Introduction
Sleep-disordered breathing (SDB) describes a group
of disorders that are characterized by breathing difficulties while
sleeping. Examples of SDB include primary snoring, upper airway
resistance syndrome and obstructive sleep apnea (OSA). SDB is
characterized by repeated narrowing of the upper airway during
sleep, which leads to partial or complete obstruction of the
airways (1,2). The symptoms of SDB are dependent on the
time of day; symptoms experienced at night include choking, pauses
in breathing and nocturia, whereas day time symptoms include
fatigue, extreme tiredness and a depressed mood (3). A number of predisposing factors have
been identified, such as age (>40 years), obesity, recent weight
gain, male gender, alcohol consumption, nasal blockage and
maxillo-mandibular abnormalities (1).
Nasopharyngeal organic lesions, particularly
malignant neoplasms, are a rare cause of SDB in adults. Chordomas
are histologically benign but clinically malignant axial skeleton
neoplasms that represent 0.1–0.2% of all intracranial neoplasms,
6–16% of skull base neoplasms and 1–4% of primary malignant bone
tumors (4,5). Furthermore, approximately one-third of
all chordomas are located within the skull base (6,7). The slow
growth of chordomas and a lack of specific initial symptoms may
prevent the diagnosis of the neoplasm, thus resulting in an
extended period of tumor development. Clinically, with the
exception of nasal patency disorders, chordoma symptoms may include
headaches, diplopia, swallowing disorders, Eustachian tube patency
disorders, tinnitus and secretion retention in the nasal cavity.
The subtle onset and benign macroscopic image of the tumor (similar
to an enlarged adenoid) may result in diagnostic errors.
The current study presents a case of incidentally
diagnosed nasopharyngeal chordoma that manifested as a patency
disorder in the upper airways and was the predominant cause of a
severe form of SDB. Written informed consent was obtained from the
patient.
Case report
The current study presents the case of a 32-year-old
female patient, with a severe form of SDB caused by the presence of
a nasopharyngeal tumor impairing nasal breathing. The patient was
diagnosed with SDB at the Department of Otorhinolaryngology at the
English Dentistry Division of the Medical University of Warsaw
(Warsaw, Poland) during the course of a research program
investigating a group of pathologically obese patients who
qualified for bariatric surgery.
In accordance with the research program's
assumptions, each patient routinely completed an interview, and
underwent a laryngological physical examination, upper airway
endoscopy, anterior rhinomanometry, computed tomography (CT) scan
of the maxillofacial region and a full in-laboratory
polysomnography. The present patient was diagnosed with
third-degree obesity, with a body mass index (BMI) of 41.9
kg/m2 (height, 1.62 meters; body weight, 110 kg). During
the interview, a five-year history of nasal patency disorders was
noted. The laryngological examination revealed a left-sided
deviation of the nasal septum to a limited degree and moderate
hypertrophy of the tonsils. The isthmus of the pharynx was not
significantly narrowed, however, an upper airway endoscopy revealed
a soft-tissue mass with a smooth surface located within the
nasopharynx, which caused almost complete impaired patency.
