Introduction
Prostate cancer is the most commonly diagnosed type
of carcinoma in men in western industrialized countries (1). Despite initially responding to
androgen-deprivation therapy, prostate cancer typically becomes
resistant and evolves into recurrent androgen-independent cancer
following approximately one year of treatment (2). The mechanism for how this resistance
develops is unclear and the development of novel therapeutic
strategies for the treatment of androgen-independent prostate
cancer are required.
Transient receptor potential melastatin 8 (TRPM8) is
a type of Ca2+ permeable cation channel that is
potentially associated with tumorigenesis and tumor progression
(3). Yang et al (4) transfected TRPM8 into
androgen-independent PC-3 prostate cancer cells, and determined
that overexpression of TRPM8 inhibits the proliferation and
malignant progression of PC-3 cells. A study conducted by Zhang and
Barritt (3) revealed that TRPM8 has a
vital role in Ca2+ homeostasis in prostate epithelial
cells, in addition to being required for cell survival. Therefore,
TRPM8 may have an effect on the growth and malignant progression of
prostate cancer.
Alternative splice variants contribute to biological
complexity and diversity by coding for functional or nonfunctional
protein isoforms. TRPM8 isoforms generated by alternative mRNA
splicing are expressed in different tissues, such as human lung
tissue (5,6) and certain types of prostate cancer
(7). The functions of various TRPM8
isoforms have previously been described in a number of studies
(8,9).
For example, short TRPM8α (sM8α) and short TRPM8β (sM8β) code for
N-terminal fragments of the full-length TRPM8 channel, and regulate
TRPM8 activity by stabilizing the closed state of the channel,
thus, reducing its activity and cold sensitivity (8). Furthermore, inhibition of TRPM8 activity
by sM8β, heat or chemical blockers revealed common mechanisms for
regulating the single-channel kinetics (9). However, the majority of previous studies
reported the functions of short TRPM8 isoforms in human embryonic
kidney (HEK) 293 cells. Therefore, research regarding the function
of short TRPM8 isoforms in prostate cancer cells is required to
elucidate their role in the progression of prostate cancer.
The aim of the present study was to detect the
expression of sM8α in various prostate cancer cell lines; to
investigate the role of sM8α expression on prostate cancer LNCaP
cell line proliferation, apoptosis, migration and invasion; and to
examine the involvement of the mitogen activated protein kinase
(MAPK) signaling pathway.
Materials and methods
Cell culture
Human prostate carcinoma LNCaP, DU145 and PC-3 cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and cultured in RPMI 1640 medium (Gibco Life Technologies,
Grand Island, NY, USA) containing 10% fetal bovine serum (FBS;
Gibco Life Technologies), 100 µg/ml streptomycin sulfate and 100
U/ml penicillin G sodium (G4003; Guge Biotech, Wuhan, China). Cells
were maintained in a humidified incubator with 5% CO2 at
a temperature of 37°C.
Reverse transcription-polymerase chain
reaction (RT-PCR) for sM8α
Total RNA was extracted from prostate carcinoma
LNCaP, DU145 and PC3 cells using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). A total of 2 µg RNA was reverse
transcribed (Beijing TransGen Biotech Co., Ltd., Beijing, China)
into complementary (c)DNA at 42°C using oligo(dT) primers and
murine leukemia virus reverse transcriptase (TransScript
First-Strand cDNA Synthesis SuperMix; Beijing TransGen Biotech Co.,
Ltd.), followed by PCR using the 2xTransTaq High Fidelity(HiFi) PCR
SuperMix II(−dye) (Beijing TransGen Biotech Co., Ltd). The PCR
primers were as follows: Forward,
5′-ATACTCGAGATGGAAGGCACCCAGATCAACCAAAGTGAGAAATGGAACT-3′ and
reverse, 5′-ATAGAATTCCTAATGATGATGATGATGATGGCAGACCTCCTCCTGTCCCA-3′
for sM8α; and forward, 5′-ACGGATTTGGTCGTATTGGG-3′ and reverse,
5′-CGCTCCTGGAAGATGGTGAT-3′ for glyceraldehyde phosphate
dehydrogenase (GAPDH). For PCR, 2 µl cDNA template of the three
prostate cancer cell lines were respectively added to a PCR mixture
(Beijing TransGen Biotech Co., Ltd.) and then ddH2O was
added up to a final volume of 20 µl. The DNA amplification
conditions were as follows: 95°C for 10 min; 35 cycles of 95°C for
30 sec, 60°C for 30 sec and 72°C for 1 min; followed by 72°C for 10
min. The length of the sM8α PCR product was 534 bp. Furthermore,
the PCR product contained two restriction enzyme sites [XhoI
(Takara Biotechnology Co., Ltd., Dalian, China) in the 5′ extremity
and EcoRI (Takara Biotechnology Co., Ltd.) in the 3′
extremity] and a His tag in the 3′ extremity. GAPDH was used as the
housekeeping gene, with the following amplification conditions:
95°C for 10 min; 28 cycles of 95°C for 30 sec, 56°C for 30 sec and
72°C for 30 sec; followed by 72°C for 10 min. The length of the
GAPDH PCR product was 214 bp. All PCR products were analyzed by gel
electrophoresis and DNA sequencing.
