Introduction
Papillary thyroid carcinoma (PTC) is a prevalent
form of thyroid cancer (1). Due to
recent developments in ultrasonography (US) and US-guided
fine-needle aspiration biopsies, impalpable small-sized papillary
thyroid microcarcinomas have been frequently detected (2).
In general, PTC has a good prognosis; however,
certain patients experience local recurrence and/or distant
metastasis. Factors that are known to significantly reduce PTC
patient prognosis include age, male gender, large tumor size,
extrathyroidal extension and metastases (3–6).
Numerous scoring systems for predicting prognosis,
including Tumor-Node-Metastases (TNM) and
Metastases-Age-Completeness of resection-Invasion-Size of tumor
(MACIS) have been used to more accurately establish prognosis in
PTC patients (3,7). Furthermore, numerous previous studies
have investigated potential molecular and cytological markers of
biological behavior (3–7).
The most prevalent type of genetic alteration in PTC
is the BRAF (V600E) mutation. BRAF is known to be a
mitogen-activated protein kinase (MAPK) signaling pathway activator
involved in regulating the growth, division and proliferation of
cells (8). The V600E amino acid
substitution in BRAF is the results of a T1799A point mutation in
exon 15 of BRAF; this mutation accounts for >90% of all the
genetic alterations detected in the BRAF gene (9).
Numerous studies have investigated potential
associations among the BRAF (V600E) mutation, clinicopathological
features of PTC and the clinical outcome of patients; however,
correlations between the incidence of the BRAF (V600E) mutation and
clinicopathological features in PTC patients remains controversial
(1,9–20).
The incidence rate of the BRAF (V600E) mutation in
PTC has been reported to vary between 29 and 83%; of note, the rate
of BRAF (V600E) mutation occurrence in Korea was reported to be
52–83%, whereas in other countries the occurrence rate was 30–49%
(9). However, the variation in these
results was suggested to be the result of a lack of prospective
studies that may reduce the selection bias, small study population
sizes, the absence of multivariate analysis and the heterogeneous
histological subtypes within PTC (9).
Mutations in the tumor suppressor gene p53 were reported to occur
in ~50% of cancers; these mutations account for the most prevalent
type of genetic alteration in cancer cells (3–8). Numerous
previous studies have demonstrated that genetic mutations of p53
often occur in undifferentiated thyroid cancers. The occurrence of
p53 mutations in well differentiated thyroid carcinomas, such as
PTC, has not been conclusively established; but the incidence of
p53 mutations has been reported as ranging between 0 and 25%
(7,21). p53 protein overexpression, as
determine by immunohistochemistry, was reported to be correlated
with the presence of p53 gene mutations (7); however, the immunohistochemical
detection of p53 overexpression has been identified in
differentiated follicular and papillary thyroid carcinomas
regardless of the occurrence of p53 gene mutations. p53 protein
overexpression was reported to have an incidence rate of between 11
and 59% (6,22,23).
Certain studies regarding the immunohistochemical analysis of p53
protein expression have demonstrated that p53 overexpression may be
used as an independent prognostic indicator for differentiated
thyroid carcinomas (3–7,22,24).
The present study aimed to investigate the
prevalence of the BRAF (V600E) mutation and the overexpression of
p53 protein in PTC, as well as to determine any potential
associations among these two factors and other clinicopathological
features of PTC.
Materials and methods
The present study was approved by the Institutional
Review Board of the Kangnam Sacred Heart Hospital of Hallym
University Medical Center (no. 2014-04-44; Seoul, Korea).
A total of 66 PTC patients (classic type, 60 cases;
follicular variant, 6 cases) who had undergone surgery for the
treatment of PTC, thyroid lobectomy or total thyroidectomy with or
without lymph node dissection, were enrolled into the present study
at the Kangnam Sacred Heart Hospital between January and December
2012. For all the cases, hematoxylin and eosin (H&E)-stained
slides and paraffin blocks for immunohistochemical staining were
reviewed. The H&E slides were examined by two of the present
authors, independently, according to the histopathological criteria
proposed by the World Health Organization (25) for the diagnosis of PTC. A multi-headed
microscope (U-MDOB3, Olympus Corporation, Tokyo, Japan) was used in
order to review any slides where the independent reviewers
diagnoses were not consistent.
