Introduction
Endometrial cancer is a common type of malignant
gynecological disease that predominantly occurs in post-menopausal
females, aged 50–60 years. It accounts for 20–30% of cases of
female cancer, with the incidence rate demonstrating an increasing
trend in recent years (1). Tumors in
endometrial cancer patients at clinical stages I/II exhibit a low
degree of differentiation and invasive ability, therefore, good
results can be obtained using surgical intervention. By contrast,
tumors in patients at stages III/IV exhibit a higher degree of
malignancy and differentiation, therefore, surgical treatments are
only able to reduce tumor volume. Instead, adjuvant endocrine
therapy, radiotherapy, and chemotherapy are typically administered
for patients at stages III and IV to effectively improve the
survival rate (2–4).
The taxol, adriamycin and carboplatin (TAC)
chemotherapy regimen has gradually been utilized for the treatment
of patients with advanced endometrial cancer. Compared with the
traditional adriamycin/cisplatin and
cyclophosphamide/adriamycin/cisplatin regimens, the effect of the
TAC regimen is more pronounced, causing fewer side effects in
patients. In particular, TAC exhibits lower hematological toxicity,
thus, resulting in more comprehensive treatment and improved
recovery for patients (5).
Endocrine therapy is an effective new method used
for the treatment of endometrial cancer. As endometrial cancer
cells typically express normal hormone receptors,
progesterone-based agents are most commonly used in endometrial
cancer treatment strategies; for example, medroxyprogesterone
acetate (MPA), megestrol and hydroxyprogesterone (6). Long-term use of progesterone agents can
gradually revert the endometrium of endometrial cancer patients
back to a healthy state by decreasing the size of the cancer
tissues. The predominant action mechanism involves the ability of
progesterone to effectively reduce the content of sulfuric acid
ester on the cancer cell surface, inhibiting binding between cancer
cells and laminin, and, thus, blocking the transfer pathway.
Furthermore, progesterone directly inhibits DNA and RNA synthesis,
as well as the expression of cancer cells. This results in mature
and well-differentiated cells, and accelerates the aging and
shrinking of cancerous tissues (7).
Previous studies have demonstrated that postoperative radiotherapy,
chemotherapy and progestin therapy effectively improved the health
of high-risk endometrial cancer patients, and prolong their
long-term survival rates (5–7). However, the currently employed progestin
endocrine therapy has common side effects associated with the
long-term use of steroids. For example, progesterone improves
patient appetite, and a small number of patients are prone to fluid
retention, gastrointestinal discomfort and depression. Additional
adverse reactions, such as rare pulmonary embolism, may also
occur.
Metastasis-associated protein 1 (MTA1) is a human
protein that is encoded by the MTA1 gene (8,9). MTA1 is a
protein that has been associated with the metastatic state of
various cancer types (10–14). MTA1 promotes tumor invasion by the
downregulation of E-cadherin in human esophageal squamous cell
carcinoma cells (10). Furthermore,
it was recently determined that MTA1 promotes the invasion and
migration of laryngeal squamous cell carcinoma cells (13). In the present study, 64 patients with
endometrial cancer who received TAC chemotherapy combined with
endocrine therapy were studied. MTA1 protein and mRNA expression
levels were determined, and compared between patients who received
TAC chemotherapy only and patients who received TAC plus endocrine
therapy.
Patients and methods
Patients
The present retrospective analysis included 124
patients with endometrial cancer who were hospitalized at the
Affiliated Hospital of Yan'an University (Yan'an, China) between
January 2011 and April 2013. All the patients were diagnosed by
performing a computed tomography scan and biopsy. No patients had
received radiotherapy, chemotherapy or endocrine therapy prior to
hospitalization. The patients were aged 29–73 years (mean age,
51.4±6.4 years) and were classified according to the following
clinical stages: Stage I, 58 cases; stage II, 37 cases; stage III,
20 cases; and stage IV, 9 cases. Furthermore, the degree of tumor
differentiation within the cohort was as follows: High
differentiation, 59 cases; medium differentiation, 42 cases; and
low differentiation, 23 cases (15).
The patients were divided into an experimental (n=64) and control
(n=60) group according to the order of admission. No significant
differences in age, clinical stage or differentiation degree were
identified between the two groups of patients (P>0.05) and all
the patients were followed up for a period of 20–45 months (mean,
30±10.4 months). All procedures were approved by the Ethics
Committee of Yan'an University. Informed consent was obtained from
all the patients or their family members.