Significant nasopharyngeal obstruction prevented an objective
assessment of nasal ventilation with the use of anterior
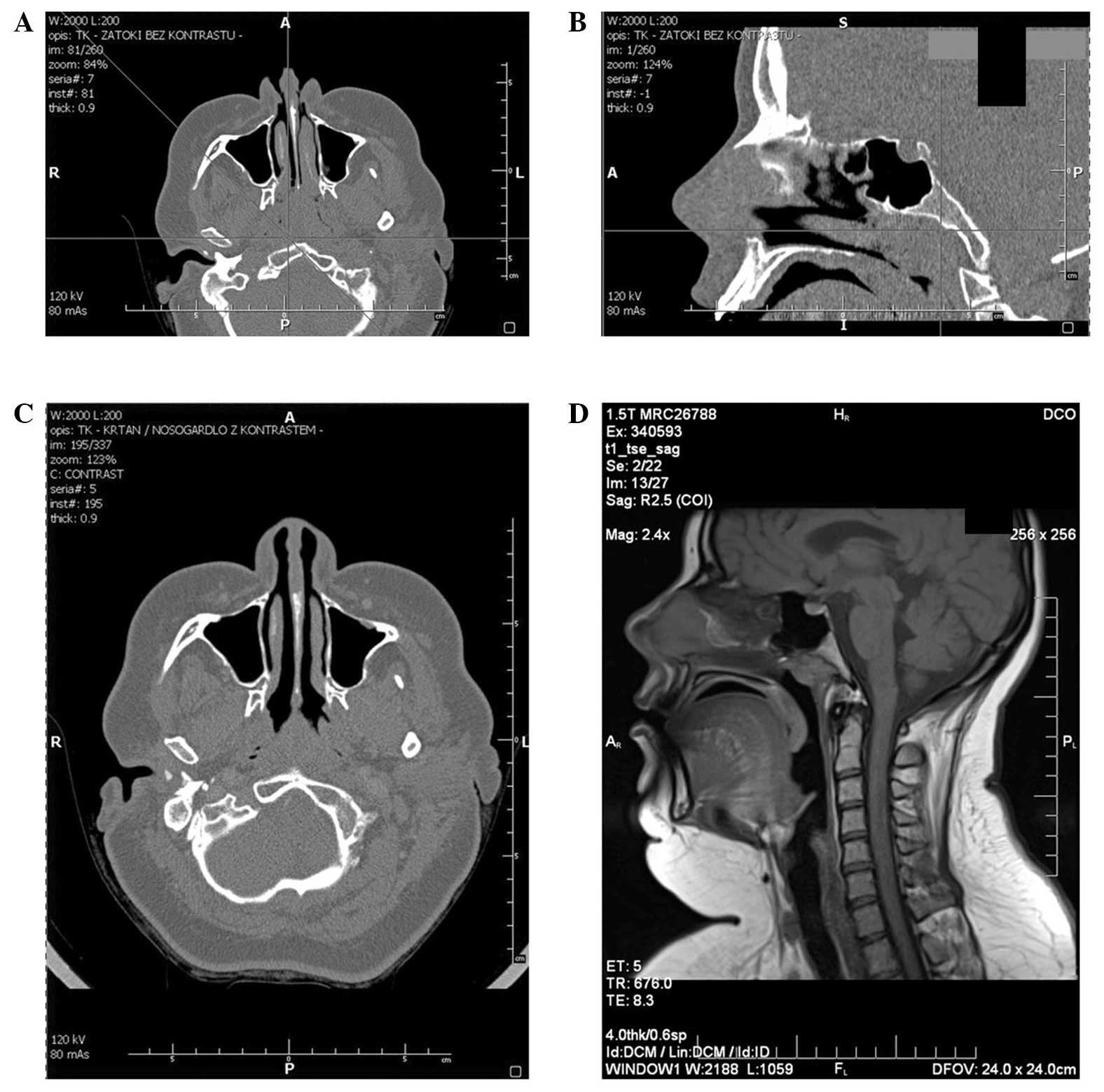
rhinomanometry. CT of maxillofacial region was consistent with the
nasopharynx being filled with soft tissue and did not reveal
features of destruction within the sphenoid bone (Fig. 1A and B).
A complete polysomnography, including
electroencephalography, electrooculography and electromyography,
was performed to assess the sleep structure of the patient. An
assessment of respiratory function was performed by recording chest
and abdomen respiratory movements, nasal and mouth air passage and
continuous arterial blood oxygen saturation levels. In addition,
continuous electrocardiogram records were kept. In the course of
the polysomnography study, the patient exhibited 392 incidents of
SDB (five obstructive, 19 central and 16 mixed apneas, and 352
hypopneas) resulting in an apnea hypopnea index (AHI) of 53.5.