Immunohistochemistry was performed on 4-µm-thick
paraffin-embedded tissue sections using the automated staining
system, the Leica Bond-Max autostainer (Leica Microsystems GmbH,
Wetzlar, Germany) with appropriate positive and negative controls
according to the manufacturer's instructions. Mouse monoclonal
anti-p53 antibody (1:3,000; DO-7; Dako, Glostrup, Denmark) was used
as the primary antibody. Expression of p53 protein was scored
according to intensity and positive cell proportion. The intensity
was graded into 0, none; 1+, weak; 2+, intermediate; 3+; strong.
The positive cell proportion was semiquantitatively evaluated
according to the estimated percentage of positive tumor cells: 0,
no positive cells; 1, <10% positively-stained cells; 2, 0–33%
positively-stained cells; 3, 33–66% positively-stained cells; 4,
>66% positively-stained cells. The two scores were combined: a
total score of <4+ was considered negative and scores of ≥4+
were considered positive for p53.
DNA extraction
Paraffin-embedded tissue was manually
micro-dissected into 10 µm sections. Genomic DNA was extracted
using the QIAamp DNA mini kit (QIAGEN, Chatsworth, CA, USA)
according to the manufacturer's instructions.
Analysis of BRAF (V600E) mutation by
polymerase chain reaction (PCR)
PCR analysis was performed using Seeplex BRAF
Autocapillary Electrophoresis Detection kits (Seegene, Seoul,
Korea). The PCR reaction mixtures were prepared as follows (total
volume, 20 µl): 4 µl 5X BRAF primer mix, 3 µl extracted DNA (10
ng/µl), 3 µl 8-methoxypsoralen (8-Mop) solution and 10 µl 2X
multiplex master mix (Seegene). Following incubation at 94°C for 15
min, amplification was performed in a 9700 Thermal Cycler (Applied
Biosystems, Foster City, CA, USA), with 35 cycles of denaturation
at 94°C for 30 sec, annealing at 63°C for 30 sec, extension at 72°C
for 60 sec and a final extension at 72°C for 10 min. In order to
confirm the presence and size of the amplified PCR products, 5 µl
was electrophoresed on 2% (wt/vol) agarose gels containing EtBr
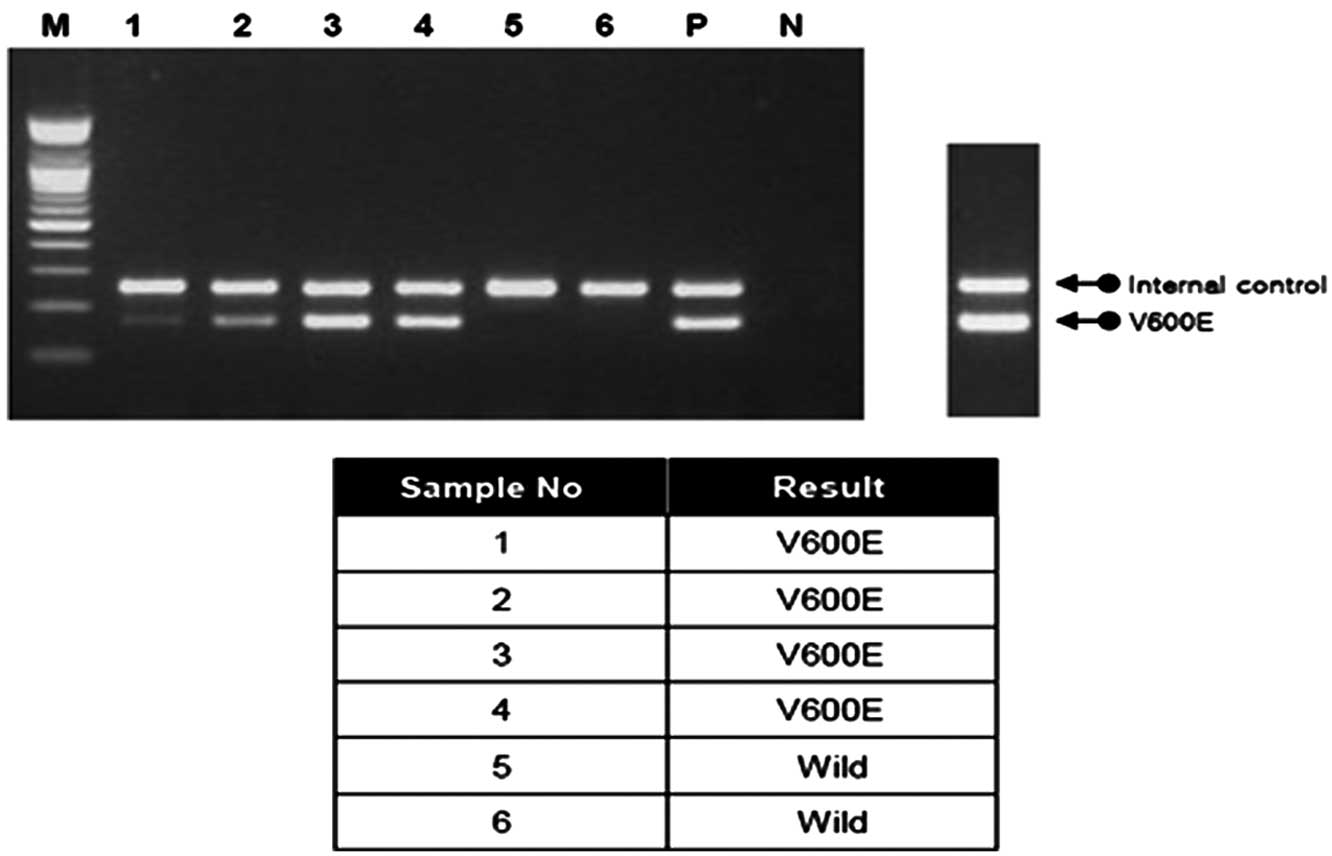
(Seegene, Inc., Seoul, Korea): Wild-type BRAF PCR amplification
results in a 251-bp amplicon (internal control); BRAF (V600E)
results in a 167-bp amplicon (Fig.
1).
For eradicating the template activity of