Plasmid construction
A pcDNA3.1(−) eukaryotic expression clone vector
(Invitrogen Life Technologies) was used in the present study. The
PCR product contained the gene of interest, a His tag and
XhoI and EcoRI restriction enzyme sites. The plasmid
and PCR product were digested by XhoI and EcoRI prior
to isolation by DNA gel extraction (Axygen, Hangzhou, China). Then,
the digested plasmid and PCR product sequences were linked using T4
DNA ligase (Thermo Fisher Scientific Inc., Beijing, China), and
transformed into DH5α cells (Biovector NTCC, Inc., Beijing, China)
for synthesis of the pcDNA3.1(−)-sM8α-His plasmid. The plasmid was
sequenced and found to contain no mutations.
Cell transfection for stable cell
clone
LNCaP cells were plated into a six-well plate at a
density of 105 cells/well, and transfected at ~90–95%
confluence with the recombinant pcDNA3.1(−)-sM8α-His and
pcDNA3.1(−) negative control (NC) plasmids using 10 µl
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer's instructions. Stably
transfected clones were selected using G418 (Sigma-Aldrich, St
Louis, MO, USA) at a concentration of 700 µg/ml. Colonies were
identified using RT-PCR and western blot analysis. In the present
study, LNCaP-sM8α cells refer to LNCaP cells transfected with and
overexpressing His-tagged sM8α, and LNCaP-NC cells refer to LNCaP
cells transfected with empty vector.
Cell proliferation
Cell proliferation was detected by performing a
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
assay. Cells were seeded in 96-well plates at a density of
1×103 cells per well and were incubated for 1–5 days.
Subsequently, 20 µl MTT (concentration, 5 mg/ml) was added to each
well and the plate was incubated for 4 h. The liquid was removed
from each well and replaced with 150 µl DMSO. The optical density
(OD) value of each well was measured at a wavelength of 570 nm
using an MD2 Microplate Reader (Molecular Devices, Sunnyvale, CA,
USA).
Quantum dots (QDs)-based
immunofluorescent imaging
LNCaP-sM8α and LNCap-NC cells were plated on 12-mm
coverslips and incubated overnight, prior to being fixed with 1%
neutral formaldehyde at room temperature for 10 min. To prepare
samples for QD-based immunofluorescent imaging, cells were first
blocked with 2% w/v bovine serum albumin (BSA) for 30 min at 37°C
and then incubated with the primary anti-His-tag antibody for 4 h
at 37°C. The cells were incubated with secondary antibody (QDs-605)
for 2 h at 37°C following an a second blocking step with 2% BSA for
30 min. Cells were subsequently incubated with DAPI (5 µg/ml) for 3
min at room temperature to stain the nuclei. The samples were
washed with Tris-buffered saline and examined under an Olympus BX51
fluorescence microscope equipped with an Olympus DP72 camera
(Olympus Corporation, Tokyo, Japan). The QDs-605 and DAPI were
excited by ultraviolet light (wavelength, 388 nm) (10).
Flow cytometry
LNCaP-NC and LNCaP-sM8α cells were prepared for cell
cycle and apoptosis assays (kit from Kaiji Biotechnology Co., Ltd.,
Nanjing, China), according to the manufacturer's instructions.
Briefly, the cells were fixed with 70% ethanol overnight at a
temperature of 4°C, washed with phosphate-buffered saline and
stained with propidium iodide. Subsequently, the cell cycle stage
was determined and analyzed by performing flow cytometry. To
evaluate apoptosis, cells were seeded in complete culture medium
for 24 h and then incubated in culture medium containing 1% fetal
bovine serum (FBS) for an additional 48 h. The percentage of
apoptotic cells were determined using flow cytometry, according to
the manufacturer's instructions (Kaiji Biotechnology Co., Ltd.).
Details of the flow cytometry experiments have been previously
described (4).
Cell migration and invasion
assays
To assay cell migration, 2×104 cells
suspended in 200 µl RPMI-1640 medium without FBS were seeded onto
the fibronectin-coated polycarbonate membrane of a Transwell®
insert [Becton Dickinson Medical Devices (Shanghai) Co., Ltd.,
Shanghai, China]. A volume of 600 µl RPMI-1640 with 10% FBS was
added as a chemoattractant in the lower chamber. Following
incubation for 12 h at 37°C in a 5% CO2 atmosphere, the
Transwell insert was washed with PBS and the cells on the top
surface of the insert were removed with a cotton swab. Cells
adhering to the lower surface were fixed with methanol for 10 min,
stained with 0.1% Giemsa solution for 10 min, thrice-washed with
PBS and finally air-dried. Five predetermined fields
(magnification, x200) were counted using a fluorescence microscope
(BX51; Olympus Corporation, Tokyo, Japan). All assays were
independently repeated in triplicate. The cell invasion assay
procedure was similar to that for cell migration, except the
Transwell membranes were pre-coated with 50 µg/µl Matrigel® (BD
Biosciences, Franklin Lakes, NJ, USA) and the cells were incubated
for 48 h at 37°C in a 5% CO2 atmosphere. Cells adhering
to the lower surface were counted in the same manner as for the
cell migration assay.