Furthermore, 367 desaturation events were recorded, with an average
saturation of 92.5% and a minimum saturation of 72.5%. Thus, a
severe form of SDB was diagnosed. The patient qualified for
surgical treatment with the aim of restoring nasal patency and
histopathologically verifying the presence of a soft-tissue mass
located within the nasopharynx. The lesion was removed using
Beckman's adenotome and histopathologically assessed to determine a
diagnosis of nasopharyngeal chordoma. Microscopic examination
revealed a lobulated tumor divided by fibrous septa, composed of
vacuolated neoplastic cells in a myxoid stroma. The resection was
not microscopically radical, therefore, additional diagnostic and
oncological treatments were planned. However, the patient did not
agree to the proposed treatment strategies. Six months after the
primary surgical procedure, the patient underwent a follow-up
imaging assessment. CT of the maxillofacial region with contrast
revealed thickening of the posterior pharyngeal wall on the border
of the nasopharynx and oropharynx, with greater enlargement on the
left side (Fig. 1C). The thickness of
the pre-spinal tissue at the C2 level had increased to ~13 mm. The
described area was contrast-enhanced within 63–80 HU limits and it
was identified that the lesion adhered to the longissimus capitis
muscle, however, unambiguous features of its infiltration were not
identified in the CT images. In addition, the neck lymph nodes were
not enlarged and no pathological remodeling within the bony
structures was identified. Magnetic resonance imaging (MRI)
examination clarified the presence of a nodular structure outlined
within the nasopharyngeal roof, situated further towards the left
side and unevenly contrast enhanced (Fig.
1D). Again, surgical and oncological treatment strategies were
proposed to the patient, however, the patient refused. Six months
after the chordoma diagnosis, polysomnography was performed as a
follow-up examination and a significant improvement was identified
in the respiratory parameters. Only 35 respiratory incidents of
desaturation were recorded, and no apneas were detected.
Furthermore, the AHI was 6.4, the average saturation during
examination was 95% and the minimum saturation was 81%.
Discussion
It is estimated that between 9 and 24% of the
general population have SDB (8).
Various risk factors for developing SDB exist, including obesity,
the male gender, older age, a positive family history, hormonal
disorders, tobacco smoke, alcohol consumption, maxillofacial region
structural abnormalities and upper airway patency disorders. A
recognized association exists between SDB and obesity, with SDB
diagnosed in 50–77% of obese patients (9). The probability of SDB occurrence rises
with the increase in the degree of obesity; in particular, the risk
of sleep apnea syndrome increases by 10-fold among individuals with
at least first-degree obesity (BMI, >30 kg/m2)
(10). Furthermore, an obstructive
form of SDB qualifying for surgical obesity treatment is diagnosed
in 70–91% of pathologically obese patients (BMI, >40
kg/m2) (11–13). The type of fatty tissue distribution
is of particular importance in the SDB pathogenesis of obese
patients (14–16). Deposits of fatty tissue within the
neck compress the walls of the pharynx from the outside, and fatty
tissue infiltrates degenerate the neck muscles, impairing the
ability of the muscles to support the upper airways; thus, patency
is more easily impaired in obese patients, particularly in those
with a high fat distribution in the neck (17).
Upper airway patency disorders resulting from
structural abnormalities, which are the result of various
pathologies within the head and neck, are important in the
pathogenesis of SDB (18). Thorough
investigation into upper airway patency at the oropharynx has
identified a number of factors involved in oropharynx obstruction,
including a lengthened soft palate, tongue and uvula hypertrophy,
and palatine tonsil hypertrophy (19). However, the role of nose and
nasopharynx patency in SDB pathogenesis (with the exception of in
children, where adenoids are the predominant cause of SDB) has yet
to be discussed (20).
The lower section of the upper airways, in contrast
to the nasal cavity, larynx, trachea and bronchi level, does not
have cartilaginous or osseous scaffolding to provide rigid support
(21). Instead, stabilization at this
level is predominantly provided by the pharynx, palate and tongue
muscles. During inspiration, a partial or complete collapse and
narrowing of the lumen of the airway occurs when the negative
pressure in the airway is greater than the tension of the
stabilizing muscles of the wall. As a consequence, OSA occurs
(19–22). The pathologies that lead to nasal
patency impairment cause a rise in negative pressure in the upper
airways during inspiration (23),
therefore, increasing the risk of obstructive apneas. Nasal patency
disorders may result from numerous anatomical abnormalities and
pathologies that make nasal ventilation difficult, such as
deviation of the nasal septum, nasal polyps, inferior nasal
turbinate hypertrophy, middle nasal turbinate pneumatization,
posterior nares underdevelopment, neoplasms, granulomatous
diseases, adenoid hypertrophy, meningocele, foreign bodies,
post-operative and post-traumatic adhesions, and allergic and
non-allergic rhinitis (20,22).