contaminating DNAs, 8-Mop solution was used, which intercalates
into double-stranded nucleic acids, forming covalent interstrand
cross-links following photoactivation with light of wavelengths
320–400 nm, using a Gel Doc XR+ system (Bio Rad, CA, USA).
Statistical analysis
Values are presented as the mean ± standard error of
the mean. All statistical analyses were performed using SPSS 21.0
software (International Business Machines, Armonk, NY, USA). The
Chi-Squared test was used to analyze categorical data and the
Student's t-test was used to evaluate continuous variables; all
other variables were analyzed using Fisher's exact test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinicopathological features, BRAF
(V600E) status and p53 protein status in PTC
A total of 66 patients were enrolled in the present
study, with 17 males (25.8%) and 49 females (74.2%), with a mean
age of 49.5±10.3 years (range, 31–74 years) at the time of surgery.
A total of 58 patients (87.9%) underwent a total thyroidectomy with
or without neck dissection. Of these 58 patients, 2 patients (3.0%)
underwent concurrent comprehensive neck dissection [level II–VI
(26)], 2 patients underwent level IV
lymph node dissection, 2 patients underwent level II and level III
lymph node dissection, 1 patient underwent level I and level II
lymph node dissection, 1 patient underwent level II and central
lymph dissection, 1 patient underwent level IV and central lymph
node dissection and 35 patients (53.0%) underwent central neck
dissection (CND). In addition, 7 patients (10.6%) underwent
lobectomy with (2 patients) or without (5 patients) CND and 1
patient underwent subtotal thyroidectomy without lymph node
dissection.
Among the 66 cases, 55 cases (83.3%) were diagnosed
as microcarcinoma and the mean tumor size was 8.7 mm ±6.7 (range,
0.1–4.0 cm). 60 cases were classic type and 6 cases were follicular
variant. One of the 6≈follicular variant cases was encapsulated
follicular variant and the remaining 5 cases were diffuse
follicular variant. BRAF (V600E) was identified in 50 cases (75.8%)
and p53 overexpression was detected in 52 cases (78.8%) (Tables I and II).
 | Table I.Associations between the BRAF (V600E)
mutation and clinicopathological features of papillary thyroid
carcinomas. |
Table I.