Western blot analysis
Protein expression levels of the sM8α-His fusion
protein, TRPM8, matrix metalloproteinase (MMP)-2, MMP-9, MAPK
signaling pathway proteins [p38, c-Jun N-terminal kinase (JNK) and
extracellular signal-regulated kinase 1/2 (ERK1/2)] and GAPDH were
assayed using western blot analysis. Equal quantities of protein
(30 µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and then transferred to
electrochemiluminescence nitrocellulose membranes (GE Healthcare
Life Sciences, Piscataway, NJ, USA). Primary antibodies against
human TRPM8 (rabbit polyclonal; catalog no. ACC-049; Alomone Labs,
Jerusalem, Israel; 1:500 dilution), MMP-2 (rabbit monoclonal IgG;
catalog no. 13132; Cell Signaling Technology, Inc., Danvers, MA,
USA; 1:1,000 dilution), MMP-9 (rabbit monoclonal IgG; catalog no.
13667, Cell Signaling Technology, Inc.; 1:1,000 dilution), GAPDH
(rabbit polyclonal IgG; catalog no. sc-25778, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; 1:1000 dilution), MAPK family
[rabbit anti-p38, ERK1/2 (p44/42) and JNK (catalog no. 9926), and
phospho (p-)p38, p-ERK1/2 and p-JNK (catalog no. 9910); Cell
Signaling Technology, Inc.] and His-tag (mouse monoclonal IgG2a;
catalog no. D291-3, Medical & Biological Laboratories Co.,
Ltd., Nagoya, Japan) were applied overnight at 4°C. Polyclonal goat
anti-rabbit (catalog no. sc-2005; 1:5,000 dilution) and goat
anti-mouse (catalog no. sc-2004; 1:5,000 dilution) IgG horseradish
