Introduction
Colorectal cancer (CRC) is one of the leading causes
of cancer-related mortality worldwide (1). Nearly half of patients newly diagnosed
with colorectal cancer will develop metastases and require systemic
chemotherapy (2). As current
chemotherapeutic drugs for colorectal cancer have clinically
significant toxicities, it is a challenging endeavor to develop
novel chemotherapeutic agents with good efficacy and selectivity
(3).
Piperlongumine (PPLGM) is a bioactive component that
was first isolated from Piper longum L., commonly referred
to as the long pepper (4).
Traditionally, PPLGM has been used for treating gastrointestinal
and respiratory diseases (5).
Notably, PPLGM has been shown to selectively target a wide spectrum
of cancer cells (6–9). PPLGM induces cancer cell death by
triggering different pathways, including apoptosis, necrosis and
autophagy (10–13). The elevation of reactive oxygen
species (ROS), characteristic of oxidative stress, is an important
mechanism by which PPLGM induces cancer-selective cell death
(7,8).
In addition, ROS-independent mechanisms, such as cellular
cross-linking events, may also contribute to the induction of
apoptosis by PPLGM (14). Extensive
research has documented the fact that PPLGM induces apoptosis in
cancer cells by interfering with redox and ROS homeostatic
regulators (8,15). The outstanding efficiency of PPLGM in
inducing cancer cell death and its low toxicity favor the potential
development of this compound as a chemotherapeutic agent against
cancer (8).
The c-Jun NH2-terminal kinases (JNKs) are
protein kinases that can phosphorylate the c-Jun transcription
factor at serine (Ser)63 and −73, resulting in the robust induction
of c-Jun transactivation (16).
Evidence has accumulated demonstrating that JNKs are involved in a
variety of cell activities, including cell apoptosis, which is an
important process for tumor suppression (17,18).
Further studies have revealed that JNKs play an essential role in
cancer cell death induced by redox chemotherapeutic agents
(19,20). In particular, PPLGM-mediated oxidative
stress has been shown to be partially induced by inhibiting
glutathione S-transferase π-1 (GSTP1), which is a direct negative
regulator of JNK, resulting in cancer cell death (8,21). We thus
hypothesize that PPLGM induces cell death in colorectal cancer
cells via activation of the JNK signaling pathway. The present
study investigates the effects of PPLGM on JNK signal transduction
and the role of the JNK signaling pathway on PPLGM-mediated cell
apoptosis in HCT116 cells.
Materials and methods
Reagents
PPLGM and SP600125 (Sigma-Aldrich, St. Louis, MO,
USA) were dissolved in dimethyl sulphoxide (DMSO) to a 50 mM
solution and stored at −20°C. Rabbit monoclonal antibodies (Abs)
against c-Jun and phospho-c-Jun at Ser63 were purchased from
Epitomics (Burlingame, CA, USA). Rabbit monoclonal Abs against
cysteinyl aspartate-specific proteinase-3 (caspase-3) and JNK, and
mouse monoclonal Abs against phospho-JNK at Thr183/Tyr185 were
obtained from Cell Signaling Technology, Inc., (Beverly, MA, USA).
Mouse monoclonal Abs against poly(adenosine diphosphate-ribose)
polymerase (PARP) were obtained from BD Biosciences (San Jose, CA,
USA). Mouse monoclonal Abs against β-actin, and anti-mouse
immunoglobulin G and anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibodies were purchased from
Proteintech Group, Inc. (Chicago, IL, USA).
Cell culture
Human epithelial colorectal adenocarcinoma HCT116
cells were purchased from Culture Collection of Chinese Academy of
Science (Shanghai, China) and cultured in RPMI 1640 medium (Gibco
Life Technologies, Carlsbad, CA, USA) supplemented with 10%
inactivated fetal bovine serum, 100 IU/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C until confluence.
Cell viability assay
An MTS assay (CellTiter 96® AQueous One Solution
Cell Proliferation Assay; Promega Corporation, Madison, WI, USA)
was used to test cell viability. A total of 3×104/ml
cells in 100 µl cell culture medium were incubated with increasing
concentrations of PPLGM for 72 h. Detailed description of the
indicated duration periods and doses is provided in Fig. 1. Control cells received DMSO for a
final concentration that was the identical to the highest
concentration of PPLGM, but less than 0.1% (v/v). At 4 h prior to
culture termination, 20 µl MTS was added to the wells. The
absorbance density was read on a 96-well plate reader (Mithras LB
940 Multimode Microplate Reader; Berthold Technologies GmbH &
Co. KG, Bad Wildbad, Baden-Württemberg, Germany). at wavelength of
490 nm.
Trypan blue dye exclusion assay
The HCT116 cells were seeded into 24-well plates to
20–30% confluency, and then treated with PPLGM in various
concentrations for the indicated duration. Detailed description of
the indicated duration periods and doses is provided in Fig. 2. The cells were then trypsinized and
stained with 0.4% (w/v) trypan blue (Sigma-Aldrich) and the viable
cells were counted using a hematocytometer (Qiujing Biology Company
Limited, Shanghai, China).
Cell cycle analysis by flow
cytometry
Subsequent to being exposed to a fixed dose of PPLGM
for various periods of time, the HCT116 cells were collected and
fixed overnight in 66% cold ethanol at 4°C. A detailed description
of the indicated duration periods and doses is provided in Fig. 3. The cells were then washed twice in
cold phosphate-buffered saline (PBS) and labeled with propidium
iodide (PI; BD Biosciences, Franklin Lakes, NJ, USA). Cell cycle
distribution was determined using a BD FACScanto II flow cytometry
analyzer equipped with BD FACSDiva software, version 6.1.3 (BD
Biosciences, Franklin Lakes, NJ, USA).
Analysis of cell apoptosis by flow
cytometry
Apoptosis was determined by flow cytometry using an
Annexin V-fluoroisothiocyanate (FITC)/PI double-staining kit
(Nanjing KeyGen Biotechnology, Co., Ltd., Nanjing, Jiangsu, China).
The HCT116 cells were incubated with increasing concentration of
drugs for the indicated times. A detailed description of the
indicated duration periods and doses is provided in Fig. 4. Following the drug treatment, the
cells were collected, washed and stained in working solution (500
µl binding buffer with 5 µl Annexin V-FITC and 5 µl PI) for 15 min
in the dark. Apoptotic cells were then determined by flow
cytometry, and the results were analyzed by BD FACSDiva software
version 6.1.3.
Western blotting
The procedures for western blotting were performed
as described previously (22).