Associations between the BRAF (V600E)
mutation and clinicopathological features of papillary thyroid
carcinomas.
| Parameter | BRAF mutation +
(%) | BRAF mutation -
(%) | P-value |
|---|
| No. of cases | 50 (75.8) | 16 (24.2) |
|
| Age, years | 49.5±10.4 | 49.3±10.4 | 0.9502 |
| Gender |
|
| 0.7433 |
|
Male | 12 (70.6) | 5 (29.4) |
|
|
Female | 38 (77.6) | 11 (22.4) |
|
| Tumor size
(mm) | 9.1±6.8 | 7.2±6.6 | 0.3272 |
| Multiplicity |
|
| 1.0000 |
|
Present | 19 (76.0) | 6 (24.0) |
|
|
Absent | 31 (75.6) | 10 (24.4) |
|
|
Extrathyroidal-extension |
|
| 0.0661 |
|
Present | 20 (90.9) | 2 (9.1) |
|
|
Absent | 30 (68.2) | 14 (31.8) |
|
 | Table II.Associations between p53 protein
overexpression and clinicopathological features of papillary
thyroid carcinomas. |
Table II.
Associations between p53 protein
overexpression and clinicopathological features of papillary
thyroid carcinomas.
| Parameter | p53 overexpression
+ (%) | p53 overexpression
- (%) | P-value |
|---|
| No. of cases | 52 (78.8) | 14 (21.2) |
|
| Age (years) | 49.7±10.1 | 48.6±11.5 | 0.7217 |
| Gender |
|
| 1.0000 |
|
Male | 14 (82.4) | 3 (17.6) |
|
|
Female | 38 (77.6) | 11 (22.4) |
|
| Tumor size
(mm) | 9.1±6.7 | 7.0±6.9 | 0.3007 |
| Multiplicity |
|
| 1.0000 |
|
Present | 20 (80.0) | 5 (20.0) |
|
|
Absent | 32 (78.0) | 9 (22.0) |
|
|
Extrathyroidal-extension |
|
| 1.0000 |
|
Present | 17 (77.3) | 5 (22.7) |
|
|
Absent | 35 (79.5) | 9 (20.5) |
|
Associations between BRAF (V600E)
status and clinicopathological features of PTC
As shown in Table I,
among the 50 patients with the BRAF (V600E) mutation, 12 were males
(24%) and 38 were females (76%), with a mean age of 49.5±10.4
years. The mean tumor size was 9.1±6.8 mm. Multiplicity and
extrathyroidal extension were present in 19 (38%) and 20 (40%) out
of 50 cases, respectively.
Among the 16 patients without the BRAF (V600E)
mutation, 5 were males (31.3%) and 11 were females (68.7%), with a
mean age of 49.3±10.4 years. The mean tumor size was 7.2±6.6 mm.
Multiplicity and extrathyroidal extension were present in 6 (37.5%)
and 2 (12.5%) of the 16 cases, respectively (Table I).
As shown in Table I,
none of the parameters exhibited a significant correlation with the
BRAF (V600E) mutation (P>0.05); however, there was a notable,
but non-significant, association between extrathyroidal extension
and the BRAF (V600E) mutation (P=0.0661).
Associations between p53 protein
overexpression and clinicopathological features of PTC
As shown in Table II,
among the 52 patients who exhibited p53 protein overexpression, 14
were males (26.9%) and 38 were females (73.1%), with a mean age of
49.7±10.1 years and a mean tumor size of 9.1±6.7 mm. Multiplicity
and extrathyroidal extension were present in 20 (38.5%) and 17
(32.7%) out of 52 cases, respectively.
Among the 14 patients who demonstrated no p53
protein overexpression, 3 were males (21.4%) and 11 were females
(78.6%), with a mean age of 48.6±11.5 years and a mean tumor size
of 7.0±6.9 mm. Multiplicity and extrathyroidal extension were each
present in 5 out of 14 (35.7%) cases (Table II).
As shown in Table II,
none of the clinicopathological parameters demonstrated a
significant correlation with p53 protein overexpression.
