Introduction
Hematopoiesis is a complex process that generates
multiple lineages of various blood cell types with distinct
functions (1). The hematopoietic stem
cells (HSCs) constantly renew themselves to prevent exhaustion of
the stem cell pool (2). Transcription
factors play a pivotal role in this orchestrated process by
manipulating the expression of lineage-specific genes. The ETS
family member PU.1, encoded by SPI1, is one of the most important
regulators involved in normal hematopoiesis, particularly in
myeloid differentiation, as PU.1 regulates the expression of almost
all myeloid genes, including granulocyte macrophage
colony-stimulating factor (CSF) receptor α (GM-CSFRα), macrophage
CSF receptor (M-CSFR) and granulocyte CSF receptor (G-CSFR)
(3–5).
PU.1 is required for the commitment and maturation of myeloid
lineages, and the expression of PU.1 increases during granulocytic
and monocytic differentiation (6,7). Previous
studies have revealed that the introduction of PU.1 at high levels
induced macrophage differentiation in primary fetal liver
progenitors. Inversely, deficient PU.1 expression severely impaired
hematopoietic development or led to leukemia (8). PU.1 knockout mice present an early block
in myeloid differentiation and lack of mature myeloid cells
(6), while graded reduction in PU.1
expression to 20% of wild-type expression has been demonstrated to
induce acute myeloid leukemia (AML) in all mice (9). Accordingly, altered PU.1 function is
possibly involved in leukemogenesis, as the PU.1 gene mutation has
been described in certain patients with AML (10). Furthermore, certain oncogenic fusion
proteins, such as AML1-eight twenty-one and promyelocytic
leukemia-retinoic acid receptor α, are also associated with PU.1
inhibition (11–14). Thus, as a tumor suppressor, PU.1
expression facilitates commitment to myeloid differentiation and
its downregulation may be crucial in the pathogenesis of AML.
However, PU.1 exerts various functions at distinct
hematopoietic stages. Although PU.1 is expressed at low levels in
the early stage of hematopoiesis, it has an indispensable function
in the maintenance of the HSC pool (6,15). Loss of
PU.1 expression in mice leads to a weakening in the self-renewal
capacity of long-term HSCs, which are then outcompeted by normal
HSCs in bone marrow (8). This role of
PU.1 provides insights into possible processes occurring in
leukemia stem cells. Previous studies have reported that PU.1 is
also required for the initiation and maintenance of AML stem cells
induced by monocytic leukemia zinc-finger protein fusion proteins
(4). Similarly, PU.1 is the immediate
cause for maintaining the leukemic phenotype in MEL cells by
promoting repopulation of transformed erythroblastic cells and
blocking the terminal differentiation program towards erythrocytes,
which are also reversed by downregulation of the expression of PU.1
(6). Collectively, PU.1 is crucial
for not only lineage differentiation, but also the leukemic
process.
Previous studies have revealed that PU.1 is
essential for leukemia harboring mixed lineage leukemia (MLL) gene
rearrangements (16), which is
characterized by high expression of the homeobox oncogene MEIS1
(17). In addition, there is a
positive association between the expression of PU.1 and MEIS1 in
MLL patients, and the regulation of MEIS1 by PU.1 is central to the
pathogenesis of leukemia harboring MLL rearrangements (16). However, the function of PU.1 and its
mechanism in non-MLL remains unclear. In the present study, in
order to investigate the role of PU.1 in acute myeloid not
harboring MLL rearrangements, the human acute myeloid leukemia U937
cell line was selected, as this cell line exhibits a relative
higher expression level of endogenous MEIS1, compared to the other
two non-MLL cell lines. Our current work reveals the regulatory
function and molecular mechanism of PU.1, which facilitates the
development of targeted therapies with potential to correct the
inappropriate MEIS1 expression for non-MLL leukemia.
Materials and methods
Cell cultures and transfection
U937 cells (American Type Culture Collection,
Manassas, VA, USA) were maintained in RPMI-1640 with 10% fetal
bovine serum (FBS). 293T cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's
medium supplemented with 10% FBS. PU.1 siRNA (accession no.,
NM_003120; catalog nos., SASI_Hs02_00335096, SASI_Hs02_00335097 and
SASI_Hs02_00335098) and MEIS1 siRNA (accession no., NM_002398;
catalog no., SASI_Hs01_00088989) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). The cells were transfected with siRNA using
X-treme GENE siRNA (Roche, Indianapolis, IN, USA), according to the
manufacturer's instructions.