Briefly, equal amounts of protein were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to a polyvinylidene difluoride membrane (Millipore, Billerica, MA,
USA). The membranes were then blocked with 5% skimmed-milk, 20 mM
PBS and 0.1% Tween-20 buffer for 1 h at room temperature, and
incubated with rabbit anti-c-Jun (dilution, 1:1,000), rabbit
anti-phospho-c-Jun at Ser63 (dilution, 1:1,000), rabbit
anti-caspase-3 (dilution, 1:1,000), mouse anti-PARP (dilution,
1:1,000), mouse anti-β-actin (dilution, 1:10,000) and rabbit
anti-JNK (dilution, 1:1,000), mouse anti-phospho-JNK at
Thr183/Tyr185 (dilution, 1:1,000) overnight at 4°C. The next day,
following incubation with horseradish peroxidase-conjugated
secondary antibodies (dilution, 1:10,000) for 1 h at room
temperature, the signals were detected using an enhanced
chemiluminescence detection kit (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA).
Treatment with JNK inhibitors
The cells were preincubated with the JNK inhibitor,
SP600125 (10 µM), for 2 h and then treated with PPLGM (10 µM) for
24 h. Cell apoptosis was analyzed by flow cytometry and the protein
levels were measured by western blotting.
Statistical analysis
All experiments were performed at least three times,
and results are expressed as the mean ± standard deviation.
Statistical analysis was performed by one-way analysis of variance