Treatment strategies
Surgical treatment was received by 46/64 patients in
the experimental group and 43/60 patients in the control group.
Thus, the ratio of patients receiving surgical treatment was
similar in the two groups. All the patients were administered the
TAC chemotherapy regimen (160 mg/m2 taxol, 45
mg/m2 adriamycin and AUC5 carboplatin) for 2–8 cycles
(cycle duration, 21 days; average number of cycles, 5.3±1.2
cycles). Patients in the experimental group were concurrently
treated with the progesterone agent MPA (dose, 250 mg/day).
Evaluation index
The patients were scanned with computed tomography
three months after treatment and followed up every six months. The
recurrence and metastasis evaluation criteria was as follows: i)
Local recurrence, the tumor appeared at the pelvic or vaginal
stump; and ii) distant metastasis, distant organ metastasis and
lymph node metastasis outside of the primary tumor the region
(3). Furthermore, survival evaluation
was conducted using the following criteria: i) Tumor-free survival
period, no reoccurrence or metastasis identified between the
termination of the treatment regimen and the termination of the
follow-up period; and ii) overall survival period, from the
termination of the treatment regimen to the date of patient
mortality. Blood cell count, liver and kidney function, urine
analysis and physical examinations were performed every week during
the treatment and follow-up period.
Biopsy
The patients laid on the examination table with
their feet in stirrups for the pelvic biopsy. A speculum was
inserted into the vagina to spread the walls of the vagina and
expose the cervix. The cervix was then cleansed with an antiseptic
solution and a tenaculum, a type of forceps, held the cervix steady
for the biopsy. The biopsy curette was inserted into the uterine
fundus and, with a scraping and rotating motion, a small quantity
of tissue was removed. The removed tissue was placed in formalin or
equivalent for preservation. The tissue was sent to a laboratory
for processing and analysis, prior to microscopic analysis by a
pathologist to provide a histological diagnosis.
Western blot analysis
Total proteins were prepared from tissue samples
scraped from the wall of the uterus, as described above. The total
proteins were separated using 10% SDS-polyacrylamide gel
electrophoresis and then transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Following incubation
with the polyclonal goat anti-human MTA1 primary antibody (sc-9445;
dilution, 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) at 4°C overnight. The membranes were rinsed with Tris-buffered
saline and Tween-20, and then incubated with a secondary antibody
conjugated to peroxidase (dilution, 1:5,000; Santa Cruz
Biotechnology, Inc.) for 1 h. After washing three times, the
membranes were detected by enhanced chemiluminescence (EMD
Millipore), with GAPDH used for normalization. Furthermore, the
relative intensity of the target bands was analyzed by Quantity One
1-D analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Each assay was independently repeated three times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and mRNA was
reverse transcribed to complementary DNA using the Reverse
Transcriptase MMLV kit (Takara Biotechnology Co., Ltd., Dalian,
China), according to the manufacturer's instructions. PCR reactions
were performed using the SYBR® PrimeScript RT-PCR kit (Takara
Biotechnology Co., Ltd.), with relative levels of MTA1 mRNA
normalized to GAPDH mRNA. The primer sequences for MTA1 and GAPDH
detection were as follows: Forward, 5′-AGCTACGAGCAGCACAACGGGGT-3′
and reverse, 5′-CACGCTTGGTTTCCGAGGAT-3′ for MTA1; and forward,
5′-CATGCGCCTCACTAGTCAGCT-3′ and reverse,
5′-TACGCTGAGGATACAGGATAC-3′ for GAPDH. qPCR was performed using the
ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA,
USA) and each experiment was repeated a minimum of three times.
Statistical analysis
SPSS software (version 16.0; SPSS, Inc., Chicago,
IL, USA) was used for data analysis. Measurement data was compared
using a paired t-test and count data was analyzed by performing a
χ2 test. Data are presented as the mean ± standard
deviation and a value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Combination with adjuvant endocrine
MPA therapy reduces the recurrence and metastasis rates of patients
treated with TAC chemotherapy alone
One hundred and twenty-four endometrial cancer
patients were enrolled in the present study, all of whom were
diagnosed by performing a computed tomography scan and uterine
biopsy (Fig. 1). The patients were
divided into two groups, with 64 patients in the experimental group
and 60 patients in the control group. The experimental group
received TAC and adjuvant endocrine MPA therapy, however, the
control group received TAC therapy only. A t-test was performed to
compare the recurrence or metastasis rate following treatment
between the experimental and control groups. The results indicated
that the total recurrence or metastasis rate for the experimental
group was significantly decreased compared with the control group
(P<0.05; Table I). Furthermore,
the time to recurrence or metastasis in the experimental group was
significantly prolonged compared with the control group (P<0.05;
Table I). Thus, recurrence or
metastasis rates and occurrence times following treatment appear to
be significantly different between the experimental and control
groups.
 | Table I.Comparison of recurrence or metastasis
between the experimental and control groups. |
Table I.