peroxidase-conjugated (Santa Cruz Biotechnology, Inc.) secondary
antibodies were then applied for 2 h at 37°C. Protein bands were
visualized using an ECL Western Blotting kit (Guge Biotech, Wuhan,
China). The results of western blot were analyzed by Image-Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) and the
integrated optical density values were normalized to GAPDH, which
was the loading control. The procedure was performed as previously
described (4) and each experiment was
repeated three times with similar results.
Statistical analysis
SPSS software for Windows (version 13.0; SPSS, Inc.,
Chicago, IL, USA) was used to perform all statistical analyses. All
data are presented as the mean ± standard error of the mean.
Statistical analyses were performed using the unpaired t-test, with
P<0.05 considered to indicate a statistically significant
difference.
Results
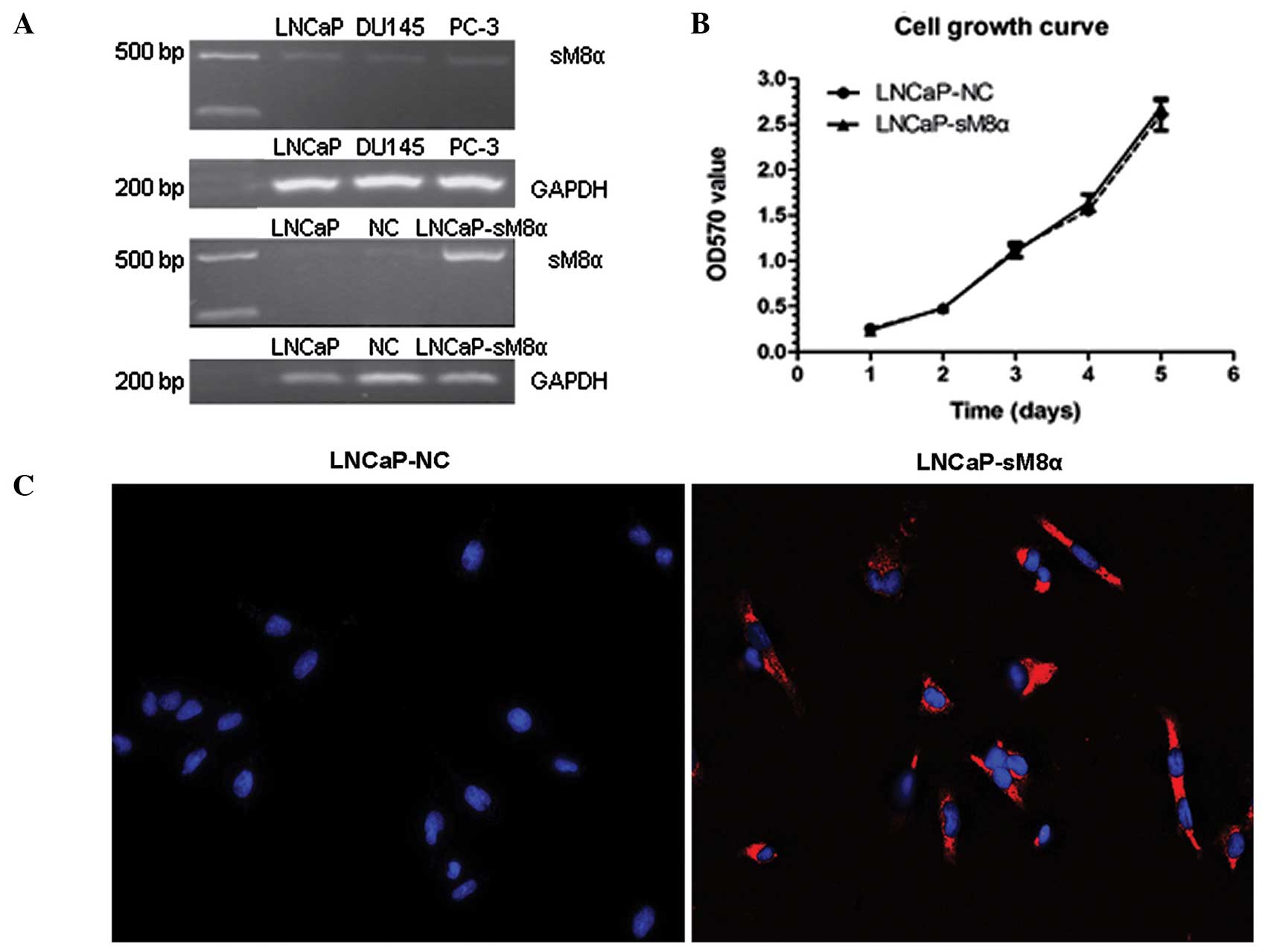
Expression of sM8α in three prostate
cancer cell lines and its effect on cell proliferation
sM8α mRNA expression levels were investigated in
three prostate cancer cell lines (LNCaP, DU145 and PC-3) using
RT-PCR, revealing that the expression level of sM8α was low in all
three lines. Following stable transfection of sM8α into LNCaP
cells, sM8α mRNA expression levels were detected in LNCaP, LNCaP-NC
and LNCaP-sM8α cells. As expected, expression of sM8α in the