followed by Tukey's test using GraphPad Prism 6.02 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
PPLGM causes concentration- and
time-dependent growth inhibition of HCT116 cells
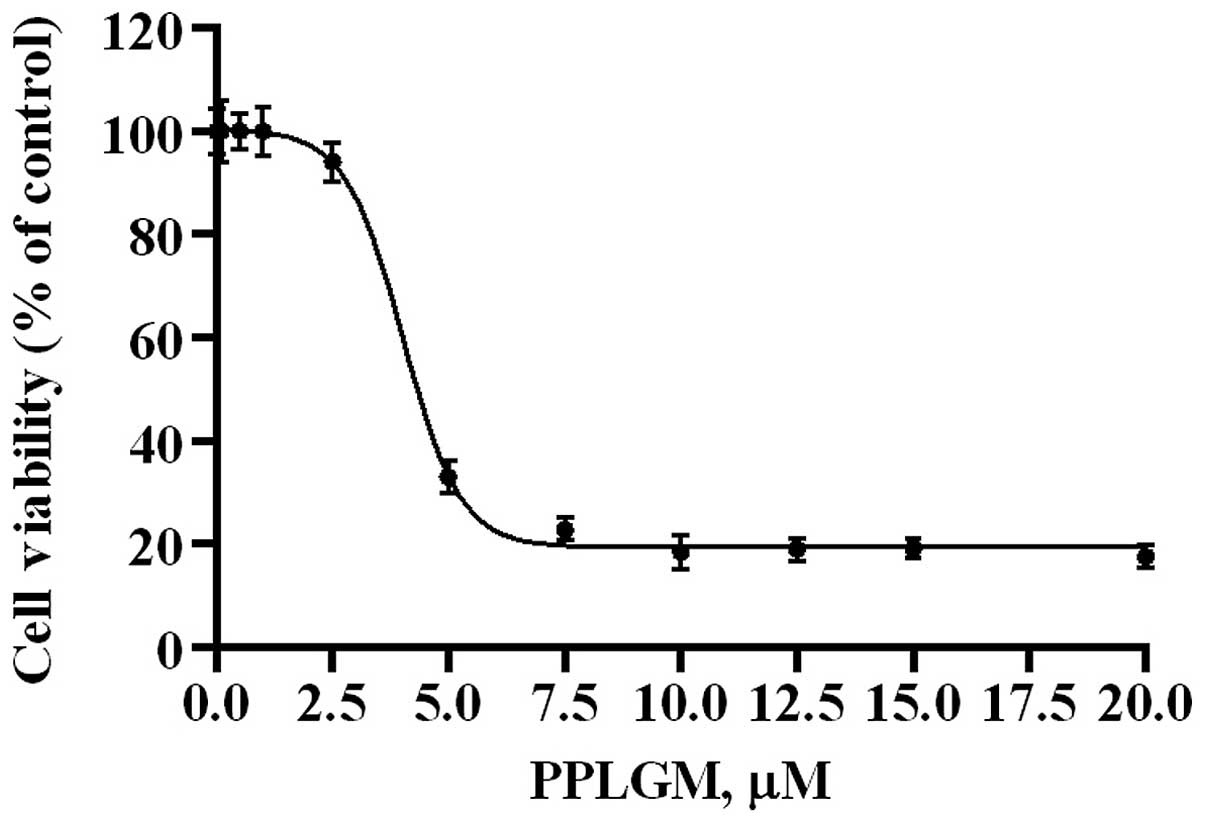
The dose-response effects of PPLGM on HCT116 were
evaluated by MTS assay (Fig. 1). The
half maximal inhibitory concentration value for PPLGM was 4.6 µM in
the HCT116 cells at 72 h. Next, the cytotoxic effects of PPLGM on
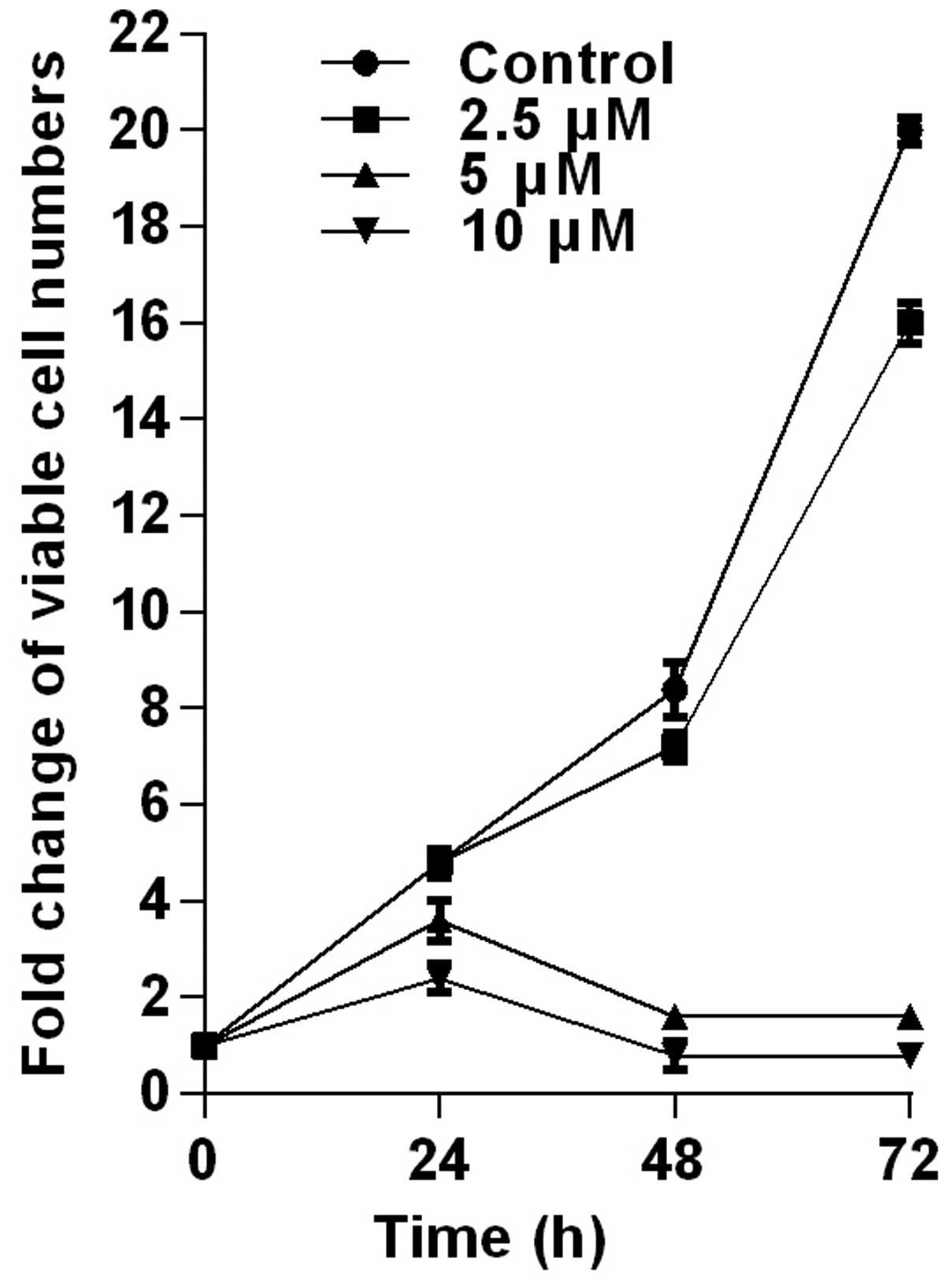
the HCT116 cells were tested by trypan-blue dye exclusion assay
(Fig. 2). PPLGM at different
concentrations inhibited the cell viability in the HCT116 cells,
and the cellular response to the drug increased markedly as the
drug concentration was raised from 2.5 to 10 µM (Fig. 2). The result showed that the
inhibition of cell proliferation by PPLGM in the HCT116 cells was
concentration- and time-dependent. After the cells had been treated
with 5 µM PPLGM for 24 h, the cell viability decreased sharply
compared with the control (Fig. 2).
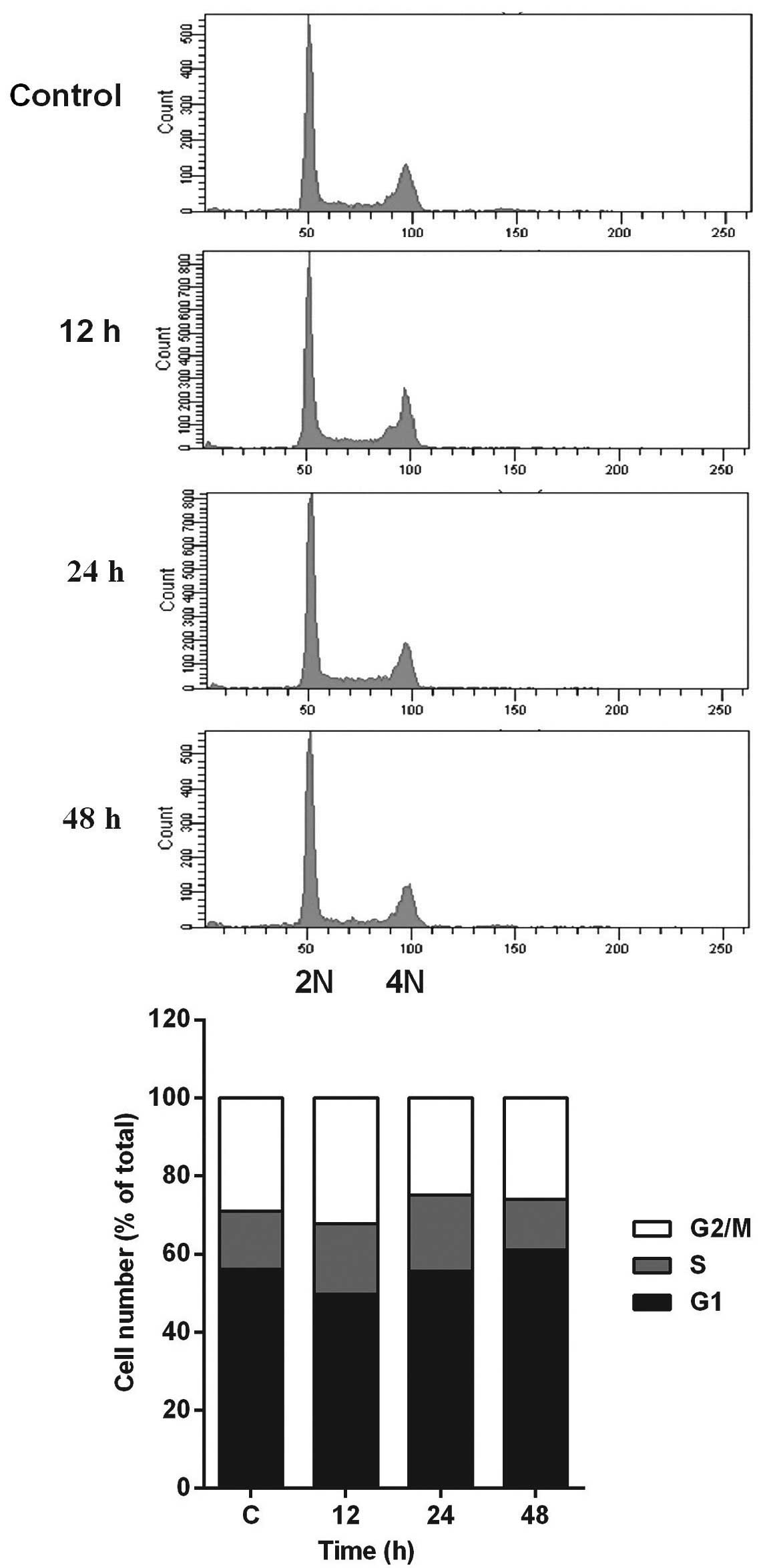
In addition, flow cytometric analysis revealed no significant
alteration in cell-cycle distribution in the HCT116 cells incubated
with 5 µM PPLGM (Fig. 3). Thus, PPLGM
inhibited the growth of HCT116 cells without significantly
affecting the cell cycle, suggesting that the loss of viability in
the HCT116 cells may be attributable to cell death, but not to cell
cycle withdrawal.