Comparison of recurrence or metastasis
between the experimental and control groups.
| Group | Cases, n | Recurrence or
metastasis, n | Time of recurrence or
metastasis, monthsa | Total recurrence or
metastasis rate, %b |
|---|
| Experimental | 64 | 3 | 22.4±15.4 | 4.69 |
| Control | 60 | 7 | 20.5±14.6 | 11.67 |
Combination with adjuvant endocrine
MPA therapy improves the long-term survival rates of patients
treated with TAC chemotherapy alone
A t-test analysis was performed to compare the one-
to three-year survival rates of the experimental and control
groups. The one- and two-year survival rates of the patients were
not significantly different between the experimental and control
groups (P>0.05; Table II).
However, the three-year survival rate of the experimental group was
significantly higher than that of the control group (P<0.05;
Table II). These results indicate
that concurrent treatment endocrine MPA therapy may significantly
improve the long-term survival rate of patients treated with TAC
chemotherapy.
 | Table II.Comparison of patient survival rates
between the experimental and control groups. |
Table II.
Comparison of patient survival rates
between the experimental and control groups.
| Group | Cases, n | One-year survival
rate, %a | Two-year survival
rate, %b | Three-year survival
rate, %c |
|---|
| Experimental | 64 | 98.2 | 93.4 | 89.4 |
| Control | 60 | 94.5 | 86.5 | 76.1 |
Adjuvant endocrine therapy with MPA
does not cause obvious toxicity in patients
To investigate the toxicity of endocrine therapy,
blood, liver and kidney tests, as well as computed tomography scans
were performed. The degree of neutropenia, nausea, vomiting and
other symptoms in the experimental group were only marginally
different from those in the control group (data not shown). In the
experimental group, two patients exhibited significant increases in
body weight, and one patient developed a cough and asthma,
resulting in a 4.69% incidence of adverse reactions. The
aforementioned adverse reactions were not identified in the control
group; however, the difference in adverse reactions between the
experimental and control group was not statistically significant
(P>0.05). These data indicate that adjuvant endocrine MPA
therapy does not cause significant toxicity in patients with
endometrial cancer.
MPA decreases MTA1 expression
levels in patients treated with TAC chemotherapy
As demonstrated in the aforementioned results, the
MTA1 protein was associated with recurrence and metastasis rates in
patients with endometrial cancer. To investigate the mechanism
underlying the inhibitory effect of MPA on the recurrence and
metastasis rates of patients treated with TAC chemotherapy, the
total proteins and RNAs were extracted from tissue samples scraped