LNCaP-sM8α cells was high compared with the LNCaP-NC cells
(Fig. 1A). Cell growth curves for the
LNCaP-sM8α and LNCaP-NC cells were generated using OD data obtained
on days 1–5. No significant difference in cell proliferation was
identified between the LNCaP-sM8α and LNCaP-NC cells (P>0.05;
Fig. 1B).
Subcellular location of the sM8α-His
fusion protein in stably transfected LNCaP cells
QD-based immunofluorescent imaging revealed clearly
observable red immunofluorescence in the majority of LNCaP-sM8α
cells examined, indicating expression of the sM8α-His fusion
protein. Furthermore the sM8α-His fusion protein was visualized in
cytoplasm. By contrast, immunostaining did not detect a red
immunofluorescence signal for the sM8α-His fusion protein in
LNCaP-NC cells; instead, only DAPI-stained blue fluorescent nuclei
were observed (Fig. 1C).
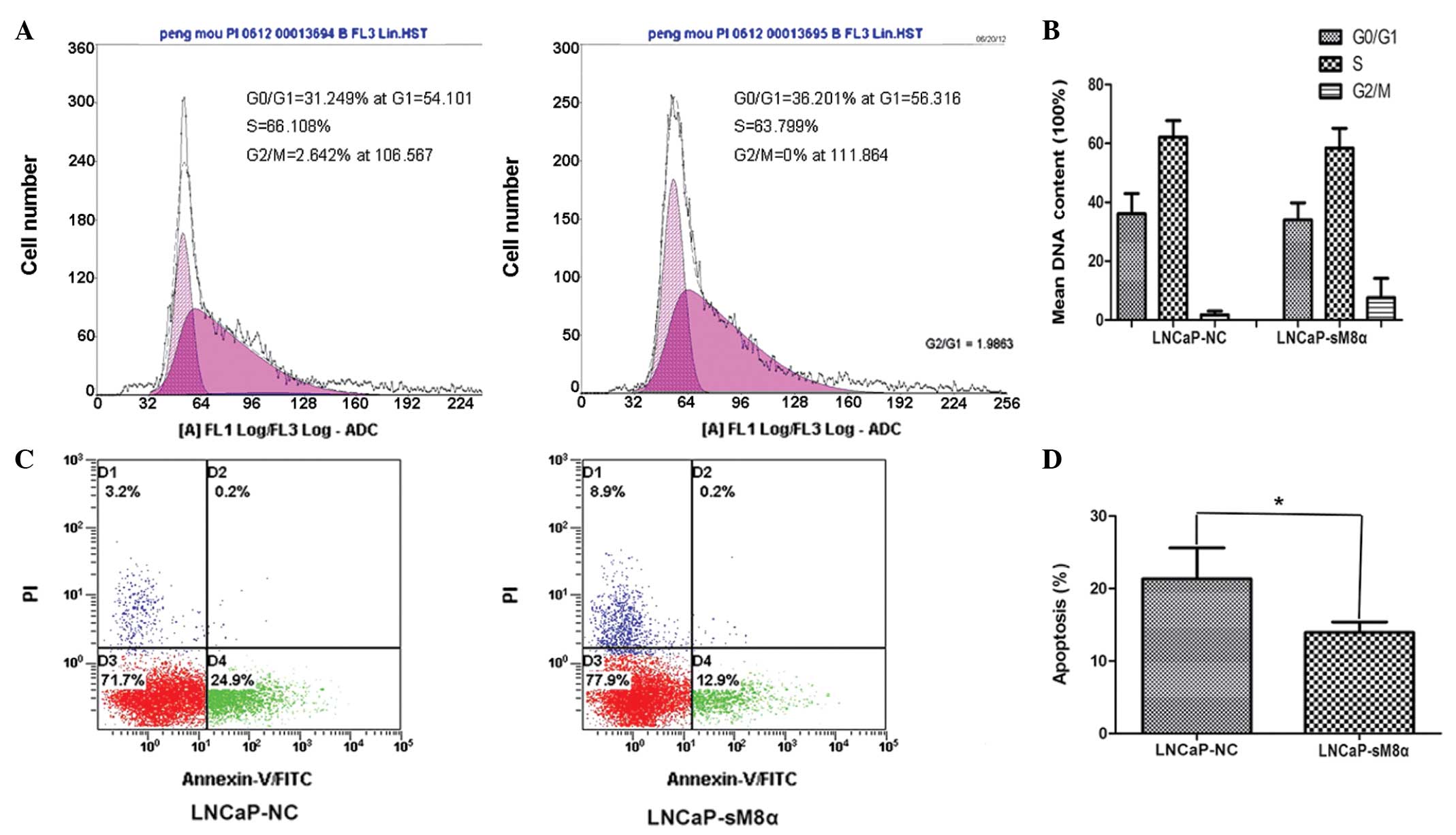
Flow cytometry analysis of cell cycle
distribution and apoptosis
The results demonstrated that there were no
significant differences in the cell cycle between LNCaP-NC and
LNCaP-sM8α cells (Fig. 2A and B). The
effect of the sM8α-His fusion protein on the apoptosis of
transfected LNCaP cells was also investigated. Following incubation
in RPMI 1640 with 1% FBS for 48 h, sM8α-His fusion protein was
identified to exhibit a significant antiapoptotic effect on LNCaP
cells. The percentage of apoptotic LNCaP-sM8α cells was