PPLGM induces apoptosis in HCT116
cells
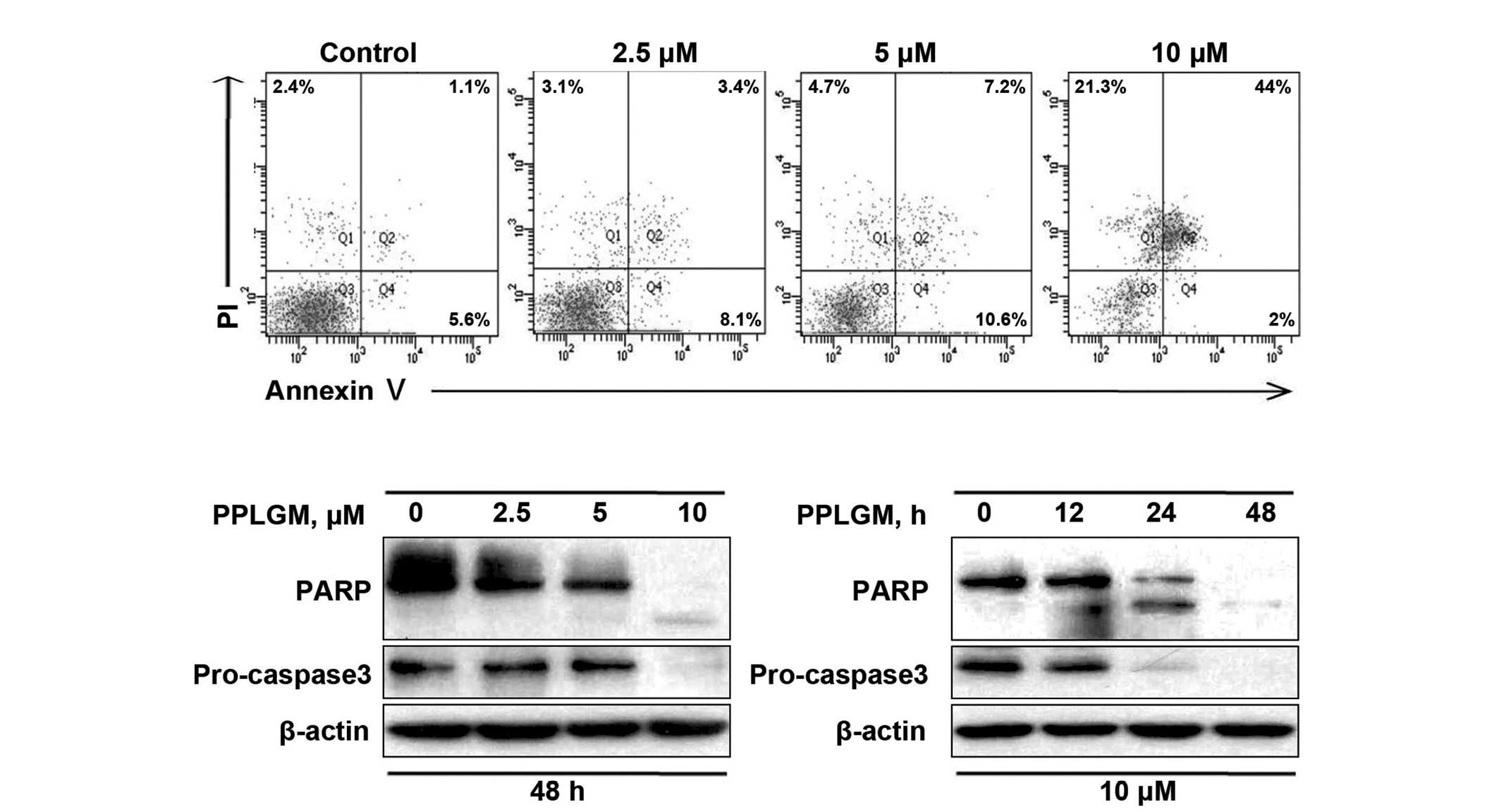
To confirm the capability of PPLGM in inducing
apoptosis, the control and PPLGM-treated HCT116 cells were assessed
by flow cytometry subsequent to staining with Annexin V and PI.
After 48 h of treatment with 10 µM PPLGM, the population of
apoptotic HCT116 cells reached 67.3% (Fig. 4A). These data supported the occurrence
of apoptosis in the HCT116 cells following PPLGM treatment. Next,
to further verify apoptotic induction, cleaved PARP and caspase-3
in the PPLGM-treated HCT116 cells were monitored by western
blotting. As illustrated in Fig. 4B,
PARP cleavage and the reduction of caspase-3 protein levels, known
hallmarks of apoptosis, appeared after 24 h of treatment with 10 µM
PPLGM. Taken together, these results show that PPLGM induces a
substantial level of apoptosis in HCT116 cells.