Various studies in the literature have indicated
that nasal patency disorders should be treated as a potential risk
factor for SDB development (24,25). For
example, in a prospective study by Lofaso et al (26), it was stated that nasal patency
disorders assessed on the basis of posterior rhinomanometry are
independent risk factors for OSA development, although their impact
was significantly weaker compared with the effect of obesity or
facial bone abnormalities. By contrast, Young et al
(27) did not identify a strong
association between increased airway resistance at the level of the
nasal cavity and SDB in the total population. In addition, the use
of surgical treatment for nasal patency disorders as a treatment
strategy for SDB is controversial, with contrary perspectives
reported in the literature. Sériès et al (28) determined that surgical correction of
nasal patency disorders is an effective method of SDB treatment,
but only in patients who were not diagnosed with significant
abnormalities of the facial bones. Isolated studies have described
cases of upper airway neoplastic lesions, which predominantly
manifest as nasal and nasopharyngeal obstruction, leading to SDB
(29–31). Furthermore, Piccin and Sorrenti
(29) described a retropharyngeal
space lipoma with severe symptoms typical of SDB that completely
subsided following surgical removal of the tumor.
The case described in the current study concerns a
rare nasopharyngeal chordoma that manifested as a nasal
obstruction, resulting in SDB. Chordomas are slow-growing malignant
neoplasms originating from the remnants of the notochord, a
primitive tissue of embryonic origin that is preserved outside of
the axial skeleton. Chordomas develop in the midline of the body,
most often in the sacrococcygeal area of the spine (50–60%), the
skull base (25–30%), the cervical spine (~10%) and the
thoracolumbar segment of the spine (~5%). Chordoma localization in
the skull base is limited to the clivus, and casuistic cases are
concerned with lesions developing outside of the bones as a
soft-tissue mass in the nasopharynx (32,33). In
addition, chordomas constitute only 0.2% of all nasopharyngeal
neoplasms, therefore, diagnosis poses a significant challenge, and
pre-surgical diagnostics based on a patient's clinical condition
and imaging examinations do not typically coincide with the final
histopathological diagnosis. Chordoma can develop at any age,
however, it is predominantly diagnosed in adults. Lesions in the
skull base typically occur in the third and fourth decade of life,
while tumors of the sacrococcygeal area typically occur in the
fifth and sixth decade (34).
Furthermore, male individuals suffer from chordoma twice as often
as females (35). Chordomas leading
to distant metastasis to the lungs, liver, bones and lymph nodes
have previously been described in the literature (36).
Nasal patency symptoms are dominant in tumors
located within the nasopharynx. Imaging diagnostics are based on CT
and MRI scans of the area occupied by the tumor, however, the two
techniques provide non-specific images of the lesion (37). Chordomas are not
chemotherapy-sensitive tumors, therefore, the typical treatment
strategy is radical surgery. However, the localization of chordomas
in the vicinity of nervous system structures makes negative tissues
margins difficult or even impossible to obtain. In such cases,
surgical resection should be complemented with post-operative
radiotherapy. Efficient chordoma radiotherapy treatment requires
the use of high-dose radiation (>60 Gy), which may damage
sensitive tissue in the brain and optic nerve chiasm area.
Promising alternatives include modern conformal radiotherapy
techniques, intensity-modulated radiation therapy and proton beam
therapy (38). Furthermore, combined
treatment strategies provide good local control. It is estimated
that the five-year survival rate of patients with chordomas of all
localizations is ≤70% (39).
The nasopharyngeal chordoma described in the current
study was incidentally diagnosed due to poor symptomatology. The
chordoma was diagnosed during SDB diagnostics performed as a part
of a research project of pathologically obese patients qualifying
for bariatric surgery. A non-typical radiogram without features of
adhering bone structure destruction and endoscopic images imitating
nasopharyngeal lymphatic tissue hypertrophy were obtained, thus,
the SDB diagnostics were not extended to incorporate neoplastic
disease at the preliminary stage. A polysomnographic control
examination, which was performed independently of oncological
proceedings, revealed a significant improvement in various
respiratory parameters following tumor removal; however, the BMI
did not change, as the patient was not subjected to bariatric
surgery. Thus, it is proposed that the obstruction of the
nasopharynx by the tumor was the predominant cause of the SDB.