significantly lower than the proportion of apoptotic LNCaP-NC cells
(13.93±0.84 vs. 21.33±2.47%; P<0.05; Fig. 2C and D).
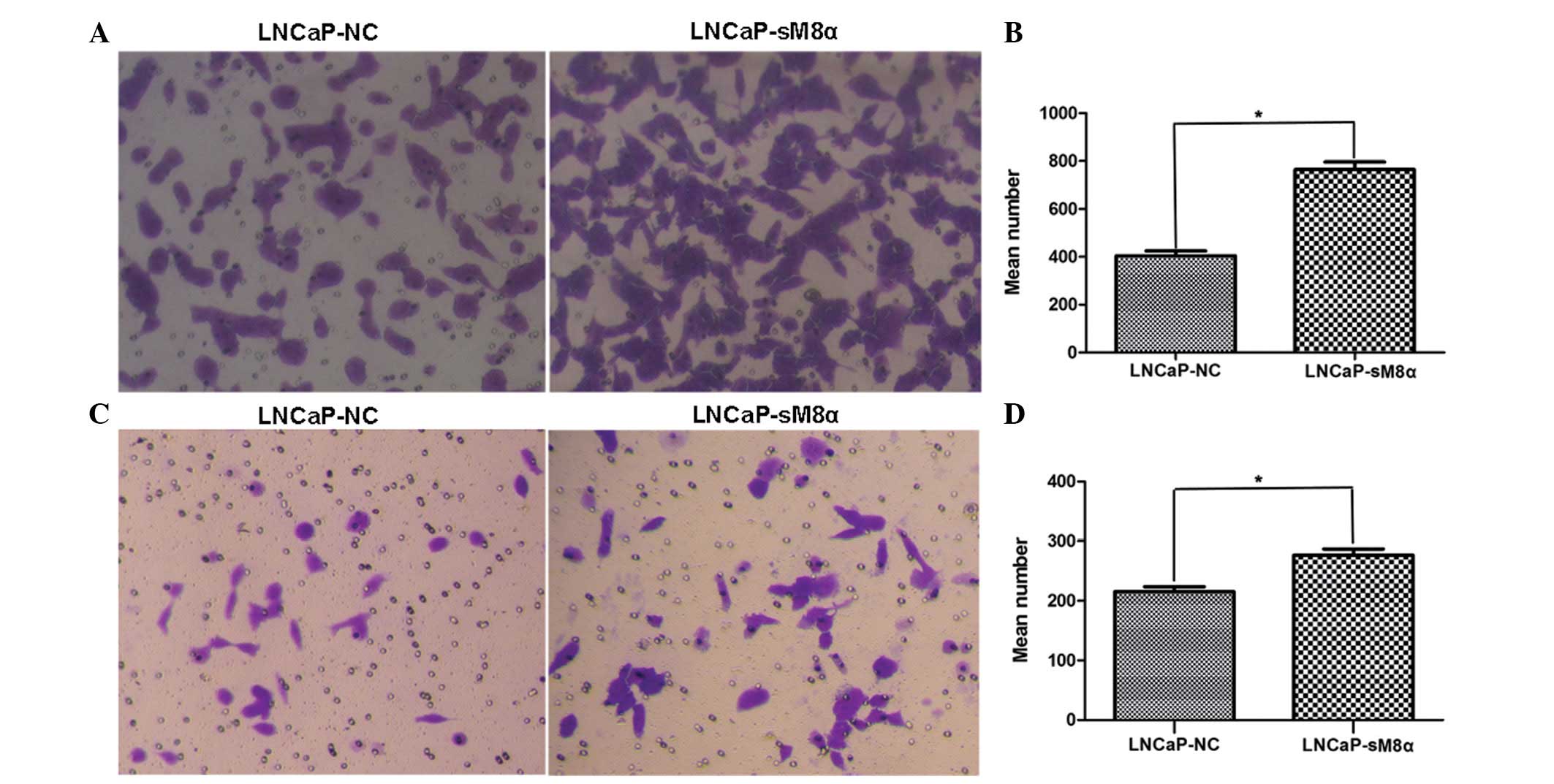
Enhanced cell migration and invasion
in LNCaP-sM8α cells
High rates of cell migration and invasiveness are
characteristics of cancer cells, indicating and contributing to
malignancy (11). Therefore, cell
migration and invasion are common targets of anticancer treatment
strategies. Cell counts of the lower surfaces of the Transwell
membranes revealed that the migration of LNCaP-sM8α cells was
significantly increased following 12 h of incubation when compared
with LNCaP-NC cells (P<0.05; Fig. 3A
and B). Subsequent analysis of the cell count data revealed a
significant increase in the invasiveness of LNCaP-sM8α cells
compared with LNCaP-NC cells (P<0.05; Fig. 3C and D). Furthermore, western blotting
(Fig. 4) of MMP-2 and MMP-9 indicated
that overexpression of sM8α may increase the migration and invasion
in LNCaP cells via significantly increasing the proportion of
active, 64-kDa MMP-2 (Fig. 4A, G and
H).
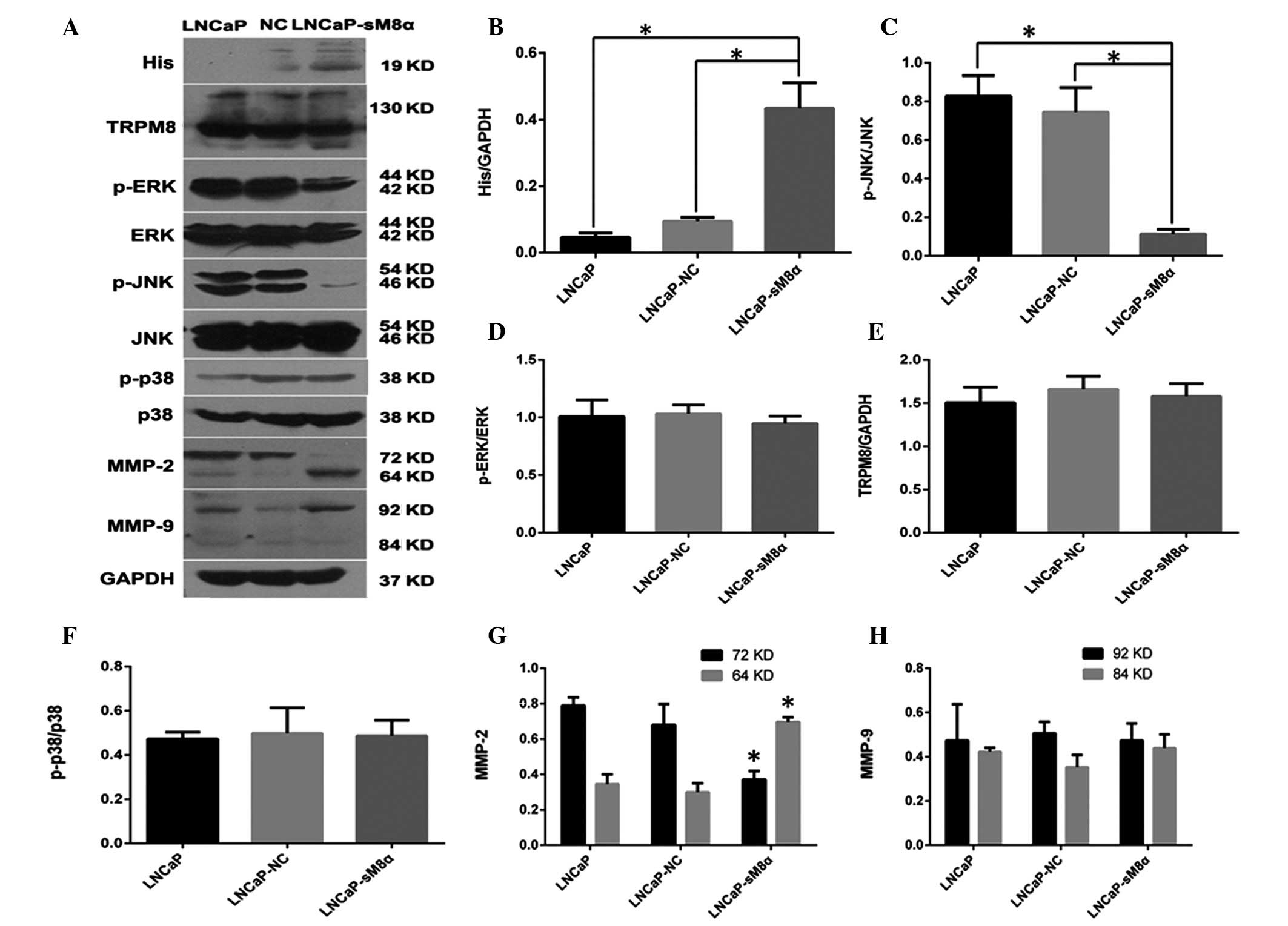 | Figure 4.(A) Western blot analysis indicating
that overexpression sM8α-His fusion protein reduced the expression
of p-JNK and increased the expression of active MMP-2 (64 kDa) in
LNCaP cells. GAPDH was used as the inner control. (B) sM8α-His
fusion protein was significantly overexpressed in LNCaP-sM8α cells.