Viable cell count
A total of 45 µl single cell suspension was mixed
with 5µl Trypan Blue (0.4%; Invitrogen Life Technologies, Carlsbad,
CA, USA) and incubated for 5 min at room temperature. Next, the
unstained (viable) cells were counted using a hemocytometer
(Hausser Scientific, Horsham, PA, USA) under a light microscope
(Professional Infinity Planachromatic Binocular Upright Microscope;
VWR, Philadelphia, PA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen Life Technologies), according to the
manufacturer's instructions. Reverse transcription was performed
according to the manufacturer's instructions (Promega, San Luis
Obispo, CA, USA). qPCR was performed using SYBR Green qPCR Master
mix (Fermentas, Pittsburgh PA, USA) on a MyiQ thermocycler
(Bio-Rad, Hercules, CA, USA). PGK, a housekeeping gene with
constitutive expression, was used as an internal control to
normalize the RNA level. The primer sequences used are listed in
Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Direction | Sequence | Usage |
|---|
| MEIS1 promoter
upstream | F |
5′-TAAGACGCGACCTGTTATGGC-3′ | ChIP-qPCR |
|
| R |
5′-CCAGAATGCTAGAACCCGGA-3′ | ChIP-qPCR |
| MEIS1 promoter | F |
5′-GCATTGTGTAAGACGCGACCTG-3′ | ChIP-qPCR |
|
| R |
5′-CGACCAGAATGCTAGAACCCGGAAG-3′ | ChIP-qPCR |
| MEIS1 intron 1 | F |
5′-TGCTGACATACAGCGATCCC-3′ | ChIP-qPCR |
|
| R |
5′-CACTCACACTGGCAGGCTTG-3′ | ChIP-qPCR |
| MEIS1 intron 2 | F |
5′-TCAGGATGCAATGGTGAGCA-3′ | ChIP-qPCR |
|
| R |
5′-TAAGGCCCTCATCACTCCCA-3′ | ChIP-qPCR |
| PGK1 | F |
5′-AGAGCCCAGAGCGACCCTT-3′ | RT-PCR |
|
| R |
5′-AAAAGCCATTCCACCACCAAT-3′ | RT-PCR |
| MEIS1 | F |
5′-ATGTGACAATTTCTGCCACCG-3′ | RT-PCR |
|
| R |
5′-CCTGAACGAGTAGATGCCGTG-3′ | RT-PCR |
| PU.1 | F |
5′-GAGCCCCCCACTGGAGGT-3′ | RT-PCR |
|
| R |
5′-TGGTACAGGCGGATCTTCTTCT-3′ | RT-PCR |
| GFI1 | F |
5′-GAGCCTGGAGCAGCACAAAG-3′ | RT-PCR |
|
| R |
5′-TCCCACAGATCTTACAGTCAAAGC-3′ | RT-PCR |
| WT Probe | F |
5′-CCACTACTTCCGGGTTCTAGC-3′ | EMSA |
|
| R |
5′-GCTAGAACCCGGAAGTAGTGG-3′ | EMSA |
| MT Probe | F |
5′-CCACTACGCGAGGGTTCTAGC-3′ | EMSA |
|
| R |
5′-GCTAGAACCCTCGCGTAGTGG-3′ | EMSA |
Chromatin immunoprecipitation
(ChIP)
The cells were cross-linked with 1% formaldehyde for
10 min at room temperature, and the reaction was subsequently
stopped with 0.125 M glycine. The cells were washed with
phosphate-buffered saline and then lysed in cell lysis buffer. The
nuclei were recovered by centrifugation and then lysed in nuclear
lysis buffer. Chromatin was sonicated and precleared overnight with
50 µl of rabbit immunoglobulin (Ig)G (catalog no., sc-3888; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and 40 µl of protein
A/G-agarose (Invitrogen Life Technologies). Precleared lysate was
incubated with 5 µg of purified rabbit anti-PU.1 antibody (catalog
no., sc-352X; Santa Cruz Biotechnology, Inc.). An aliquot of
precleared lysate (10%) was reserved as input. Immunoprecipitates
were washed and eluted with 100 mM NaHCO3 and 1% SDS.
Cross-links were reversed at 65°C for 12 h. RNA and protein were
digested with RNase A and proteinase K. Isolated DNA was purified
by MinElute Reaction Cleanup kit (Qiagen, Valencia, CA, USA). The
amount of purified DNA was subjected to qPCR using SYBR Green
Master Mix (Applied Biosystems, Grand Island, NY, USA). The data
are shown as fold enrichment over input DNA. The primer sequences
were listed in Table I.
Electrophoretic mobility shift assay
(EMSA)
In total, 107 cells were harvested and
resuspended with 400 µl cold buffer A, which consisted of 10 mM
Hepes (pH 7.9), 10 mM KCl, 1.5 mM MgCl2, 0.5 mM
phenylmethylsulfonyl fluoride (PMSF) and 0.5 mM DTT. Subsequent to
being maintained on ice for 10 min, the cell suspension was
centrifuged with 4,000 × g for 10 sec and the supernatant fraction
was discarded. The pellet cells were resuspended in 80 µl cold
buffer B, which consisted of 20 mM Hepes (pH 7.9), 25% glycerol,
0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM PMSF and
0.5 mM DTT, in a 1.5 ml eppendorf tube and incubated on ice for 20
min for high-salt extraction. Cellular debris was removed by
centrifugation at top speed (12,500 × g) for 30 min at 4°C, and the
supernatant was reserved as nuclear extract.