SDB is a common phenomenon in pathologically obese
individuals and polysomnography should be the diagnostic gold
standard in this group of patients. Although the predominant risk
factor in SDB patients with a high BMI is the obesity itself, this
does not prejudice the duty of additional laryngological
diagnostics with the aim of excluding organic and anatomical causes
of SDB.
Laryngological assessment of patients with SDB
should be based on a detailed interview and physical examination,
followed by an endoscopic examination of the upper airways. When
necessary, this should be accompanied by a head and neck imaging
examination.
In conclusion, nasopharyngeal chordoma is a rare
type of slow-growing nasopharyngeal malignant neoplasm that may
remain unnoticed for a long period of time. Due to its non-specific
clinical presentation and, in a number of cases, a lack of
radiological features consistent with infiltration of the
nasopharyngeal bone structures, this neoplasm is often misdiagnosed
as a benign lesion of the nasopharynx, such as an enlarged
adenoids.
Acknowledgements
This study was supported by the EEA/Norway Grants
(grant number POL/NOR/196258/2013.
References
|
1
|
Taranto Montemurro L and Kasai T: The
upper airway in sleep-disordered breathing: UA in SDB. Minerva Med.
105:25–40. 2014.PubMed/NCBI
|
|
2
|
Budhiraja R, Budhiraja P and Quan SF:
Sleep-disordered breathing and cardiovascular disorders. Respir
Care. 55:1322–1332; discussion. 1330–1332. 2010.PubMed/NCBI
|
|
3
|
Ioachimescu OC and Collop NA:
Sleep-disordered breathing. Neurol Clin. 30:1095–1136. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McMaster ML, Goldstein AM, Bromley CM, et
al: Chordoma: incidence and survival patterns in the United States,
1973–1995. Cancer Causes Control. 12:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kłosiński P, Lisiecki J, Goździewicz J and
Kowalska B: Chordoma - treatment and prognosis. Współczesna
Onkologia. 7:107–114. 2003.(In Polish).
|
|
6
|
Harbour JW, Lawton MT, Criscuolo GR,
Holliday MJ, Mattox DE and Long DM: Clivus chordoma: a report of 12
recent cases and review of the literature. Skull Base Surg.
1:200–206. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heffelfinger MJ, Dahlin DC, MacCarty CS
and Beabout JW: Chordomas and cartilaginous tumors at the skull
base. Cancer. 32:410–420. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Young T, Palta M, Dempsey J, Skatrud J,
Weber S and Badr S: The occurrence of sleep-disordered breathing
among middle-aged adults. N Engl J Med. 328:1230–1235. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vgontzas AN, Tan TL, Bixler EO, Martin LF,
Shubert D and Kales A: Sleep apnea and sleep disruption in obese
patients. Arch Intern Med. 154:1705–1711. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kyzer S and Charuzi I: Obstructive sleep
apnea in the obese. World J Surg. 22:998–1001. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frey WC and Pilcher J: Obstructive
sleep-related breathing disorders in patients evaluated for
bariatric surgery. Obes Surg. 13:676–683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Keeffe T and Patterson EJ: Evidence
supporting routine polysomnography before bariatric surgery. Obes
Surg. 14:23–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hallowell PT, Stellato TA, Schuster M, et
al: Potentially life-threatening sleep apnea is unrecognized
without aggressive evaluation. Am J Surg. 193:364–367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schäfer H, Pauleit D, Sudhop T, et al:
Body fat distribution, serum leptin, and cardiovascular risk
factors in men with obstructive sleep apnea. Chest. 122:829–839.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shelton KE, Woodson H, Gay S and Suratt
PM: Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis.
148:462–466. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Horner RL, Mohiaddin RH, Lowell DG, et al:
Sites and sizes of fat deposits around the pharynx in obese
patients with obstructive sleep apnoea and weight matched controls.
Eur Respir J. 2:613–622. 1989.PubMed/NCBI
|
|
17
|
Schwartz AR, Patil SP, Laffan AM, et al:
Obesity and obstructive sleep apnea: pathogenic mechanisms and
therapeutic approaches. Proc Am Thorac Soc. 5:185–192. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fogel RB, Malhotra A and White DP: Sleep.
2: Pathophysiology of obstructive sleep apnoea/hypopnoea syndrome.
