussion
Numerous cancer cells exhibit an increased demand
for iron (10). Consequently,
proteins involved in iron regulation are often deregulated in
cancers and play a significant role during the tumor development
process. Ferroportin is a transmembrane protein that transports
iron from the inside of cells to the outside, and is the only known
mammalian iron exporter to date. High levels of ferroportin protein
have been detected in intestinal epithelial cells, placenta cells,
liver cells and macrophages regulating iron export by the
hepcidin-ferroportin axis (11).
Previous studies have demonstrated that the expression levels of
ferroportin and hepcidin affect the growth and progression of
breast cancer cells, and that the stability of ferroportin is
regulated by hepcidin (12). Compared
with normal breast epithelial cells, the expression level of
hepcidin is increased in breast cancer cells, whereas ferroportin
exhibits reduced expression, which is associated with reduced iron
export leading to enhanced iron availability to the tumor cells.
Moreover, another previous study also found that decreased levels
of ferroportin gene expression are associated with a significant
reduction in metastasis-free and disease-specific survival that is
independent of other breast cancer risk factors (12). It has been reported that the
ferroportin protein is a strong and independent predictor of
prognosis in breast cancer, and a novel potential target for
molecular therapy (13). However,
only two studies have reported that hepcidin is closely associated
with anemia in patients with advanced prostate cancer (14). In the present study, the expression
levels of ferroportin were measured in prostate tissues and
prostate cancer cell lines; the results suggested that the
decreased hepcidin-ferroportin axis may involved, as hepcidin
predominantly mediates the degradation of ferroportin (15).
According to the Gleason score, prostate cancer
tissue samples were divided into highly-differentiated,
moderately-differentiated and poorly-differentiated prostatic
hyperplasia groups and a normal group. The expression levels of
ferroportin protein were measured by immunohistochemistry in each
group, and reduced ferroportin protein expression was found in the
prostate cancer tissues. With decreased prostate cancer cell
differentiation and an increased degree of malignancy, the
expression levels of ferroportin protein were decreased, together
with less ferroportin immunostaining in the cytoplasm. By contrast,
the expression level of ferroportin was increased in BPH. The
different expression levels of ferroportin protein in the prostate
cancer and BPH tissues at different differentiation statuses
suggested that the ferroportin protein is closely associated with
the prostate cancer cell development process.
In order to further investigate whether ferroportin
protein regulates the development process of prostate cancer, the
mRNA and protein expression levels of ferroportin were compared in
the prostate cancer PC3, DU145 and LNCAP cell lines, and the normal
prostate RWPE2 cell line. RT-qPCR analysis suggested that the mRNA
levels of ferroportin were lower compared with the control group,
whereas there was no marked difference between the prostate cancer
cell lines. This observation was confirmed by western blotting.
To the best of our knowledge, the present study is
the first to reveal and compare the expression levels of
ferroportin protein in prostate cancer cells and normal prostate
cells. We believe that ferroportin protein plays a significant role
in the development of prostate cancer. It is possible that
decreased expression levels of ferroportin in tumor cells inhibits
intracellular iron export, causing intracellular iron overload and
activating reactive oxygen species, consequently inducing DNA
damage and impairing proteins and lipids, leading to tumorigenesis.
Increased intracellular iron further promotes the growth of tumor
cells (16). Collectively, data from
the present study and other studies suggests that ferroportin
protein could be a potential indicator for the diagnosis and
prognosis of patients with prostate cancer. Moreover, ferroportin
protein has clinical significance in prostate cancer as a potential
target for molecular-targeted therapy by regulating cellular iron
metabolism, and inhibiting the growth and progression of prostate
cancer.
References
|
1
|
Ye L, Kynaston HG and Jiang WG: Bone
metastasis in prostate cancer: Molecular and cellular mechanisms.
Int J Mol Med. 20:103–111. 2007.PubMed/NCBI
|
|
2
|
Aihara M, Lebovita RM, Wheeler TM, Kinner
BM, Ohori M and Scardino PT: Prostate specific antigen and gleason
grade: An immunohistochemical study of prostate cancer. J Urol.
151:1558–1564. 1994.PubMed/NCBI
|
|
3
|
Melchior SW and Brawer MK: Role of
transrectal ultrasound and prostate biopsy. J Clin Ultrasound.
24:463–471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torti SV and Torti FM: Ironing out cancer.
Cancer Res. 71:1511–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nemeth E and Ganz T: Regulation of iron
metabolism by hepcidin. Annu Rev Nutr. 26:323–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nemeth E, Rivera S, Gabayan V, Keller C,
Taudorf S, Pedersen BK and Ganz T: IL-6 mediates hypoferremia of
inflammation by inducing the synthesis of iron regulatory hormone
hepcidin. J Clin Invest. 113:1271–1276. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pogribny IP: Ferroportin and hepcidin: a
new hope in diagnosis, prognosis and therapy for breast cancer.
Breast Cancer Res. 12:3142010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinnix ZK, Miller LD, Wang W, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43–56. 2010. View Article : Google Scholar
|
|
9
|
Gleason DF: Histologic grading of
prostatic carcinoma. Pathology of the prostate (Contemporary Issues
in Surgical Pathology). Bostwick MD and David G: Churchill
Livingstone; New York: pp. 83–87. 1990
|
|
10
|
Cairo G, Bernuzzi F and Recalcati S: A
precious metal: Iron, an essential nutrient for all cells. Genes
Nutr. 1:25–39. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merlot AM, Kalinowski DS and Richardson
DR: Novel chelators for cancer treatment: Where are we now?
Antioxid Redox Signal. 18:973–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pinnix ZK, Miller LD, Wang W, D'Agostino R
Jr, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, et al:
Ferroportin and iron regulation in breast cancer progression and
prognosis. Sci Transl Med. 2:43–56. 2010. View Article : Google Scholar
|
|
13
|
Song S, Christova T, Perusini S, Alizadeh
S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, et
al: Wnt inhibitor screen reveals iron dependence of β-catenin
signaling in cancers. Cancer Res. 71:7628–6737. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanno T, Rabel A, Alleyne M, Lee YT, Dahut
WL, Gulley JL and Miller JL: Hepcidin, anaemia and prostate cancer.
BJU Int. 107:678–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Domenico I, Ward DM, Langelier C,
Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G and Kaplan J:
The molecular mechanism of hepcidin-mediated ferroportin
down-regulation. Mol Biol Cell. 18:2569–2578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ellervik C, Tybjaerg-Hansen A and
Nordestgaard BG: Risk of cancer by transferrin saturation levels
and haemochromatosis genotype: Population-based study and
meta-analysis. J Intern Med. 271:51–63. 2012. View Article : Google Scholar : PubMed/NCBI
|