Introduction
Gastric cancer (GC) is a major cause of morbidity
and mortality and has become a major public health concern
worldwide (1). The early symptoms of
GC are atypical; therefore, when patients develop apparent
symptoms, the disease has already progressed to an advanced stage.
Therefore, the opportunity for radical surgery for these patients
is missed, and chemotherapy becomes the primary means of
treatment.
RNA interference (RNAi) is the phenomenon of
post-transcriptional gene silencing caused by double-stranded
(ds)RNA (2). RNAi is considered a
revolutionary novel post-gene transcriptional regulation technology
due to its unique efficiency and specificity. This technology has
become a powerful tool for the study of gene function and has
provided novel techniques and application prospects for specific
gene therapy. The RNAi technology is becoming known for gene
function and regulation analysis in the post-genomic era.
Zinc finger proteins (ZNFs) are a class of proteins
widely distributed in the human genome. ZNFs have been shown to
regulate gene transcription, thus playing an important role in the
occurrence and development of GC (3).
It was previously demonstrated that the ZNF139 gene is closely
associated with GC (4).
Proteomics relies on the bilateral fluorescence
two-dimentional difference gel electrophoresis (2D-DIGE) to
separate different proteins, which are then identified and analysed
by mass spectrometry and bioinformatics (5). Proteomics technology is currently being
used in various fields of biology due to its high sensitivity,
accuracy and throughput. A previous study demonstrated that the
ZNF139 gene is closely associated with GC (4). The aim of the present study was to
further investigate the mechanisms underlying the role of ZNF139 in
the occurrence, development and chemosensitivity of GC.
Based on the principle of RNAi, a ZNF139-targeted
small interfering (si)RNA plasmid was developed in this study. The
different proteins prior to and following siRNA-ZNF139 transfection
were analyzed to provide a theoretical basis for the mechanisms
underlying the role of ZNF139 in the occurrence, development and
chemosensitivity of GC.
Materials and methods
Construction of siRNA-ZNF139
The 19-bp siRNA and ineffective interference control
fragment were designed by the siRNA online software (http://i.cs.hku.hk/~sirna/software/sirna.php). The
siRNA-ZNF139 template sequences were 5′-ACCTCGGAAGATTCAGCAT-3′
(sense strand) and 5′-ATGCTGAATCTTCCGAGGT-3′ (antisense strand).
The ineffective interference control sequences were
5′-GACGAGTTGACTGCGATTG-3′ (sense strand) and
5′-CAATCGCAGTCAACTCGTC-3′ (antisense strand). The pSilencerTM
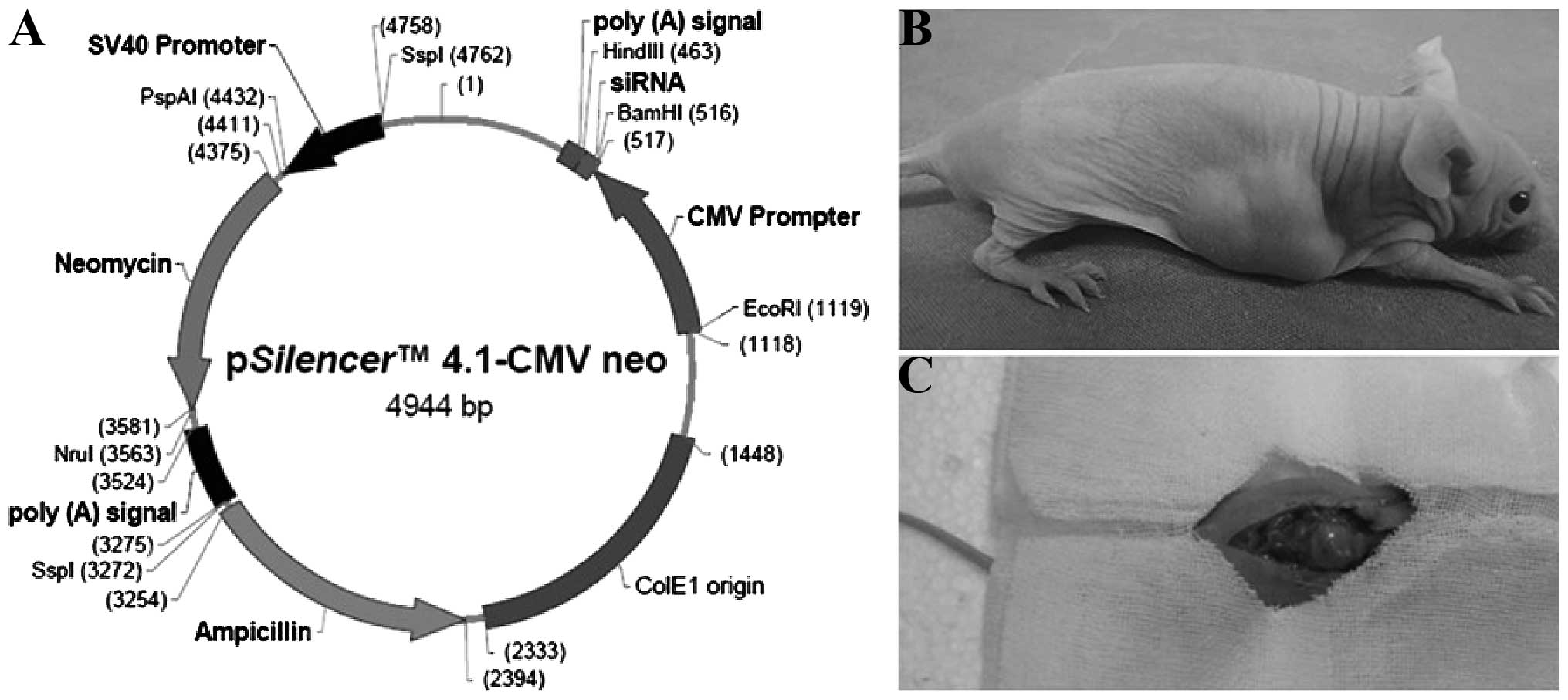
4.1-CMV neo-ZNF139 siRNA plasmid (Shanghai GenePharma Co., Ltd.,
Shanghai, China; Fig. 1A) and
Escherichia coli were used to construct the engineered bacteria,
then the endotoxin plasmid gross extraction kit was applied to
extract the plasmid. Each recombinant plasmid was amplified and
purified for the subsequent experiments.
RNAi-mediated inhibition of ZNF139 in
vitro
Experiments were divided into the blank control,
siRNA-ZNF139 and negative control groups. Based on the transfection
reagent instructions of the X-tremeGENE HP DNA transfection reagent