(C) The expression of p-JNK protein was significantly reduced in
LNCaP-sM8α cells compared with LNCaP and LNCaP-NC cells. However,
there were no significant changes in the expression of (D) p-ERK1/2
and (F) p-p38 or (E) full-length TRPM8 protein. (G) MMP-2 was
activated, as indicated by upregulation of 64-kDa MMP-2 and
downregulation of 72-kDa MMP-2 in LNCaP-sM8α cells. (H) There were
no remarkable differences in the expression of MMP-9 in LNCaP,
LNCaP-NC and LNCaP-sM8α cells. *P<0.05. NC, negative control;
sM8α, short transient receptor potential melastatin 8α; TRPM8,
transient receptor potential melastatin 8; p-ERK,
phosphorylated-extracellular signal-regulated kinase 1/2; p-JNK,
phosphorylated-c-Jun N-terminal kinase; MMP, matrix
metalloproteinase; GAPDH, glyceraldehyde phosphate
dehydrogenase. |
p-JNK/MAPK signaling pathway may be
important in antiapoptosis
The MAPK signaling pathway is an important signaling
pathway, and is involved in the regulation of cell proliferation
and apoptosis (12). Therefore,
western blotting was performed to detect possible changes in the
expression levels of proteins in this signaling pathway. The
expression of p-JNK was significantly reduced compared in
LNCaP-sM8α cells with the control (P<0.05; Fig. 4A and C), indicating its possible
involvement in the antiapoptotic mechanism of mS8α. By contrast,
p38 and ERK1/2 expression exhibited no significant change in
expression between LNCaP-sM8α cells and control cells (P<0.05;
Fig. 4A, D and F). Changes in the
expression level of full-length TRPM8, which is known to be
regulated by sM8α and sM8β in terms of cold sensitivity and channel
activity in HEK293 cells (9), were
not observed in the LNCaP-sM8α and LNCaP-NC cells (P>0.05;
Fig. 4A and E).
Discussion
Alternative splice variants are the product of mRNA
post-transcriptional processing and have an important effect on the
biological behavior of cancer cells. Thus far, the splice variants
of numerous ion channels, including TRP channels, have been
described (13,14). The role of these TRP-channel splice
variants in biological behavior is becoming increasingly clear;
however, at present, there is little information regarding the
functions of TRPM8 isoforms in the field of carcinogenesis and
cancer progression. Therefore, the role of sM8α, which encodes the
N-terminal fragment of TRPM8, was investigated in prostate cancer
cells in the present study.
At least three short TRPM8 isoforms, each exhibiting
their own pathophysiological functions, have been reported in
previous studies. Sabnis et al (6) reported that the full-length TRPM8
transcript was absent in human lung epithelial cells and identified
a novel truncated TRPM8 variant that was selectively expressed as a
modulator of respiratory physiology in cold air. Furthermore,
Bidaux et al (8) reported the
following two novel short splice variants of TRPM8, which were
cloned from prostate cancer cells, using a model of HEK293 cells:
sM8α and sM8β. The results demonstrated that the two variants were
in a closed configuration with the C-terminal tail of the
full-length TRPM8 channel, resulting in stabilization of its closed
state, and reducing its cold sensitivity and activity.
Additionally, Fernández et al (9) identified that, in addition to increased
temperature, or treatment with BCTC or clotrimazole, short sM8-6
isoforms of TRPM8 inhibited the channel. The present study
investigated sM8α by generating an expression vector containing
only the sM8α coding sequence and a His-tag, encoding a protein of
19 kDa. This was different to the sM8α splice variant reported by
Bidaux et al (8), which
encoded two protein isoforms of 6 and 18 kDa. This discrepancy may
result from the existence of a regulatory sequence in the sM8α
plasmid allowing for further splicing. Subsequent QDs-based
immunofluorescent imaging revealed the sM8α-His fusion protein
located in the cytoplasm of the LNCaP cells.
Apoptosis is a fundamental cellular process
regulated by precise gene expression (15). In the present study, LNCaP cell
apoptosis was induced by starvation and it was identified that
overexpression of sM8α could significantly reduce the percentage of
apoptotic LNCaP cells. MMP-2 and MMP-9 are key proteins in cancer
progression, and are involved in the initial breakdown of collagen
and basement membrane components during tumor growth and invasion
(16). The present study used
Transwell chambers to simulate the basement membrane for migration
and invasion, and the results indicated that overexpression of
sM8α, through activation of MMP-2, may increase the migration and
invasion of LNCaP cells.