Associations between lymph node
metastasis and clinicopathological parameters of PTC
As shown in Table II,
among the 14 patients with lymph node metastasis, 4 were males
(28.6%) and 10 were females (71.4%), with a mean age of 47.4±10.0
years and a mean tumor size of 9.8±7.0 mm. Multiplicity and
bilaterality were present in 3 (21.4%) and 0 of the 14 cases,
respectively. Extrathyroidal extension and lymphovascular invasion
were present in 6 (42.9%) and 0 of the 14 cases, respectively. In
addition, p53 overexpression and the BRAF (V600E) mutation were
present in 10 (71.4%) and 11 (78.6%) of the cases,
respectively.
As shown in Table
III, only one parameter, bilaterality was demonstrated to be
significantly associated with lymph node metastasis (P=0.0280).
 | Table III.Associations between lymph node
metastasis and clinicopathological parameters of papillary thyroid
carcinomas. |
Table III.
Associations between lymph node
metastasis and clinicopathological parameters of papillary thyroid
carcinomas.
| Parameter | Lymph node
metastasis + (%) | Lymph node
metastasis - (%) | P-value |
|---|
| No. of cases | 14 (21.2) | 52 (78.8) |
|
| Age (years) | 47.4±10.0 | 50.0±10.4 | 0.3964 |
| Gender |
|
| 0.7441 |
|
Male | 4 (23.5) | 13 (76.5) |
|
|
Female | 10 (20.4) | 39 (79.6) |
|
| Tumor size
(mm) | 9.8±7.0 | 8.4±6.7 | 0.4881 |
| Multiplicity |
|
| 0.2182 |
|
Present | 3 (12.0) | 22 (88.0) |
|
|
Absent | 11 (26.8) | 30 (73.2) |
|
| Bilaterality |
|
| 0.0280 |
|
Present | 0 (0.0) | 15 (100.0) |
|
|
Absent | 14 (27.5) | 37 (72.5) |
|
|
Extrathyroidal-extension |
|
| 0.5244 |
|
Present | 6 (27.3) | 16 (72.7) |
|
|
Absent | 8 (18.2) | 36 (81.8) |
|
|
Lymphovascular-invasion |
|
| – |
|
Present | 0 (0.0) | 0 (0.0) |
|
|
Absent | 14 (21.2) | 52 (78.8) |
|
| p53
overexpression |
|
| 0.4734 |
|
Present | 10 (19.2) | 42 (80.8) |
|
|
Absent | 4 (28.6) | 10 (71.4) |
|
| BRAF mutation |
|
| 1.0000 |
|
Present | 11 (22.0) | 39 (78.0) |
|
|
Absent | 3 (18.7) | 13 (81.3) |
|
Association between BRAF (V600E)
mutation and p53 protein overexpression in PTC
Among the 50 patients with the BRAF (V600E)
mutation, 42 patients exhibited p53 protein overexpression (84%).
In addition, among the 16 patients without BRAF (V600E) mutation,
10 patients demonstrated p53 protein overexpression (62.5%)
(Table IV). Therefore, there was a
notable, but not a statistically significant, correlation between
the BRAF (V600E) mutation and p53 protein overexpression
(P=0.0854).
 | Table IV.Associations between BRAF (V600E)
mutation and p53 protein overexpression in papillary thyroid
carcinomas. |
Table IV.
Associations between BRAF (V600E)
mutation and p53 protein overexpression in papillary thyroid
carcinomas.
| Parameter | BRAF mutation +
(%) | BRAF mutation -
(%) | P-value |
|---|
| No. of cases | 50 (75.8) | 16 (24.2) |
|
| p53
overexpression |
|
| 0.0854 |
|
Present | 42 (80.8) | 10 (19.2) |
|
|
Absent | 8
(57.1) | 6
(42.9) |
|
Discussion
BRAF is the most prevalent type of genetic
alteration in thyroid cancer and has been widely investigated
(27). In thyroid cancer, BRAF was
reported to be activated via certain point mutations, including a
nucleotide position 1799 substitution of thymine to adenine, which
subsequently results in the replacement of valine with glutamate at
residue 600 (V600E). Out of all the BRAF mutations that may occur
in thyroid cancer, the BRAF (V600E) mutation accounts for >90%.