(Roche, Basel, Switzerland), the recommended amount of plasmid (0.8
µg/µl) and the appropriate transfection reagents were added for
cell transfection. Transfected cells (~200 µl) were seeded in each
well of the 96-well plate and then incubated in 5% CO2
at 37°C for 0, 24, 48 and 72 h post-transfection. Approximately 20
µl of MTT solution (5 mg/ml) was added into each well of the plate
and incubated for another 4 h. The supernatant was carefully
discarded and an absorbent paper absorbed the residual liquid. A
total of 150 µl dimethyl sulfoxide (DMSO) was added to each well
and agitated for 10 min. The optical density (OD) of each well was
measured at 570 nm to illustrate the cell growth curve.
RNAi-mediated inhibition of ZNF139 in
vivo
The in vitro cultured SGC7901 human GC cells
were collected and subcutaneously injected into nude mice. In
total, 45 BALB/C male nude mice (age, 4 weeks; weight, 17–23 g)
were used, including 18 mice for inter-mouse passage subcutaneous
tumor transplantation and 27 mice for orthotopic tumor
transplantation. The mice were obtained from the Fourth Hospital of
Hebei Medical University Animal Center specific-pathogen-free
animal laboratory (Shijiazhuan, China). Inter-mouse passage of the
subcutaneous tumor was performed (Fig.
1B) and orthotopic transplantation was conducted on the sixth
generation of subcutaneous tumor using the OB biological glue
method (Fig. 1C) (6). The incision and growth of the abdominal
orthotopic tumor graft were observed daily. Interference was
conducted when the orthotopic tumor graft grew to ≥10 mm in
diameter. The orthotopic grafted nude mice were randomly divided
into the aforementioned three groups when the grafted tumor was
developed.
The orthotopic xenografted nude mice were randomly
divided into three groups (n=9 per group). The grouping and
intervention methods were as follows: In the blank control group,
50 µl of saline was injected at multiple points at the tumor spot
every other day. In the siRNA-ZNF139 group, 50 µl of the
intervention complex was administered by multipoint injection at
the tumor spot every other day. The intervention complex included
20 µg of the siRNA-ZNF139 plasmid and 20 µl of the transfection
reagent. The medium was added to a total volume of 50 µl. In the
negative control group, 50 µl of the intervention complex was
administered by multipoint injection at the tumor spot every other
day. The complex included 20 µg of the negative-control plasmid and
20 µl of the transfection reagent. The medium was added to a total
volume of 50 µl.
During the intervention period following formation
of the orthotopic tumor graft, the long and short diameters of the
tumor were measured once every other day. The orthotopic tumor
volume was also calculated with the following equation:
V=ab2/2 (where a is the long diameter and b is the short
diameter of the tumor). Two weeks after the intervention treatment,
the nude mice were sacrificed using the cervical dislocation
method. The in situ tumor was removed and the specimens were
stored at −80°C for future use. The experiment was performed in
strict accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Hebei Medical University (permit no.