PPLGM activates the JNK signaling
pathway in HCT116 cells
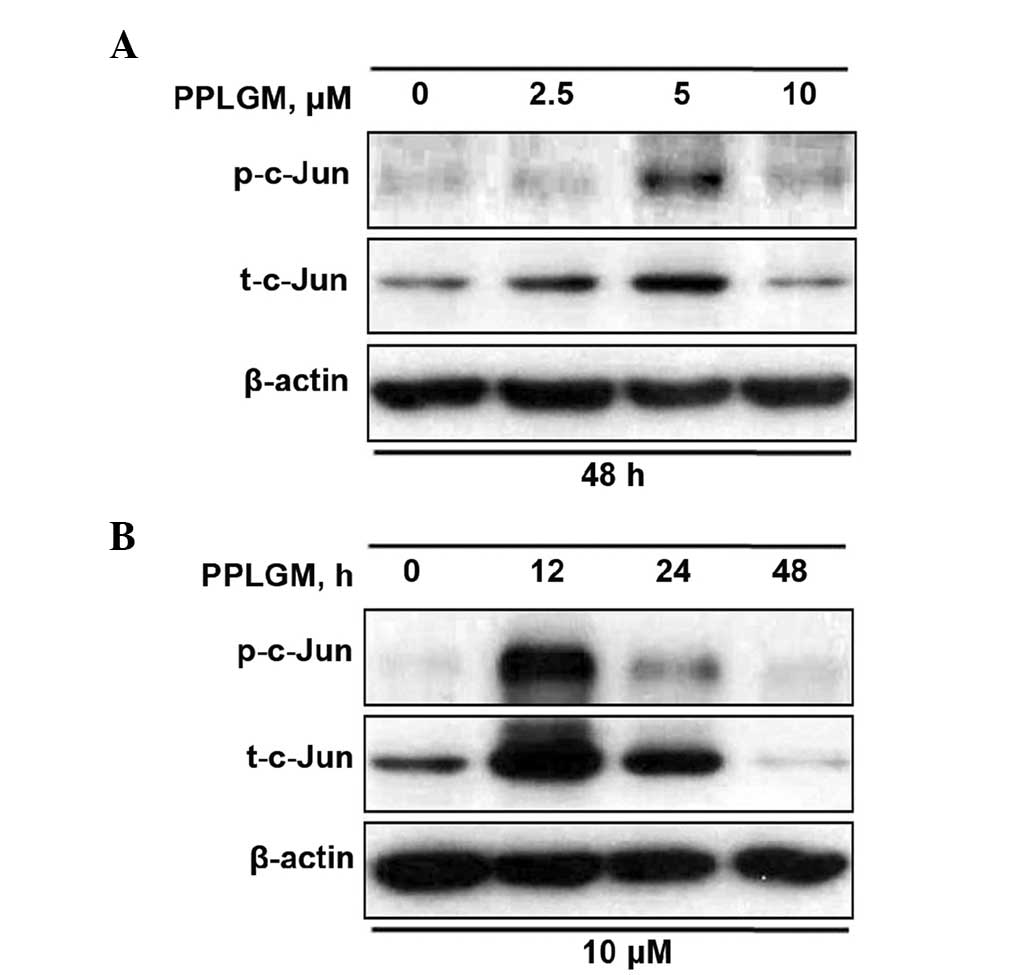
As JNK is the protein kinase that can phosphorylate
c-Jun at Ser63 and −73, c-Jun phosphorylation at site Ser63 was
selected for evaluation of the JNK signaling pathway. When the
cells were incubated with increasing concentrations of PPLGM for 48
h, a moderate increase of c-Jun phosphorylation was detected at 5
µM only (Fig. 5A). According to the
results of previous studies (8,9) and the
current results (Fig. 4), 10 µM PPLGM
induced cancer cell death more significantly than 5 µM PPLGM.
Therefore, the concentration of 10 µM PPLGM was used in subsequent
experiments. Treatment with 10 µM PPLGM for 12 h resulted in a
marked increase in c-Jun phosphorylation in the HCT116 cells when
the cell lysates were immunoblotted with the appropriate
phospho-specific antibodies (Fig.
5B). The 10-µM PPLGM treatment resulted in sustained c-Jun
phosphorylation, which was still apparent after 24 h in the HCT116
cells (Fig. 5B). In addition, the
c-Jun expression level was elevated in parallel with increased
c-Jun phosphorylation in the PPLGM-treated HCT116 cells (Fig. 5). The 10-µM PPLGM treatment induced
c-Jun phosphorylation prior to PARP cleavage and loss of cell
viability, suggesting that the JNK signal pathway may mediate
PPLGM-induced apoptosis.
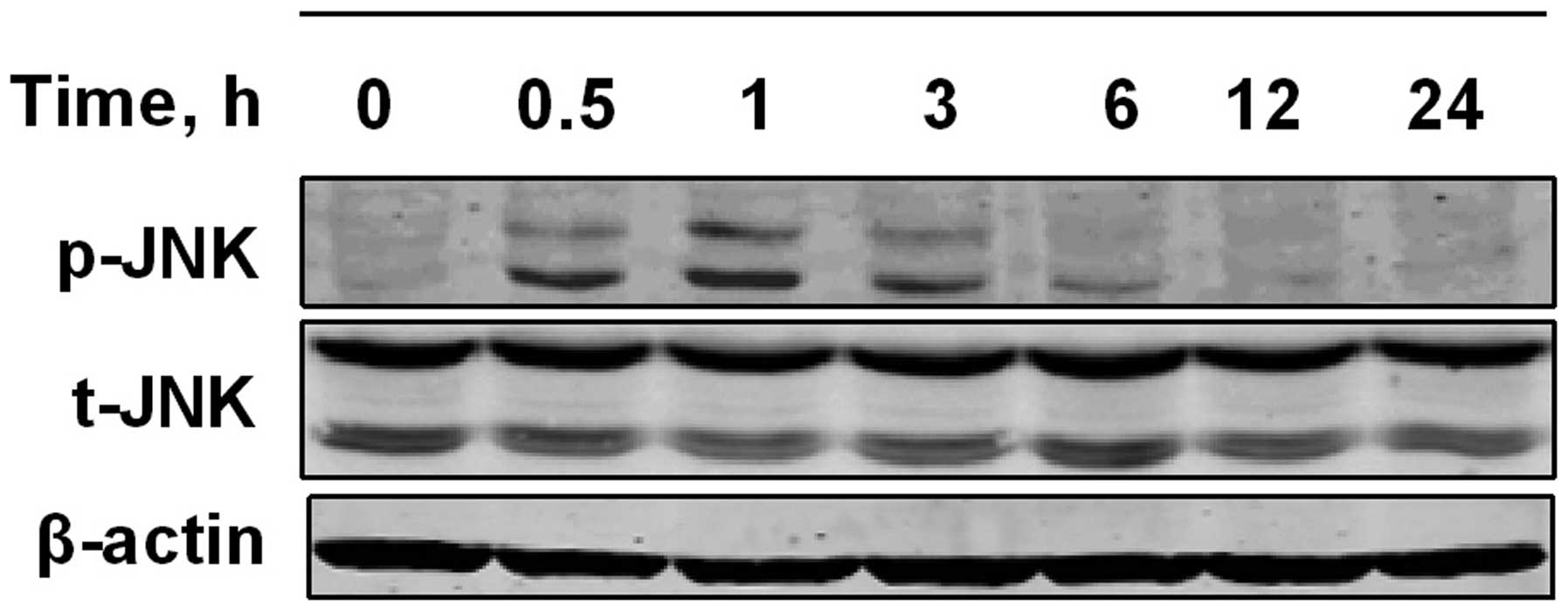
To further examine whether the JNK signaling pathway
is activated by PPLGM in HCT116 cells, the cells were exposed to 10
µM PPLGM for various periods of time, and analyzed for
PPLGM-induced changes in JNK phosphorylation by western blot
analysis using dual phospho-specific JNK antibodies. As shown in
Fig. 6, JNK phosphorylation levels
increased in the HCT116 cells within 1 h of PPLGM incubation and
then decreased again from 3 h onwards.