Double-stranded probes were generated by annealing
the following oligomers to their respective complementary
sequences: Wild-type, 5′-CCACTACTTCCGGGTTCTAGC-3′; and point
mutated, 5′-CCACTACGCGAGGGTTCTAGC-3′. Electrophoretic mobility
shift assay (EMSA) was performed using the Lightshift
Chemiluminescent EMSA kit (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA), according to the manufacturer's instructions.
For supershift bands, the same rabbit IgG or rabbit anti-PU.1
antibody were added to the EMSA reaction.
Luciferase reporter assay
The U937 cells were cultured in 12 well plates and
transfected with 0.2 µg of luciferase reporter plasmids (pGL3 or
pGL3-wild type MEIS1 promoter or pGL3-mutated MEIS1 promoter) using
Fugene HD (Roche), following the manufacturer's instructions.
Plasmid pCMV-LacZ was co-transfected as an internal control. The
activity of β-galactosidase and luciferase was measured 48 h
subsequent to transfection using Galacto-Light Plus (Applied
Biosystems) and luciferase assay system (Promega), respectively.
The luciferase activity of each sample was normalized to the
β-galactosidase. The transfection was performed in triplicate wells
and replicated with similar results in three independent
experiments.
Results
PU.1 and MEIS1 each play a crucial
role in the proliferation of human AML U937 cells
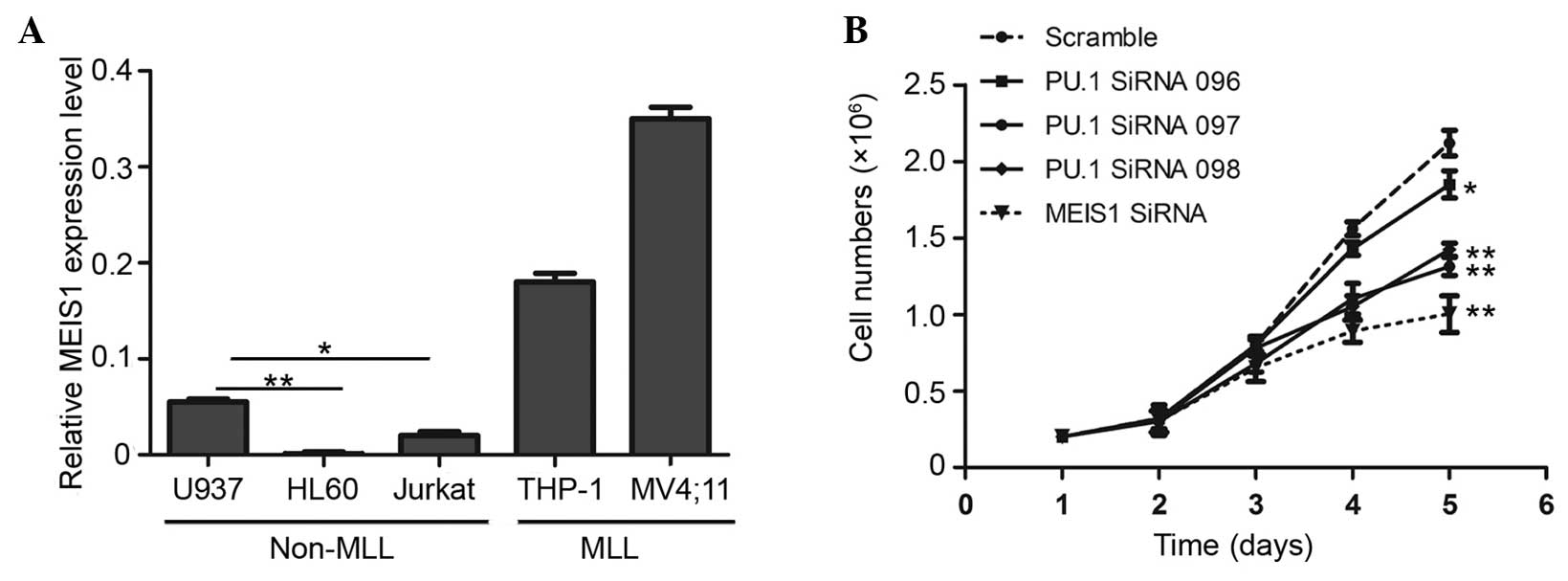
High expression of MEIS1 is one of the
characterizations of leukemia harboring MLL gene rearrangements,
whereas a limited expression level is generally demonstrated in
leukemia without MLL gene fusion (17). In the present study, a panel of
leukemic cell lines was initially compared using RT-qPCR, and the
human AML U937 cell line was selected to explore the function of
PU.1 and MEIS1 in leukemia without MLL gene rearrangements, as this
cell line demonstrated a relatively increased expression of MEIS1
compared with the two other non-MLL cell lines (Fig. 1A). In the present study, which aimed
to investigate the biological effects, three PU.1 short interfering
sequences and one MEIS1 short interfering sequence were applied to
knock down the expression of PU.1 and MEIS1. Subsequently, trypan
blue staining and cell counting were used to assess the number of
viable cells at 1–5 days after transfection. As shown in Fig. 1B, MEIS1 knock down markedly inhibited
the rate of cell growth after 3 days, compared with the cells
transduced with scrambled control siRNA. Notably, PU.1 exerted the
similar function as suppressed cell proliferation with a one-day
delay. These suggest that PU.1 and MEIS1 are each required for cell
maintenance and MEIS1 may be a downstream gene of PU.1 in non-MLL
leukemia.