off of the uterus wall for analysis. Western blot analyses were
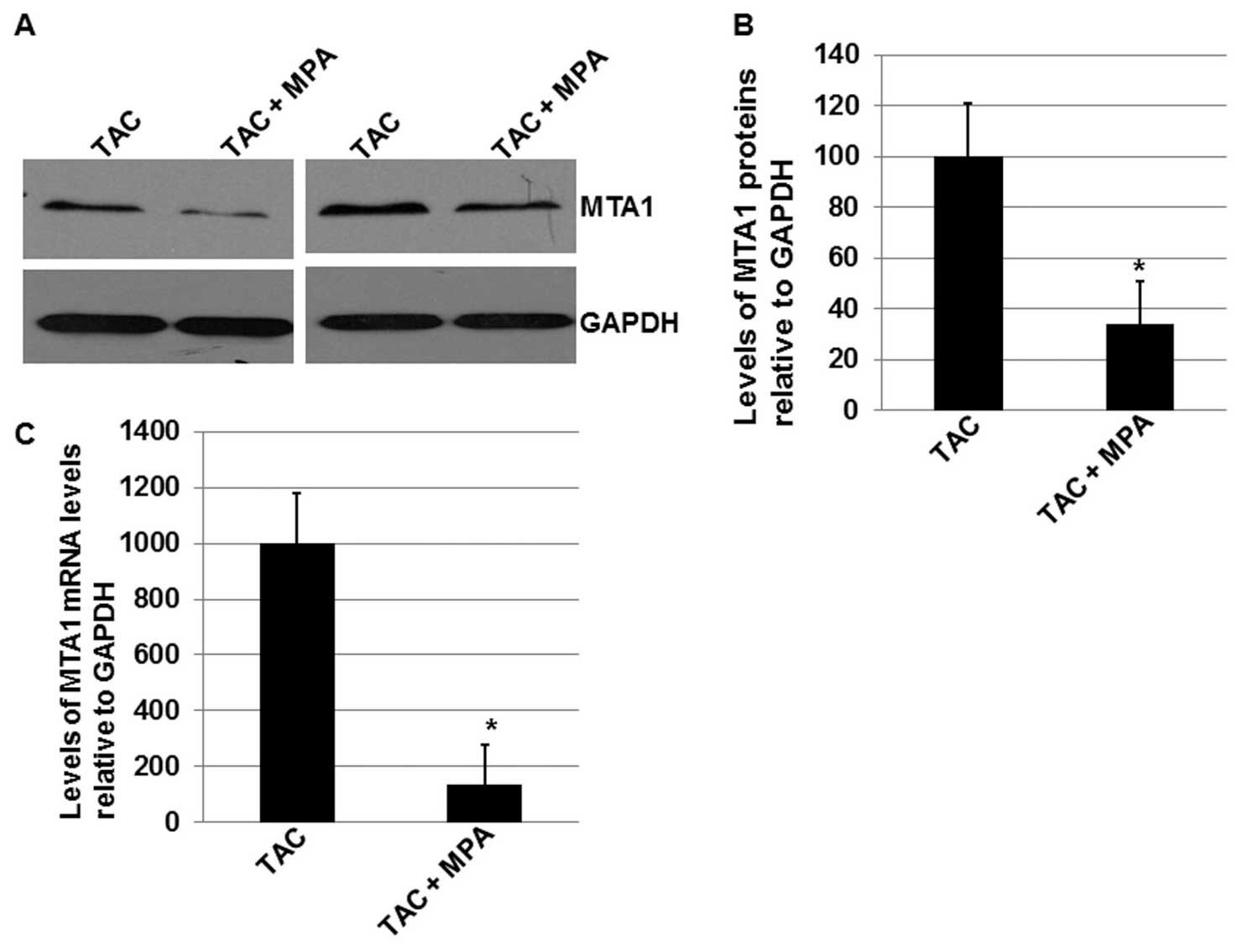
performed to determine the MTA1 protein expression levels in the 60
control group and 64 experimental group patients (Fig. 2A). As indicated in Fig. 2B, combined treatment with TCA and MPA
significantly enhanced the inhibitory effects of TAC therapy on the
expression levels of the MTA1 protein (P<0.05). Furthermore, the
mean MTA1 expression level in the experimental group was ~30% of
the level in the control group. These results indicate that MPA may
reduce MTA1 expression levels in patients that receive TAC
chemotherapy.
MPA decreases MTA1 expression levels
in patients treated with TAC chemotherapy via a transcriptional
mechanism
To determine whether the effect of MPA treatment on
MTA1 expression is mediated via a translational or transcriptional
mechanism, the total RNAs were isolated from the tissue samples
scraped off of the uterus wall of all patients in the present
study. Using RT-qPCR, it was identified that the mRNA expression
levels of MTA1 were significantly affected by treatment with MPA
(P<0.05; Fig. 2C), with the mean
level of MTA1 mRNA ~15% of the mean level in the control group
(P<0.05; Fig. 2C). These results
indicate that MTA1 expression may be downregulated via a
transcriptional mechanism.
Discussion
By performing adjuvant endocrine therapy on 65 stage
I endometrial cancer patients, Xie et al (16) demonstrated that the recurrence and
metastasis rates declined in comparison to the patients who did not
receive adjuvant endocrine MPA therapy. However, this difference
was not significant. In addition, following a treatment period of
>12 months, the three-year tumor-free survival rate of 100% was
significantly increased compared with the control group. Similarly,
the present study identified that the recurrence or metastasis rate
of the experimental group was significantly lower than in the
control group, and the recurrence or metastasis occurrence time of
the experimental group was significantly prolonged compared with
the prognosis of the control group. Furthermore, the one-year and
two-year survival rates of the experimental group were not
significantly different from that of the control group, however,
the three-year survival rate of the experimental group was
significantly higher compared with that of the control group
(P<0.05). A number of patients in the experimental and control
groups exhibited certain degrees of hematologic toxicity following
the treatment regime, however, the toxicity responses were not
significantly different between the two groups. Therefore, the
results of the present study demonstrated that TAC chemotherapy
combined with endocrine MPA therapy may effectively improve the
prognosis and survival rates of patients with endometrial
cancer.