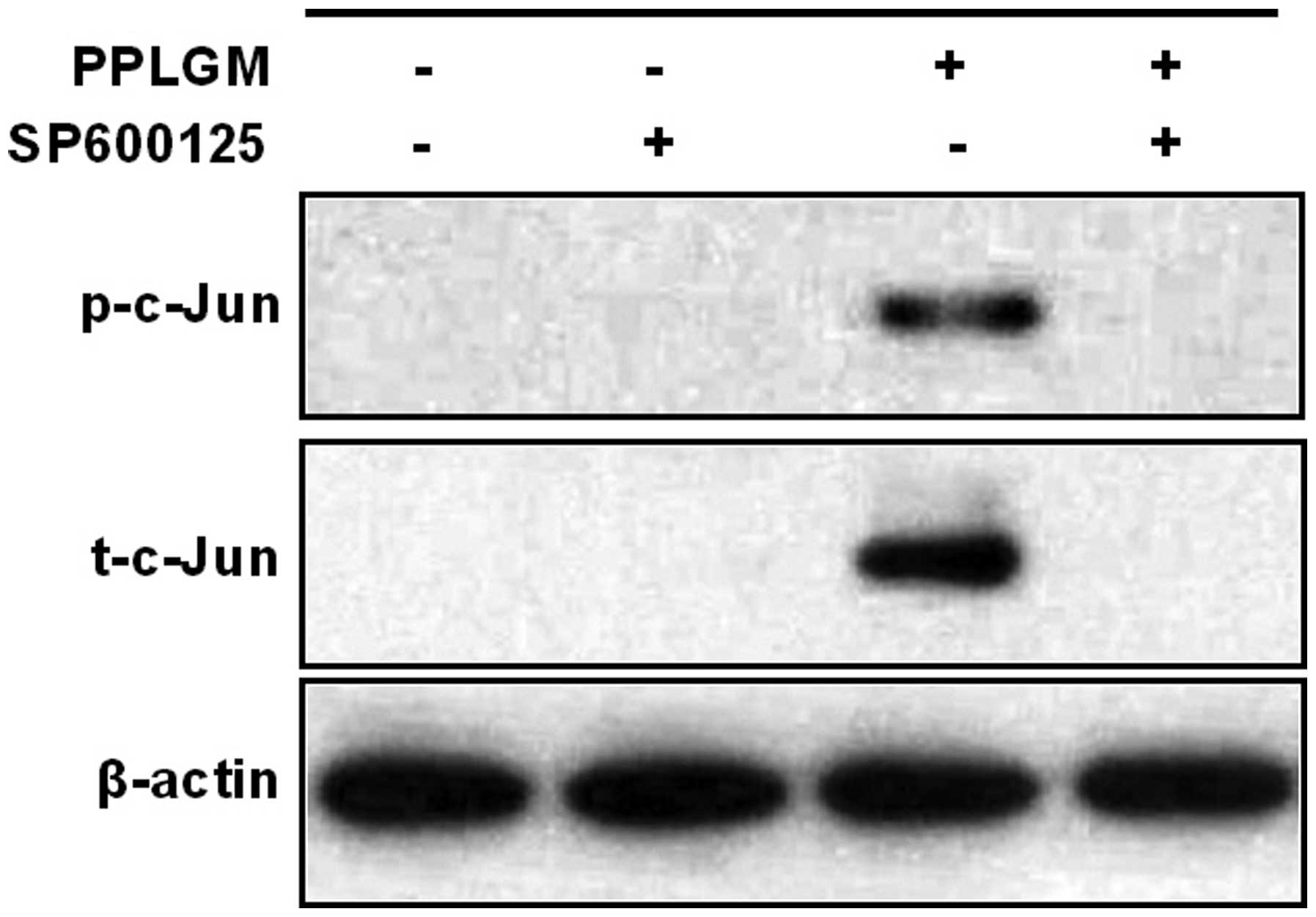
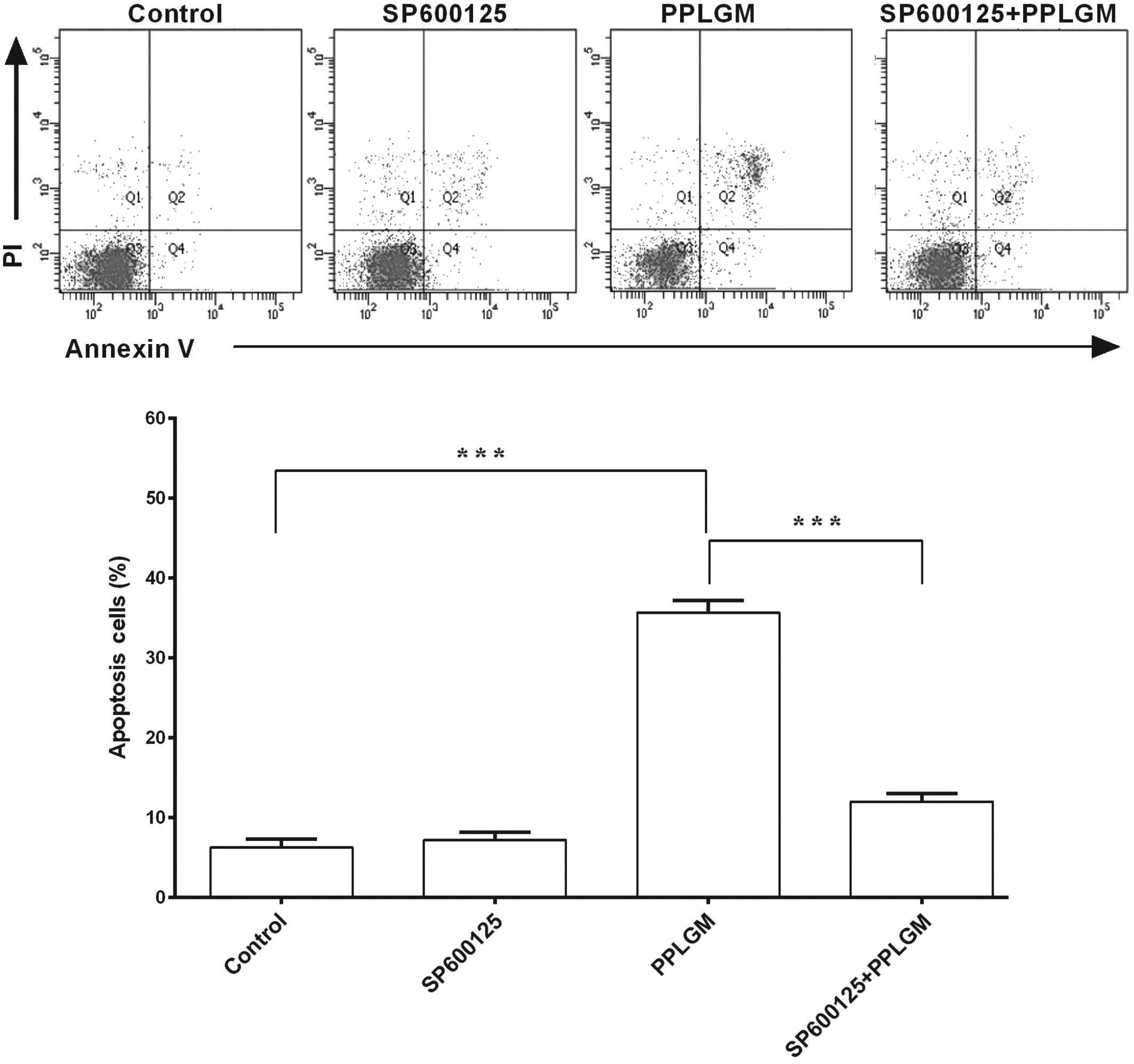
SP600125 inhibits PPLGM-mediated
apoptosis through restraining JNK signal pathway activation in
HCT116 cells
To identify whether the JNK signaling pathway was
involved in PPLGM-mediated apoptosis, SP600125, a general inhibitor
of JNK, was co-incubated with PPLGM in the HCT116 cells and cell
apoptosis was then determined using flow cytometry. Pre-treatment
with the SP600125 JNK inhibitor completely blocked the
PPLGM-induced phosphorylation of c-Jun (Fig. 7) and significantly inhibited
PPLGM-induced cell apoptosis in the HCT116 cells (Fig. 8). The percentage of apoptotic cells
following treatment with 20 µM SP600125, 10 µM PPLGM, or 20 µM
SP600125 plus 10 µM PPLGM were 7.2, 35.7 and 12.0%, respectively.
The difference between PPLGM alone and PPLGM plus SP600125 was
significant (P<0.0001).
Discussion
Recent data has showed that PPLGM can selectively
kill various cancer cells, including human colorectal cancer cells
(8,9).
To the best of our knowledge, the present study is the first to
indicate that the JNK signaling pathway is involved in
PPLGM-induced cell apoptosis in human colorectal cancer cells. The
results showed that PPLGM reduced cell viability independently of
cell cycle withdrawal and induced cell death in a time- and
concentration-dependent manner. At the same time, JNK signaling was
activated during PPLGM treatment in the HCT116 cells. In addition,
SP600125 inhibited PPLGM-induced JNK signaling and apoptosis in the
HCT116 cells, suggesting that PPLGM-mediated apoptosis was at least
partially dependent on the activation of the JNK signal pathway in
the HCT116 cells (23).
Accumulated data have shown that PPLGM induces cell
death through different pathways in multiple types of cancer cells
(7–12). Previous studies have demonstrated that
caspase-3-mediated PARP cleavage and cell cycle arrest at the
G2/M phase are involved in PPLGM-induced cell apoptosis
in human prostate cancer PC-3 cells (11). In the present study, HCT116 cells
treated with PPLGM demonstrated likewise up-regulation of
PARP/procaspase-3 cleavage (Fig. 4B),
but not cell cycle arrest (Fig. 3);
this discrepancy in the effect of PPLGM on cell cycle distribution
may be a result of the different responses to PPLGM in different
cell lines.
c-Jun, a cognate substrate for JNK, is a labile
protein that is degraded by JNK under non-stressed conditions
(24). However, a variety of cellular
stresses, such as oxidative stress, can strongly activate the JNKs,
which inhibit c-Jun ubiquitination and promote c-Jun transcription
through c-Jun phosphorylation (24,25).