The incidence rate of the BRAF (V600E) mutation varies greatly,
ranging from 29 to 83% in PTC. The reason for this variation is
unclear, but it is suggested that geographic, genetic factors, or
other factors may account for these differences (9). In addition, it was reported that in
Korean populations, the BRAF (V600E) mutation in PTC was markedly
more prevalent at 52–83% compared with 30–49% in other countries
(9). It has not been determined why
this variation occurs, although it was suggested that geographic or
genetic factors may be involved (9).
BRAF has been identified in classic papillary and tall cell thyroid
cancer as well as in ~1 out of 3 cases of poorly differentiated and
anaplastic thyroid carcinomas (27).
The results of the present study determined the prevalence of the
BRAF (V600E) mutation to be 75.8%.
Numerous studies have evaluated the association
between the BRAF (V600E) mutation, clinicopathological features and
clinical outcomes of PTC; however, controversial results have been
obtained for a correlation between the BRAF (V600E) mutation and
clinicopathological features (1,9–20). Furthermore, multiple previous studies
have demonstrated a correlation between the BRAF (V600E) mutation
and the high-risk clinicopathological characteristics of PTC,
including older age at diagnosis, male gender, large tumor size,
the presence of extrathyroidal extension, lymph node and distant
metastasis and an advanced stage (9–11,13,15,16,18–20,28,29).
By contrast, certain studies were unable to identify marked
associations between the BRAF (V600E) mutation and the high-risk
clinicopathological characteristics of PTC (12,14). The
reason for this variation is unclear, but it may be that geographic
or genetic factors may account for these differences. However, the
variation in these results is also considered to be the result of a
lack of prospective studies that may reduce the selection bias,
small study population sizes, the absence of multivariate analysis
and the heterogeneous histological subtypes within PTC (9)
The results of the present study did not demonstrate
any significant correlations between the BRAF (V600E) mutation and
the clinicopathological features of PTC, including age, gender,
tumor size, multiplicity, lymph node metastasis and extra thyroidal
extension. However, extrathyroidal extension exhibited a borderline
correlation with the BRAF (V600E) mutation (P=0.0661).
The tumor suppressor gene p53 encodes a DNA-binding
protein that has important functions in cell cycle arrest, DNA
repair, differentiation and apoptosis. p53 gene mutations have been
observed in ~50% of the human cancers and are one of the most
prevalent types of genetic modifications identified in malignant
cells; in addition, these mutations primarily occur in exons
(3,4,5,7,8,23). In the thyroid gland, mutations of the
p53 gene were reported to occur in 40–62% of undifferentiated
carcinomas and 0–25% in well-differentiated carcinomas (4,8). The
overexpression of p53, as determined by immunohistochemistry was
suggested to attributed to the mutation of a p53 gene in up to 95%
of PTC cases (5). p53 protein
overexpression was reported to have an incidence rate of between 11
and 59% (4,21,23).
Due to its short half-life, wild-type p53 protein is
undetectable; however, mutated p53 exhibits greater stability and a
prolonged half-life (24). Therefore,
it was previously hypothesized that immunohistochemistry was only
able to detect mutated p53. By contrast, it has been reported that
the overexpression of p53 may not always be attributed to gene
mutations, as wild-type overexpression may occur due to factors
that have not yet been elucidated and may provide a protection
mechanism against tumors (5,23). In addition, mutations of p53 were
proposed to induce the expression of abnormal proteins or lead to a
deficient expression of p53 (23).
In thyroid cancers, it was suggested that the
presence of p53, as detected by immunohistochemistry, was
associated with the occurrence of p53 gene mutations (5); however, the detection of p53 protein
expression was also reported in differentiated papillary and
follicular thyroid carcinomas regardless of the occurrence of p53
gene mutations; of note, the incidence rate of p53 protein
overexpression in PTC was reported to be between 11 and 59%
(4,21–25).
Certain studies regarding the immunohistochemical
analysis of p53 expression have demonstrated that p53
overexpression may act as a significant and independent prognostic
indicator for differentiated thyroid carcinomas (3,4,7,22,24. However, this association has provided
controversial results (3,5,21,30,31. Morita
et al (4) reported a
significant correlation among p53 protein expression in primary
tumors, larger tumors, the presence of lymph node metastasis and
the mean number of lymph node metastases (4). Furthermore, Horie et al (7) indicated that p53 protein overexpression
had a marked correlation with large tumor size and the occurrence
of capsular invasion (7). However,
several studies have reported no significant associations between
p53-positive tumor cells and clinicopathological data (5,6,21,30,31.