Deregulation of MEIS1 upon loss of
PU.1 expression in the human U937 cell line
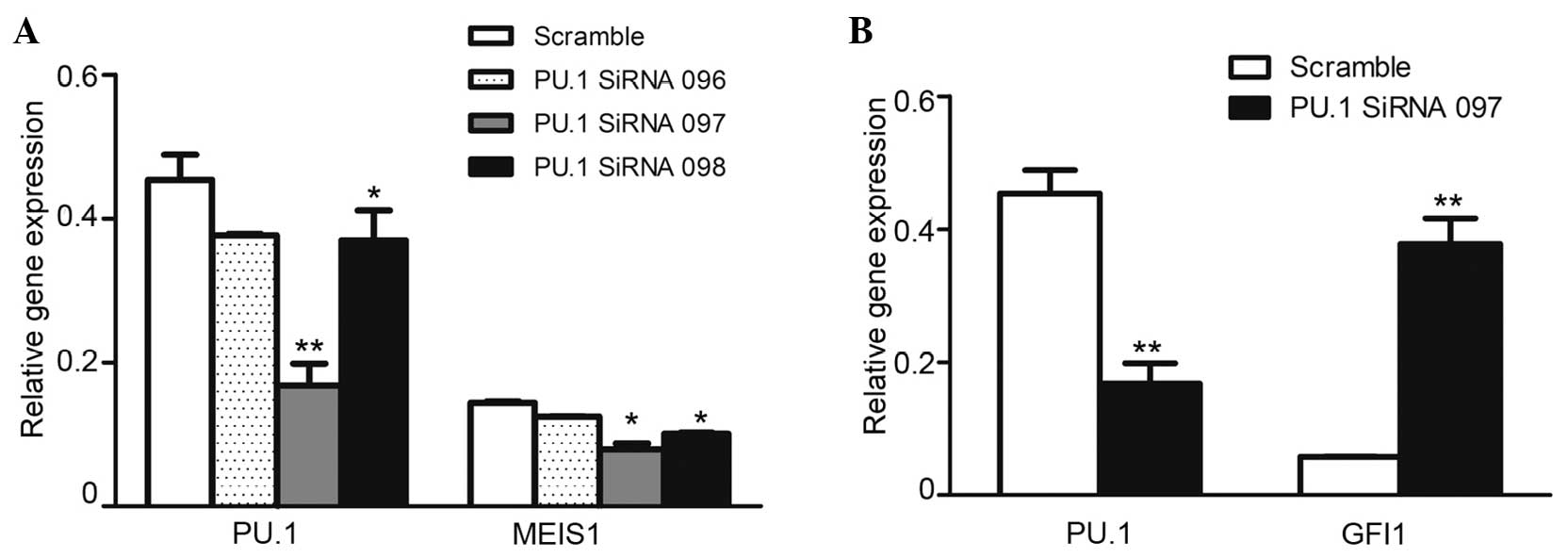
In order to confirm the regulatory function of PU.1
on MEIS1, the U937 cells were transfected with PU.1 siRNA. The
RT-PCR results revealed that the knockdown was efficient, with a
50–80% reduction of PU.1 RNA expression in the cells transfected
with PU.1 siRNA compared with the cells transfected with scrambled
control siRNA. As expected, the downregulated PU.1 significantly
inhibited leukemia oncogene MEIS1 expression in U937 cells
(Fig. 2A). However, the expression of
Gfi1, a well-known agonist gene of PU.1 (18), was markedly increased (Fig. 2B), and was considered to be the
PU.1-knockdown experiment monitor control. The present data
identified that PU.1 was positively involved in MEIS1 transcription
in U937 cells.