Inhibition of the ubiquitin-proteasome system induced by PPLGM in
cancer cells may also reduce the ubiquitin-dependent degradation of
c-Jun (26). Collectively, a steady
elevation of c-Jun expression is associated with the expression and
stabilization of c-Jun. Hence, in the present study, it followed
that sustained c-Jun phosphorylation was concomitant with the c-Jun
overexpression observed during PPLGM treatment (Fig. 5).
The positive association between c-Jun activation
and cell apoptosis has been well documented in neurons, endothelial
and myeloma cells, fibroblasts and colorectal cancer cells
(3,27–30).
Similarly, the activation of JNK and the subsequent phosphorylation
of c-Jun have been linked with apoptotic cell death induced by
PPLGM in HCT116 cells (Figs. 7 and
8). Conversely, other studies have
shown that the inhibition of JNK by SP600125 sensitizes tumor cells
to CD95-induced apoptosis in HCT116 cells and that this effect is
cell line-specific (31). Whether
c-Jun activation leads to the inhibition or promotion of apoptosis
should be dependent on the stimuli and the cell type.
Recent data have indicated that the
mitogen-activated protein kinase (MAPK) core pathways are involved
in PPLGM-induced cancer cell death. Notably, p38 MAPK activation
leads to cell death through autophagy in human osteosarcoma U2OS
cells (12). The present results
showed that JNK signaling is involved in PPLGM-induced apoptosis in
HCT116 cells (Figs. 7 and 8). As reported earlier in the colorectal
cancer HT-29 cell line (9), the
extracellular signal-regulated kinase (ERK) signaling pathway is
also activated in PPLGM-treated HCT116 cells (data not shown),
strongly arguing that a decrease in ERK and an increase in JNK are
required for the induction of apoptosis (17). A previous study in hamster fibroblast
CC139 cells demonstrated that phosphoinositide 3′-kinase (PI3K)
inhibition is necessary for JNK-mediated cell death (32). In addition, PPLGM causes PI3K
inhibition to induce caspase-dependent apoptosis in human
triple-negative breast cancer cells (13). Altogether, the mechanisms of signal
transduction are complicated during the course of PPLGM-induced
cancer cell death and the cross-talk between different signaling
pathways should be further elucidated.
In summary, the present results suggested that in
the HCT116 cells, PPLGM reduced cell viability and triggered cell
apoptosis through the JNK signal pathway in a concentration- and
time-dependent manner. A clear understanding of the molecular
mechanisms of PPLGM-mediated cell apoptosis may shed light on the
further clinical development of PPLGM for chemotherapy.