In the present study, no significant correlations
were identified between p53 protein overexpression and
clinicopathological features of PTC, including age, gender, tumor
size, multiplicity, lymph node metastasis and extrathyroidal
extension. Limitations of the present study included an
insufficient number of prospective studies to reduce selection
bias, a relatively small population size and the lack of
multivariate analysis.
In conclusion, the results of the present study
revealed that the BRAF (V600E) mutation and overexpression of p53
were not significantly correlated with clinicopathological features
of PTC; however, the BRAF (V600E) mutation demonstrated a notable,
but non-significant, association with p53 overexpression (P=0.0854)
and extrathyroidal extension (P=0.0661). In addition, a significant
correlation was observed between lymph node metastasis and
bilaterality (P=0.0280). Further prospective studies are required,
with a larger study population in order to determine the exact role
of the BRAF (V600E) mutation and p53 protein overexpression in the
clinicopathological significance of PTC.
References
|
1
|
Koperek O, Kornauth C, Capper D, Berghoff
AS, Asari R, Niederle B, von Deimling A, Birner P and Preusser M:
Immunohistochemical detection of the BRAF V600E-mutated protein in
papillary thyroid carcinoma. Am J Surg Pathol. 36:844–850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng X, Wei S, Han Y, Li Y, Yu Y, Yun X,
et al: Papillary microcarcinoma of the thyroid: Clinical
characteristics and BRAF (V600E) mutational status of 977 cases.
Ann Surg Oncol. 20:2266–2273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balta AZ, Filiz AI, Kurt Y, Sucullu I,
Yucel E and Akin ML: Prognostic value of oncoprotein expressions in
thyroid papillary carcinoma. Med Oncol. 29:734–741. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morita N, Ikeda Y and Takami H: Clinical
significance of p53 protein expression in papillary thyroid
carcinoma. World J Surg. 32:2617–2622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zafon C, Obiols G, Castellvi J, Tallada N,
Baena JA, Simo R, et al: Clinical significance of RET/PTC and p53
protein expression in sporadic papillary thyroid carcinoma.
Histopathology. 50:225–231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamzany Y, Soudry E, Strenov Y, Lipschitz
N, Segal K, Hadar T, et al: Early death from papillary thyroid
carcinoma. Am J Otolaryngol. 33:104–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horie S, Maeta H, Endo K, Ueta T,
Takashima K and Terada T: Overexpression of p53 protein and MDM2 in
papillary carcinomas of the thyroid: Correlations with
clinicopathologic features. Pathol Int. 51:11–15. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parameswaran R, Brooks S and Sadler GP:
Molecular pathogenesis of follicular cell derived thyroid cancers.
Int J Surg. 8:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SJ, Lee KE, Myong JP, Park JH, Jeon
YK, Min HS, et al: BRAF V600E mutation is associated with tumor
aggressiveness in papillary thyroid cancer. World J Surg.
36:310–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KH, Kang DW, Kim SH, Seong IO and Kang
DY: Mutations of the BRAF gene in papillary thyroid carcinoma in a
Korean population. Yonsei Med J. 45:818–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Musholt TJ, Schonefeld S, Schwarz CH,
Watzka FM, Musholt PB, Fottner C, et al: Impact of pathognomonic
genetic alterations on the prognosis of papillary thyroid
carcinoma. ESES vienna presentation. Langenbecks Arch Surg.