The activity of TRPM8 is regulated by a number of
cellular signaling pathways, most notably by phosphoinositides and
the activation of phospholipase C (17). However, the cellular signaling
pathways regulated by sM8α in prostate cancer cells are unclear.
The three major MAPKs (p38, JNK, and ERK1/2) are signal transducers
involved in a broad range of prostate cancer cell functions,
including survival, apoptosis and cell differentiation (18). The present study demonstrated a role
for the MAPK signaling pathway in the regulation of sM8α. Although
the ERK1/2 and p38 signaling pathways did not appear to be
regulated by sM8α, significantly reduced activation of p-JNK was
identified in LNCaP-sM8α cells. We hypothesize that this may be
associated with the reduction of LNCaP-sM8α cell apoptosis.
The physiological roles of short TRPM8 isoforms
require further investigation. For example, the functions of short
TRPM8 isoforms are unclear in prostate cancer cells not expressing
full-length TRPM8 and it remains to be elucidated whether short
TRPM8 isoforms can influence the release of Ca2+ from
the endoplasmic reticulum. Furthermore, additional evidence is
required to determine if short TRPM8 isoforms negatively regulate
TRPM8 in non-cancerous prostate cells (including normal prostate
and benign hyperplasia of the prostate tissue), as well as in
cancerous prostate cells.
In conclusion, the present study demonstrated that
LNCaP cells express low levels of sM8α. The results indicate that
overexpression of sM8α has no detectable affect on the
proliferation of LNCaP cells; however, sM8α overexpression did
appear to increase cell migration and invasion by activation of
MMP-2. Furthermore, the antiapoptotic effect of sM8α may be
regulated by activation of p-JNK in LNCaP cells. Additional studies
of short TRPM8 isoforms should be performed to gain a greater
understanding of the functions of the TRPM8 channel.
Acknowledgements
The present study was supported by the Program of
Chinese National Natural Science Fund (grant no. 81172734).
References
|
1
|
Spies E, Reichardt W, Alvarez G, et al: An
artificial PAP gene breaks self-tolerance and promotes tumor
regression in the TRAMP model for prostate carcinoma. Mol Ther.
20:555–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L and Barritt GJ: Evidence that
TRPM8 is an androgen-dependent Ca2+ channel required for
the survival of prostate cancer cells. Cancer Res. 64:8365–8373.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang ZH, Wang XH, Wang HP and Hu LQ:
Effects of TRPM8 on the proliferation and motility of prostate
cancer PC-3 cells. Asian J Androl. 11:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sabnis AS, Reilly CA, Veranth JM and Yost
GS: Increased transcription of cytokine genes in human lung
epithelial cells through activation of a TRPM8 variant by cold
temperatures. Am J Physiol Lung Cell Mol Physiol. 295:L194–L200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabnis AS, Shadid M, Yost GS and Reilly
CA: Human lung epithelial cells express a functional cold-sensing
TRPM8 variant. Am J Respir Cell Mol Biol. 39:466–474. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bidaux G, Flourakis M, Thebault S, et al:
Prostate cell differentiation status determines transient receptor
potential melastatin member 8 channel subcellular localization and
function. J Clin Invest. 117:1647–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bidaux G, Beck B, Zholos A, et al:
Regulation of activity of transient receptor potential melastatin 8
(TRPM8) channel by its short isoforms. J Biol Chem. 287:2948–2962.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernández JA, Skryma R, Bidaux G, et al:
Short isoforms of the cold receptor TRPM8 inhibit channel gating by
mimicking heat action rather than chemical inhibitors. J Biol Chem.
287:2963–2970. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XL, Peng CW, Chen C, Yang XQ, Hu MB,
Xia HS, Liu SP, Pang DW, Li Y, et al: Quantum dots-based
double-color imaging of HER2 positive breast cancer invasion.
Biochem Biophys Res Commun. 409:577–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim EK and Choi EJ: Compromised MAPK
signaling in human diseases: An update. Arch Toxicol. Feb
18–2015.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Frühwald J, Camacho Londoño J, Dembla S,
et al: Alternative splicing of a protein domain indispensable for
function of transient receptor potential melastatin 3 (TRPM3) ion
channels. J Biol Chem. 287:36663–36672. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu X, Tong Q, Wozney J, et al:
Identification of an N-terminal TRPC2 splice variant which inhibits
calcium influx. Cell Calcium. 37:173–182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular Mechanisms of Apoptosis and Roles in Cancer
Development and Treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015.PubMed/NCBI
|
|
16
|
Schütz A, Schneidenbach D, Aust G,
Tannapfel A, Steinert M and Wittekind C: Differential expression
and activity status of MMP-1, MMP-2 and MMP-9 in tumor and stromal
cells of squamous cell carcinomas of the lung. Tumour Biol.
23:179–184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yudin Y and Rohacs T: Regulation of TRPM8
channel activity. Mol Cell Endocrinol. 353:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodríguez-Berriguete G, Fraile B,
Martínez-Onsurbe P, Olmedilla G, Paniagua R and Royuela M: MAP
Kinases and Prostate Cancer. J Signal Transduct.
2012:1691702012.PubMed/NCBI
|