PU.1 protein is enriched by the MEIS1
promoter locus in vivo
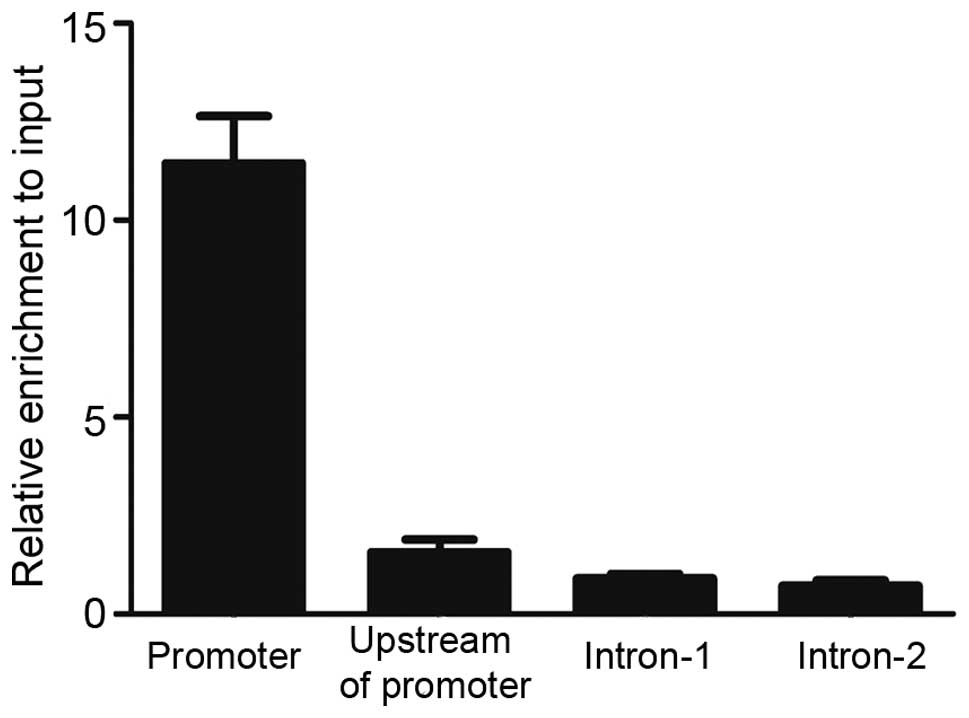
To understand the mechanism of transcriptional
regulation of MEIS1 by PU.1, evolutionary conserved genomic
sequences of the MEIS1 promoter (hg19 version) were identified
using the UCSC Genome Browser (Genome Bioinformatics Group,
University of California Santa Cruz, Santa Cruz, CA, USA). To test
the possible recruitment of PU.1 to this conserved promoter region
in vivo, ChIP-qPCR primers were designed to amplify various
locations. As Fig. 3 revealed, marked
enrichment of PU.1 at the promoter region of MEIS1 was detected,
but no visible binding in upstream of the promoter and two intron
regions were found. PU.1 significantly bound to the MEIS1 promoter
region with ~10 fold enrichment over input DNA, indicating that
MEIS1 may be directly regulated by PU.1 in the U937 cell line.
Predicted PU.1 binding site is
essential for MEIS1 promoter activity
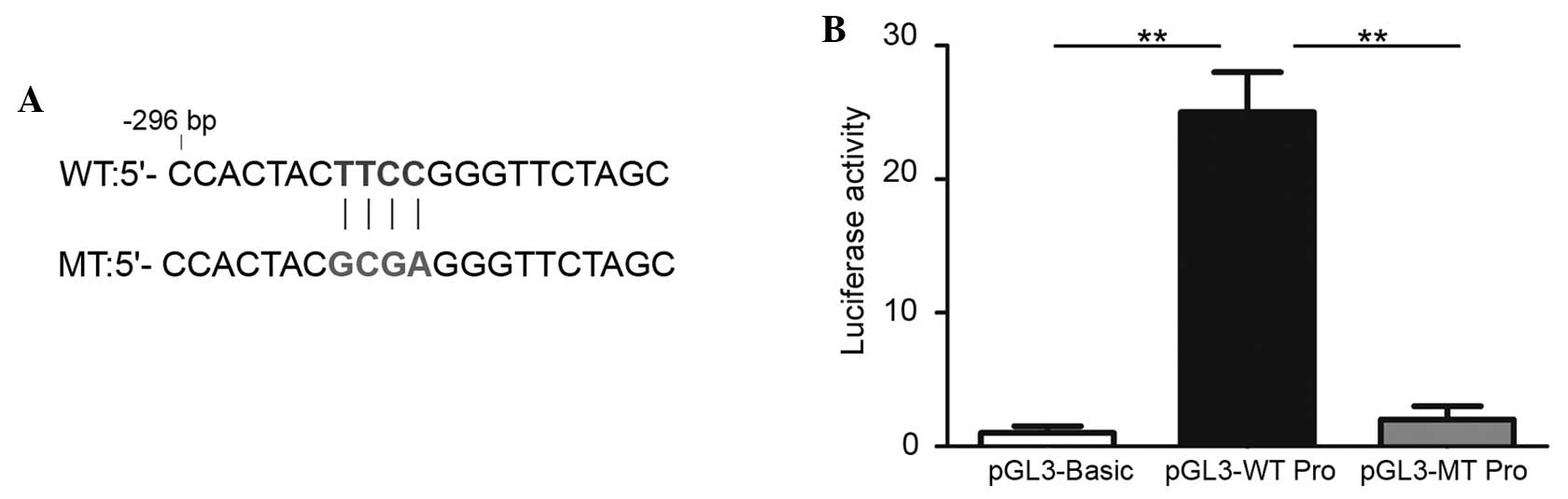
To identify whether the PU.1 binding region is of
functional importance, the MEIS1 promoter (898 bp upstream to 2 bp
downstream of transcription start site) was cloned into the
pGL3-basic vector. Additional point mutation was performed by PCR
mutagenesis (CTTCCG to CGCGAG) (Fig.