Thorax. 59:159–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryan CM and Bradley TD: Pathogenesis of
obstructive sleep apnea. J Appl Physiol (1985). 99:2440–2450. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rappai M, Collop N, Kemp S and deShazo R:
The nose and sleep-disordered breathing: what we know and what we
do not know. Chest. 124:2309–2323. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rombaux P, Liistro G, Hamoir M, et al:
Nasal obstruction and its impact on sleep-related breathing
disorders. Rhinology. 43:242–250. 2005.PubMed/NCBI
|
|
22
|
Kohler M, Bloch KE and Stradling JR: The
role of the nose in the pathogenesis of obstructive sleep apnoea
and snoring. Eur Respir J. 30:1208–1215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anch AM, Remmers JE and Bunce H III:
Supraglottic airway resistance in normal subjects and patients with
occlusive sleep apnea. J Appl Physiol Respir Enveron Exerc Physiol.
53:1158–1163. 1982.
|
|
24
|
Suratt PM, Turner BL and Wilhoit SC:
Effect of intranasal obstruction on breathing during sleep. Chest.
90:324–329. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olsen KD, Kern EB and Westbrook PR: Sleep
and breathing disturbance secondary to nasal obstruction.
Otolaryngol Head Neck Surg. 89:804–810. 1981.PubMed/NCBI
|
|
26
|
Lofaso F, Coste A, d'Ortho MP, et al:
Nasal obstruction as a risk factor for sleep apnoea syndrome. Eur
Respir J. 16:639–643. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Young T, Finn L and Kim H: Nasal
obstruction as a risk factor for sleep-disordered breathing. The
University of Wisconsin Sleep and Respiratory Research Group. J
Allergy Clin Immunol. 99:S757–S762. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sériès F, St Pierre S and Carrier G:
Surgical correction of nasal obstruction in the treatment of mild
sleep apnoea: importance of cephalometry in predicting outcome.
Thorax. 48:360–363. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piccin O and Sorrenti G: Adult obstructive
sleep apnea related to nasopharyngeal obstruction: a case of
retropharyngeal lipoma and pathogenetic considerations. Sleep
Breath. 11:305–307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Girolamo S, Marinelli L, Galli A and
Ottaviani F: Retropharyngeal lipoma causing sleep apnea syndrome. J
Oral Maxillofac Surg. 56:1003–1004. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hockstein NG, Anderson TA, Moonis G, et
al: Retropharyngeal lipoma causing obstructive sleep apnea: case
report including five-year follow-up. Laryngoscope. 112:1603–1605.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nguyen RP, Salzman KL, Stambuk HE, Ahuja
AT and Harnsberger HR: Extraosseous chordoma of the nasopharynx.
AJNR Am J Neuroradiol. 30:803–807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
DiFrancesco LM, Davanzo Castillo CA and
Temple WJ: Extra-axial chordoma. Arch Pathol Lab Med.
130:1871–1874. 2006.PubMed/NCBI
|
|
34
|
Klingler L, Trammell R, Allan DG, Butler
MG and Schwartz HS: Clonality studies in sacral chordoma. Cancer
Genet Cytogenet. 171:68–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mizerny BR and Kost KM: Chordoma of the
cranial base: the McGill experience. J Otolaryngol. 24:14–19.
1995.PubMed/NCBI
|
|
36
|
Weber AL, Brown EW, Hug EB and Liebsch NJ:
Cartilaginous tumors and chordomas of the cranial base. Otolaryngol
Clin North Am. 28:453–471. 1995.PubMed/NCBI
|
|
37
|
Yan ZY, Yang BT, Wang ZC, Xian JF and Li
M: Primary chordoma in the nasal cavity and nasopharynx: CT and MR
imaging findings. AJNR Am J Neuroradiol. 31:246–250. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hug EB, Loredo LN, Slater JD, et al:
Proton radiation therapy for chordomas and chondrosarcomas of the
skull base. J Neurosurg. 91:432–439. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chugh R, Tawbi H, Lucas DR, Biermann JS,
Schuetze SM and Baker LH: Chordoma: the nonsarcoma primary bone
tumor. Oncologist. 12:1344–1350. 2007. View Article : Google Scholar : PubMed/NCBI
|