Acknowledgements
The authors would like to thank Dr Xinhui Fu, Dr
Zihuan Yang, Dr Jun Hu and Ms. Zhiting Chen for providing technical
assistance. This study was supported by Sun Yat-sen University ‘100
Talents Program’, the Guangdong Innovative Research Team Program
(grant no. 2009010058), the Science and Information Technology
Bureau of Guangzhou, Guangdong (grant no. 2011J5200009), the
Guangdong Provincial Department of Science and Technology (grant
no. 2012B050500004), Overseas Excellent Professor Project, and the
Japanese Ministry of Education, Culture, Sports, Science and
Technology, Program of Japanese Initiative for Global Research
Network on Infectious Diseases.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Köhne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cance. N Engl J Med. 360:1408–1417. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ham J, Babij C, Whitfield J, et al: A
c-Jun dominant negative mutant protects sympathetic neurons against
programmed cell death. Neuron. 14:927–939. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chatterjee A and Dutta CP: Alkaloids of
Piper longum Linn. I. Structure and synthesis of piperlongumine and
piperlonguminine. Tetrahedron. 23:1769–1781. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bezerra DP, Pessoa C, de Moraes MO,
Saker-Neto N, Silveira ER and Costa-Lotufo LV: Overview of the
therapeutic potential of piplartine (piperlongumine). Eur J Pharm
Sci. 48:453–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han SS, Son DJ, Yun H, Kamberos NL and
Janz S: Piperlongumine inhibits proliferation and survival of
Burkitt lymphoma in vitro. Leuk Res. 37:146–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu JM, Pan F, Li L, et al: Piperlongumine
selectively kills glioblastoma multiforme cells via reactive oxygen
species accumulation dependent JNK and p38 activation. Biochem
Biophys Res Commun. 437:87–93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Raj L, Ide T, Gurkar AU, et al: Selective
killing of cancer cells by a small molecule targeting the stress
response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Randhawa H, Kibble K, Zeng H, Moyer MP and
Reindl KM: Activation of ERK signaling and induction of colon
cancer cell death by piperlongumine. Toxicol In Vitro.
27:1626–1633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bezerra DP, Militão GC, de Castro FO, et
al: Piplartine induces inhibition of leukemia cell proliferation
triggering both apoptosis and necrosis pathways. Toxicol In Vitro.
21:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong EH, Kim YJ, Cho HJ, et al: Piplartine
induces caspase-mediated apoptosis in PC-3 human prostate cancer
cells. Oncol Rep. 20:785–792. 2008.PubMed/NCBI
|
|
12
|
Wang Y, Wang JW, Xiao X, et al:
Piperlongumine induces autophagy by targeting p38 signaling. Cell
Death Dis. 4:e8242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shrivastava S, Kulkarni P, Thummuri D, et
al: Piperlongumine, an alkaloid causes inhibition of PI3K/Akt/mTOR
signaling axis to induce caspase-dependent apoptosis in human
triple-negative breast cancer cells. A Internat J Program Cell
Death. 19:1148–1164. 2014. View Article : Google Scholar
|
|
14
|
Adams DJ, Dai M, Pellegrino G, et al:
Synthesis, cellular evaluation, and mechanism of action of
piperlongumine analogs. Proc Natl Acad Sci USA. 109:15115–15120.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TH, Song J, Kim SH, et al:
Piperlongumine treatment inactivates peroxiredoxin 4, exacerbates
endoplasmic reticulum stress, and preferentially kills high-grade
glioma cells. Neuro Oncol. 16:1354–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
a 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu R, Kim BR, Chen C, Hebbar V and Kong
AN: The roles of JNK and apoptotic signaling pathways in
PEITC-mediated responses in human HT-29 colon adenocarcinoma cells.
Carcinogenesis. 24:1361–1367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu C, Shen G, Yuan X, et al: ERK and JNK
signaling pathways are involved in the regulation of activator
protein 1 and cell death elicited by three isothiocyanates in human
prostate cancer PC-3 cells. Carcinogenesis. 27:437–445. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang T, Arifoglu P, Ronai Z and Tew KD:
Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal
kinase (JNK1) signaling through interaction with the C terminus. J
Biol Chem. 276:20999–21003. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song M, Chen D, Lu B, et al: PTEN loss
increases PD-L1 protein expression and affects the correlation
between PD-L1 expression and clinical parameters in colorectal
cancer. PLoS One. 8:e658212013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bennett BL, Sasaki DT, Murray BW, et al:
SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase.
Proc Natl Acad Sci USA. 98:13681–13686. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fuchs SY, Dolan L, Davis RJ and Ronai Z:
Phosphorylation-dependent targeting of c-Jun ubiquitination by Jun
N-kinase. Oncogene. 13:1531–1535. 1996.PubMed/NCBI
|
|
25
|
Musti AM, Treier M and Bohmann D: Reduced
ubiquitin-dependent degradation of c-Jun after phosphorylation by
MAP kinases. Science. 275:400–402. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jarvius M, Fryknäs M, D'Arcy P, Sun C,
Rickardson L, Gullbo J, Haglund C, Nygren P, Linder S and Larsson
R: Piperlongumine induces inhibition of the ubiquitin-proteasome
system in cancer cells. Biochem Biophys Res Commun. 431:117–123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang N, Verna L, Hardy S, et al: c-Jun
triggers apoptosis in human vascular endothelial cells. Circ Res.
85:387–393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Podar K, Raab MS, Tonon G, et al:
Up-regulation of c-Jun inhibits proliferation and induces apoptosis
via caspase-triggered c-Abl cleavage in human multiple myeloma.
Cancer Res. 67:1680–1688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bossy-Wetzel E: Bakiri L and Yaniv M:
Induction of apoptosis by the transcription factor c-Jun. EMBO J.
16:1695–1709. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Teraishi F, Wu S, Zhang L, et al:
Identification of a novel synthetic thiazolidin compound capable of
inducing c-Jun NH2-terminal kinase-dependent apoptosis in human
colon cancer cells. Cancer Res. 65:6380–6387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuntzen C, Sonuc N, De Toni EN, et al:
Inhibition of c-Jun-N-terminal-kinase sensitizes tumor cells to
CD95-induced apoptosis and induces G2/M cell cycle arrest. Cancer
Res. 65:6780–6788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Molton SA, Todd DE and Cook SJ: Selective
activation of the c-Jun N-terminal kinase (JNK) pathway fails to
elicit Bax activation or apoptosis unless the phosphoinositide
3-kinase (PI3K) pathway is inhibited. Oncogene. 22:4690–4701. 2003.
View Article : Google Scholar : PubMed/NCBI
|