4A), based on the locus of the PU.1 enrichment peak determined
by the present ChIP-qPCR data. The luciferase reporter assay was
then performed in U937 cells transfected with pGL3-basic vectors
inserted with the wild-type or mutated MEIS1 promoter region. The
results revealed that the wild-type promoter was able to evidently
increase the downstream luciferase activity by 30-fold (Fig. 4B), compared with the empty pGl3-basic
vector. By contrast, mutating the PU.1 binding site entirely
reduced this increase of the promoter activity. These data reveal
that this binding site contributes strongly to the activity of the
MEIS1 promoter and indicate that a key transcription factor exists
at this promoter locus.
Functional MEIS1 promoter binding site
is occupied by PU.1 protein
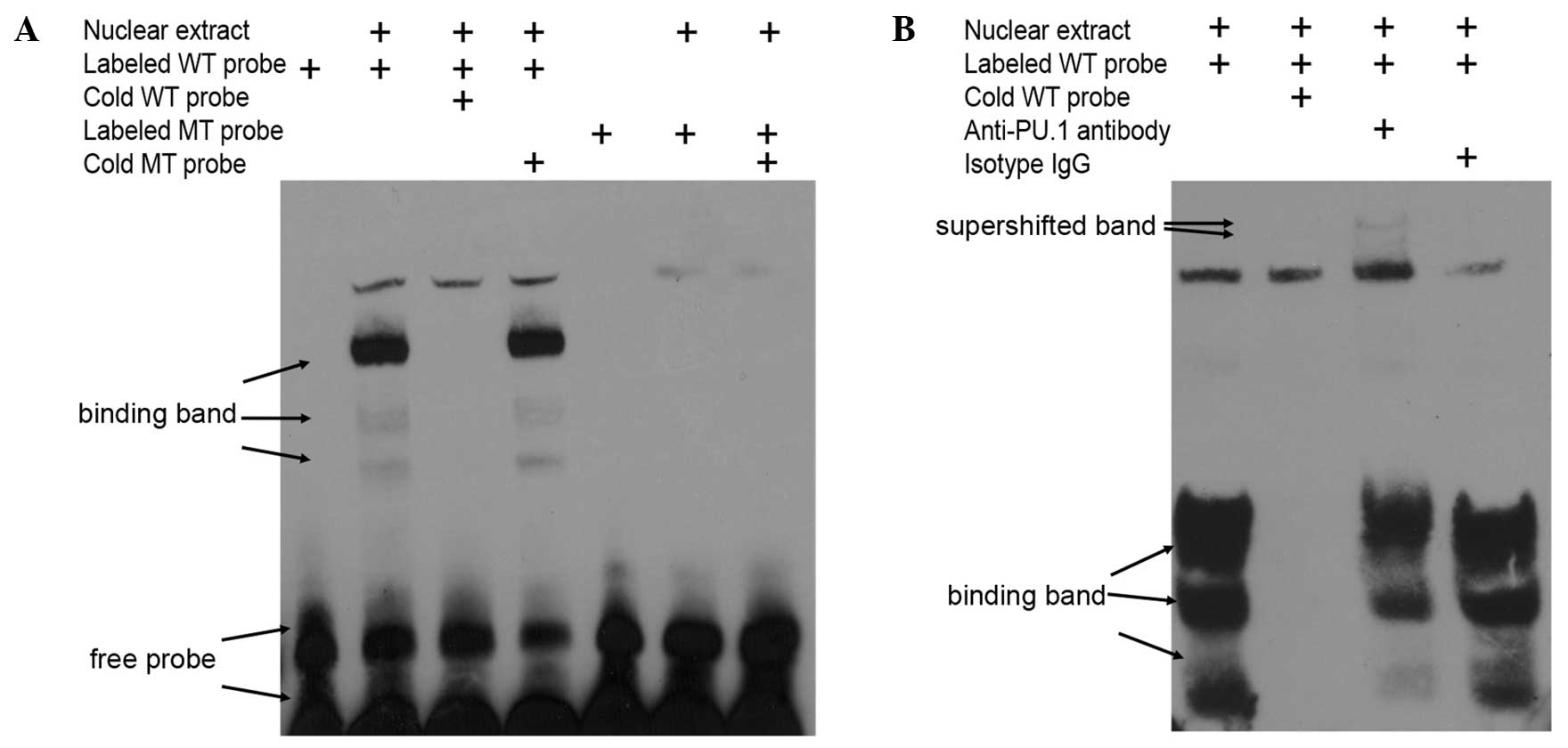
In vitro, it was determined that the transcription
factor PU.1 bound to this bio-functional site in the MEIS1 promoter
by EMSA with nuclear exacts from non-MLL leukemia cells. Using
biotin-labeled oligonucleotide probes corresponding to the putative
binding region between nucleotides −296 and −276 of the promoter
(Fig. 4A), which is upstream of the
MEIS1 transcription start site, specific bands were detected that
were readily competed off with wild type cold probes, but not with
mutated cold probes (Fig. 5A). In
addition, the obtained bands were supershifted by PU.1-specific
antibody, but not isotype IgG in 293T cells overexpressing PU.1
(Fig. 5B). Overall, the present data
indicated that the regulatory function of PU.1 on MEIS1 is mediated
by direct protein-DNA binding in the promoter region of the MEIS1
gene.