395:877–883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn D, Park JS, Sohn JH, Kim JH, Park SK,
Seo AN and Park JY: BRAF (V600E) mutation does not serve as a
prognostic factor in Korean patients with papillary thyroid
carcinoma. Auris Nasus Larynx. 39:198–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricarte-Filho J, Ganly I, Rivera M, Katabi
N, Fu W, Shaha A, et al: Papillary thyroid carcinomas with cervical
lymph node metastases can be stratified into clinically relevant
prognostic categories using oncogenic BRAF, the number of nodal
metastases and extra-nodal extension. Thyroid. 22:575–584. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurt B, Yalcin S, Alagoz E, Karslıoğlu Y,
Yigit N, Gunal A, et al: The relationship of the BRAF (V600E)
Mutation and the established prognostic factors in papillary
thyroid carcinomas. Endocr Pathol. 23:135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prescott JD, Sadow PM, Hodin RA, Le LP,
Gaz RD, Randolph GW, et al: BRAF V600E status adds incremental
value to current risk classification systems in predicting
papillary thyroid carcinoma recurrence. Surgery. 152:984–990. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elisei R, Viola D, Torregrossa L, Giannini
R, Romei C, Ugolini C, et al: The BRAF (V600E) mutation is an
independent, poor prognostic factor for the outcome of patients
with low-risk intrathyroid papillary thyroid carcinoma:
single-institution results from a large cohort study. J Clin
Endocrinol Metab. 97:4390–4398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Daglar-Aday A, Toptas B, Ozturk T, Seyhan
F, Saygili N, Eronat A, et al: Investigation of BRAF V600E mutation
in papillary thyroid carcinoma and tumor-surrounding nontumoral
tissues. DNA Cell Biol. 32:13–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeong D, Jeong Y, Park JH, Han SW, Kim SY,
Kim YJ, et al: BRAF (V600E) mutation analysis in papillary thyroid
carcinomas by peptide nucleic acid clamp real-time PCR. Ann Surg
Oncol. 20:759–766. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brahma B, Yulian ED, Ramli M, Setianingsih
I, Gautama W, Brahma P, et al: Surgical perspective of T1799A BRAF
mutation diagnostic value in papillary thyroid carcinoma. Asian Pac
J Cancer Prev. 14:31–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, et al: Association between BRAF V600E
mutation and mortality in patients with papillary thyroid cancer.
JAMA. 309:1493–1501. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gauchotte G, Philippe C, Lacomme S,
Leotard B, Wissler MP, Allou L, et al: BRAF, p53 and SOX2 in
anaplastic thyroid carcinoma: evidence for multistep
carcinogenesis. Pathology. 43:447–452. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park KY, Koh JM, Kim YI, Park HJ, Gong G,
Hong SJ, et al: Prevalences of Gs alpha, ras, p53 mutations and
ret/PTC rearrangement in differentiated thyroid tumours in a Korean
population. Clin Endocrinol (Oxf). 49:317–323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu MC and Gelmann EP: P53 gene mutations:
case study of a clinical marker for solid tumors. Semin Oncol.
29:246–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omar E, Madhavan M and Othman NH:
Immunohistochemical localisation of RET and p53 mutant protein of
thyroid lesions in a North-Eastern Malaysian population and its
prognostic implications. Pathology. 36:152–159. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dellis RA, Lloyd RV, Heitz U and Eng C:
Tumours of endocrine organsPathology and Genetics. International
Agency for Research on Cancer Press; Lyon: pp. 57–66. 2004
|
|
26
|
Mitzner R: Neck Dissection Classification.
http://emedicine.medscape.com/article/849834-overviewJune
9th–2015
|
|
27
|
Witt RL, Ferris RL, Pribitkin EA, Sherman
SI, Steward DL and Nikiforov YE: Diagnosis and management of
differentiated thyroid cancer using molecular biology.
Laryngoscope. 123:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Min HS, Lee C and Jung KC: Correlation of
immunohistochemical markers and BRAF mutation status with
histological variants of papillary thyroid carcinoma in the Korean
population. J Korean Med Sci. 28:534–541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith RA, Salajegheh A, Weinstein S,
Nassiri M and Lam AK: Correlation between BRAF mutation and the
clinicopathological parameters in papillary thyroid carcinoma with
particular reference to follicular variant. Hum Pathol. 42:500–506.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cvejic D, Selemetjev S, Savin S, Paunovic
I, Petrovic I and Tatic S: Apoptosis and proliferation related
molecules (Bcl-2, Bax, p53, PCNA) in papillary microcarcinoma
versus papillary carcinoma of the thyroid. Pathology. 40:475–480.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karlidag T, Cobanoglu B, Keles E, Alpay
HC, Ozercan I, Kaygusuz I, et al: Expression of Bax, p53 and
p27/kip in patients with papillary thyroid carcinoma with or
without cervical nodal metastasis. Am J Otolaryngol. 28:31–36.
2007. View Article : Google Scholar : PubMed/NCBI
|