MTA1 is associated with the metastatic state in
various types of cancer (9–15). For example, recent studies have
demonstrated that MTA1 promotes tumor invasion in human esophageal
(11) and laryngeal (14) squamous cell carcinoma cells. In the
present study, it was identified that combined use of MPA and TAC
chemotherapy may significantly decrease the expression levels of
MTA1 in patients with endometrial cancer. In addition, it was
determined that this inhibitory effect on MTA1 is mediated in a
transcriptional manner.
In conclusion, TAC chemotherapy combined with
endocrine MPA therapy appears to effectively reduce the recurrence
or metastasis rates, improve the prognosis, and increase the
long-term survival rates of patients with endometrial cancer.
Furthermore, such an effect may be mediated by downregulation of
the expression levels of MTA1 via a transcriptional mechanism.
Acknowledgements
The present study was supported by Yan'an University
(Yan'an, China).
References
|
1
|
Xing X, Li X, Qiao S, et al: Endocrine
cells of endometrial cancer and its endocrine differentiation
mechanism. Hebei Med J. 32:3542–3544. 2010.(In Chinese).
|
|
2
|
Wang J: Scientific understanding of
standard treatment for endometrial cancer. J Zhengzhou Univ (Med
Sci). 47:1–3. 2012.(In Chinese).
|
|
3
|
Cao Z: Common gynecological cancer
treatment guidelines. People's Health Publishing House; Beijing:
pp. 41–45. 2007
|
|
4
|
Wang J and Wei L: New Progress in the
adjuvant treatment of endometrial cancer. Chinese J Obstet Gynecol.
3:156–158. 2004.
|
|
5
|
Cui M and Xu T: The research progress of
endometrial cancer. Prac J Oncol. 27:202–205. 2013.
|
|
6
|
Li X: Clinical diagnosis and treatment of
endometrial cancer. Guangdong Med J. 33:1185–1187. 2012.(In
Chinese).
|
|
7
|
Zhu Y and Gao Q: Progestin therapy for
endometrial cancer. Chinese J Prac Gynecol Obstet. 18:204–205.
2002.(In Chinese).
|
|
8
|
Toh Y, Pencil SD and Nicolson GL: A novel
candidate metastasis-associated gene, mta1, differentially
expressed in highly metastatic mammary adenocarcinoma cell lines.
cDNA cloning, expression and protein analyses. J Biol Chem.
269:22958–22963. 1994.PubMed/NCBI
|
|
9
|
Toh Y, Pencil SD and Nicolson GL: Analysis
of the complete sequence of the novel metastasis-associated
candidate gene, mta1, differentially expressed in mammary
adenocarcinoma and breast cancer cell lines. Gene. 159:97–104.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weng W, Yin J, Zhang Y, Qiu J and Wang X:
Metastasis-associated protein 1 promotes tumor invasion by
downregulation of E-cadherin. Int J Oncol. 44:812–818.
2014.PubMed/NCBI
|
|
11
|
Kang HJ, Lee MH, Kang HL, et al:
Differential regulation of estrogen receptor α expression in breast
cancer cells by metastasis-associated protein 1. Cancer Res.
74:1484–1494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bettum IJ, Vasiliauskaite K, Nygaard V, et
al: Metastasis-associated protein S100A4 induces a network of
inflammatory cytokines that activate stromal cells to acquire
pro-tumorigenic properties. Cancer Lett. 344:28–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Yang D, Wang H, et al:
Metastasis-associated gene 1 promotes invasion and migration
potential of laryngeal squamous cell carcinoma cells. Oncol Lett.
7:399–404. 2014.PubMed/NCBI
|
|
14
|
Nagaraj SR, Shilpa P, Rachaiah K and
Salimath BP: Crosstalk between VEGF and MTA1 signaling pathways
contribute to aggressiveness of breast carcinoma. Mol Carcinog. Nov
22–2013.(Epub ahead of print). PubMed/NCBI
|
|
15
|
Hoffman BL, Schorge JO, Schaffer JI,
Halvorson LM, Bradshaw KD, Cunningham FG and Calver LE: Endometrial
CancerWilliams Gynecology. 2nd. McGraw-Hill; New York, NY: pp.
8252012
|
|
16
|
Xie L: A clinical analysis of the effects
of adjuvant endocrine therapy for stage I endometrial carcinoma. J
Clin Exp Med. 12:1063–1064. 2013.
|