Discussion
Numerous factors that activate the expression of
MEIS1 genes in leukemia have been identified, such as the MLL
fusion protein (19), Hoxa9 (20) and E74-like factor 1 (21). The majority of previous studies have
focused on leukemia with MLL rearrangements (17,19,22,23),
as this leukemia is characterized by high expression of the
homeobox gene MEIS1. Downregulation of MEIS1 in MLL rearranged
acute leukemia results in the reduced expression of genes
associated with cell cycle entry and inhibition of cell
proliferation (17), and also impairs
engraftment (22), indicating that
MEIS1 gene activation is a key event in leukemia with MLL
rearrangements. Compared with the leukemia harboring MLL
rearrangement, MEIS1 demonstrates decreased or limited expression
in non-MLL rearranged leukemia, which confers certain challenges to
the associated studies. At present, the regulation and bio-function
of MEIS1 in leukemia without MLL rearrangement remains unknown. The
present preliminary data reveals that the limited expression of
MEIS1 also functions as an essential oncogene in the human acute
leukemia U937 cell line, a non-MLL leukemia cell line. In addition,
the present results provide evidence that the activity of MEIS1 is
regulated tightly by the transcription factor PU.1.
The transcription factor PU.1 is a
hematopoietic-specific ETS family member involved in the
development of all hematopoietic lineages (6,8) and acts
as an activator and repressor to regulate the transcription of
various genes (24,25). Traditionally, PU.1 functions as a
tumor suppressor in the majority of leukemia types. Dysregulation
of PU.1 leads to loss of lineage development and leukemia in
vitro and in vivo (9,26–28). Previous studies, however, have also
demonstrated that PU.1 is required for the repopulation or
self-renewal capacity of normal hematopoietic stem cells, and
sustained PU.1 levels also balance cell cycle-associated regulators
to prevent the exhaustion of adult HSC (8,15). These
studies indicated that the presence of PU.1 activity may be
required to favor the growth of myeloid leukemia stem cells.
Previous studies have reported that PU.1 demonstrated an essential
expression and activated a well-known oncogene MEIS1 pathway in
MLL, accompanied by MEIS1 overexpression (16). In addition, the expression of the PU.1
gene and the survival rate appeared to be inversely associated in
human AML samples with MLL rearrangement (16). Despite the requirement of PU.1 in the
development of MLL, as a pro-tumor gene, the key function of PU.1
may not be limited to MLL in AML.
In the current study, it was identified that
knockdown of PU.1 caused inhibition of cell proliferation in the
human non-MLL U937 cell line and the function of PU.1 was mediated
by MEIS1 transcriptional regulation. This result is consistent with
the observation in MLL, indicating that PU.1 may function as a
pro-tumor gene ubiquitously, or not specifically in MLL leukemia.
In the present study, which aimed to investigate the regulatory
mechanism, the activity of putative PU.1 binding site in MEIS1
promoter region as a positive regulatory motif in U937 cells was
confirmed by the Luciferase report system. Importantly, the present
data initially revealed that PU.1 exhibited strong enrichment in
the MEIS1 promoter region using in vivo and in vitro
assays. Notably, this PU.1 binding genomic locus in U937 cells
varies from the regulation via intron regions in MLL (16), indicating that MEIS1 regulatory sites
by PU.1 may be multiple in various type of leukemia.
Overall, the present study identifies that
transcription factor PU.1 is required for cell proliferation in
U937 cells and its biological function, at least in part, is
mediated by regulating the expression of target oncogene MEIS1
directly. This indicates that the potential tumor activator effect
of PU.1 may be a universal phenomenon and interference of PU.1
expression may be an alternative target for non-MLL acute myeloid
leukemia treatment. The present finding may potentially lead to a
novel direction for non-MLL studies. However, the roles of PU.1 in
other non-MLL cell lines as well as other subtypes of leukemia
remain to be addressed in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no., 81100381).
References
|
1
|
Orkin SH and Zon LI: Hematopoiesis: An
evolving paradigm for stem cell biology. Cell. 132:631–644. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kehrl JH: Hematopoietic lineage
commitment: role of transcription factors. Stem Cells. 13:223–241.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gangenahalli GU, Gupta P, Saluja D, Verma
YK, Kishore V, Chandra R, Sharma RK and Ravindranath T: Stem cell
fate specification: Role of master regulatory switch transcription
factor PU.1 in differential hematopoiesis. Stem Cells Dev.
14:140–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aikawa Y, Katsumoto T, Zhang P, Shima H,
Shino M, Terui K, Ito E, Ohno H, Stanley ER, Singh H, et al:
PU.1-mediated upregulation of CSF1R is crucial for leukemia stem
cell potential induced by MOZ-TIF2. Nat Med. 16:580–585, 1p
following 585. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houston IB, Huang KJ, Jennings SR and
DeKoter RP: PU.1 immortalizes hematopoietic progenitors in a
GM-CSF-dependent manner. Exp Hematol. 35:374–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mak KS, Funnell AP, Pearson RC and
Crossley M: PU.1 and Haematopoietic Cell Fate: Dosage Matters. Int
J Cell Biol. 2011:8085242011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friedman AD: Transcriptional control of
granulocyte and monocyte development. Oncogene. 26:6816–6828. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwasaki H, Somoza C, Shigematsu H, Duprez
EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram
T, et al: Distinctive and indispensable roles of PU.1 in
maintenance of hematopoietic stem cells and their differentiation.
Blood. 106:1590–1600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nutt SL, Metcalf D, D'Amico A, Polli M and
Wu L: Dynamic regulation of PU.1 expression in multipotent
hematopoietic progenitors. J Exp Med. 201:221–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Renneville A, Roumier C, Biggio V, et al:
Cooperating gene mutations in acute myeloid leukemia: A review of
the literature. Leukemia. 22:915–931. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seshire A, Rößiger T, Frech M, et al:
Direct interaction of PU.1 with oncogenic transcription factors
reduces its serine phosphorylation and promoter binding. Leukemia.
26:1338–1347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mueller BU, Pabst T, Fos J, et al: ATRA
resolves the differentiation block in t (15;17) acute myeloid
leukemia by restoring PU.1 expression. Blood. 107:3330–3338. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vangala RK, Heiss-Neumann MS, Rangatia JS,
et al: The myeloid master regulator transcription factor PU.1 is
inactivated by AML1-ETO in t (8;21) myeloid leukemia. Blood.
101:270–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang K, Wang P, Shi J, et al: PML/RARalpha
targets promoter regions containing PU.1 consensus and RARE half
sites in acute promyelocytic leukemia. Cancer Cell. 17:186–197.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Staber PB, Zhang P, Ye M, et al: Sustained
PU.1 levels balance cell-cycle regulators to prevent exhaustion of
adult hematopoietic stem cells. Mol Cell. 49:934–946. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou J, Wu J, Li B, et al: PU.1 is
essential for MLL leukemia partially via crosstalk with the
MEIS/HOX pathway. Leukemia. 28:1436–1448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar AR, Li Q, Hudson WA, Chen W, Sam T,
Yao Q, Lund EA, Wu B, Kowal BJ and Kersey JH: A role for MEIS1 in
MLL-fusion gene leukemia. Blood. 113:1756–1758. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dahl R, Iyer SR, Owens KS, Cuylear DD and
Simon MC: The transcriptional repressor GFI-1 antagonizes PU.1
activity through protein-protein interaction. J Biol Chem.
282:6473–6483. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zeisig BB, Milne T, García-Cuéllar MP,
Schreiner S, Martin ME, Fuchs U, Borkhardt A, Chanda SK, Walker J,
Soden R, et al: Hoxa9 and Meis1 are key targets for
MLL-ENL-mediated cellular immortalization. Mol Cell Biol.
24:617–628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu YL, Fong S, Ferrell C, Largman C and
Shen WF: HOXA9 modulates its oncogenic partner Meis1 to influence
normal hematopoiesis. Mol Cell Biol. 29:5181–5192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang P, Lo C, Argiropoulos B, et al:
Identification of E74-like factor 1 (ELF1) as a transcriptional
regulator of the Hox cofactor MEIS1. Exp Hematol. 38:798, 808
e1-e2. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orlovsky K, Kalinkovich A, Rozovskaia T,
et al: Down-regulation of homeobox genes MEIS1 and HOXA in
MLL-rearranged acute leukemia impairs engraftment and reduces
proliferation. Proc Natl Acad Sci USA. 108:7956–7961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wong P, Iwasaki M, Somervaille TC, So CW
and Cleary ML: Meis1 is an essential and rate-limiting regulator of
MLL leukemia stem cell potential. Genes Dev. 21:2762–2774. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta P, Gurudutta GU, Saluja D and
Tripathi RP: PU.1 and partners: Regulation of haematopoietic stem
cell fate in normal and malignant haematopoiesis. J Cell Mol Med.
13:4349–4363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dakic A, Metcalf D, Di Rago L, Mifsud S,
Wu L and Nutt SL: PU.1 regulates the commitment of adult
hematopoietic progenitors and restricts granulopoiesis. J Exp Med.
201:1487–1502. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosenbauer F, Wagner K, Kutok JL, et al:
Acute myeloid leukemia induced by graded reduction of a
lineage-specific transcription factor, PU.1. Nat Genet. 36:624–630.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suraweera N, Meijne E, Moody J,
Carvajal-Carmona LG, Yoshida K, Pollard P, Fitzgibbon J, Riches A,
van Laar T, Huiskamp R, et al: Mutations of the PU.1 Ets domain are
specifically associated with murine radiation-induced, but not
human therapy-related, acute myeloid leukaemia. Oncogene.
24:3678–3683. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walter MJ, Park JS, Ries RE, Lau SK,
McLellan M, Jaeger S, Wilson RK, Mardis ER and Ley TJ: Reduced PU.1
expression causes myeloid progenitor expansion and increased
leukemia penetrance in mice expressing PML-RARalpha. Proc Natl Acad
Sci USA. 102:12513–12518. 2005. View Article : Google Scholar : PubMed/NCBI
|