20060828001).
MTT assay
The SGC7901 human GC cells from different
experimental groups and nude mice with in situ grafted tumors were
prepared. Approximately 180 µl of the cell suspension were seeded
in each well of the 96-well plate. The three chemotherapeutic
agents used were 5-fluorouracil (5-FU), cisplatin (CDDP) and
mitomycin C (MMC). Each agent (~20 µl) was added into each well of
the 96-well plate to a total volume of 200 µl per well. The
procedure was repeated five times. The final concentrations of the
chemotherapeutic agents were 25 µg/ml 5-FU, 4 µg/ml CDDP and 4
µg/ml MMC. The plate was incubated in 5% CO2 at 37°C for
48 h. Following incubation, 20 µl of MTT (5 mg/ml) was added to
each well and incubated for another 4 h. The supernatant was
carefully discarded and the residual liquid was absorbed with an
absorbent paper. Following addition of 150 µl DMSO to each well and
vortex mixing for 10 min, the OD value of each well was measured at
570 nm with a microplate reader (Anthos 2010; Anthos Labtec
Instruments GmBH, Wals-Siezenheim, Austria). The mean cell growth
inhibitory rate (IR) was calculated as follows: IR = (1 - mean
ODdrug treatment well/mean ODcontrol well) ×
100%.
Preparation of protein samples
The Bradford method (7) was used to detect the protein sample
concentrations. Protein samples from the two experimental groups
(~50 µg) were collected and labelled with CyDye5 and CyDye3.
Approximately 50 µg of the protein samples in the control and
experimental groups were replenished. Active hydration was
conducted at 50 V for 10 h and then at 500 V for 1 h, 1,000 V for 1
h, 8,000 V for 1 h and 8,000 V for 3 h. The final voltage increased
from 10,000 to 65,000 V. The strips were transferred to 10 ml of
balance solution A [75 mmol/l Tris-HCl (pH 8.8), 6 mol/l urea, 30%
glycerol, 2% sodium dodecyl sulfate (SDS) and 1% disulfide
dithiothreitol (DTT); Sigma-Aldrich, St Louis, MO, USA] for 15 min
and then to balance solution B (2.5% indole-3-acetic acid was used
to replace the 1% DTT in solution A) for another 15 min. The 12.5%
SDS-polyacrylamide gel (PAGE) electrophoresis was performed at 50 V
for 2 h and then at 120 V until the frontier of bromophenol blue
was ~1 cm from the lower end of the gel. Scanning was performed by
Typhoon 9400 (GE Healthcare, Little Chalfont, UK), and image
analysis was conducted by DeCyder difference analysis software (GE
Healthcare).
Liquid chromatography-mass
spectrometry (LC-MS) and database query
Following Deep Purple staining, an Ettan Spot Picker
(GE Healthcare) was used to dig the spot. LTQXL™ (Thermo Fisher
Scientific, Waltham, MA, USA) was used for the mass identification
of the samples following in-gel digestion. BioWorks 3.3.1 software
(Thermo Fisher Scientific) was used for database searching. The
FASTA-form protein database was obtained from the National Center
for Biotechnology Information human protein sequence database.
Cysteine reductive alkylation and methionine oxidation were set as
the fixed and variable modifiers, respectively.
Western blot analysis
Approximately 50 µg of the prepared protein sample
from each experimental group was separated by SDS-PAGE
electrophoresis. The gel was sliced and transferred onto the
polyvinylidene fluoride membrane, which was initially treated with
the closure solution at room temperature and agitated for 1 h. The
primary antibodies [rabbit anti-human polyclonal antibodies against
ZNF139 (cat. no. ab126124), pyridoxal kinase (PDXK; cat. no.
ab38208), annexin A2 (ANXA2; cat. no. ab41803) and fascin (cat. no.
ab183891), and mouse anti-human monoclonal antibody against β-actin
(cat. no. ab6276); Abcam, Cambridge, UK] were added and then
incubated at 4°C overnight. The membrane was washed, followed by
the addition of horseradish peroxidase enzyme-labelled goat
anti-rabbit IgG secondary antibody (cat. no. ab6721; Abcam) and
incubated at room temperature for 1 h. The membrane was washed
again and stained by chemiluminescence. The Bio-Rad Image Analysis
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
with β-actin as the internal reference. The experiment was
performed thrice.
Statistical analysis
Measured data are expressed as the mean ± standard
deviation and analysed by SPSS 13.0 statistical software (SPSS,
Inc., Chicago, IL, USA). The intergroup comparison was conducted by
analysis of variance.
Results
RNAi-mediated inhibition of ZNF139 in
vitro
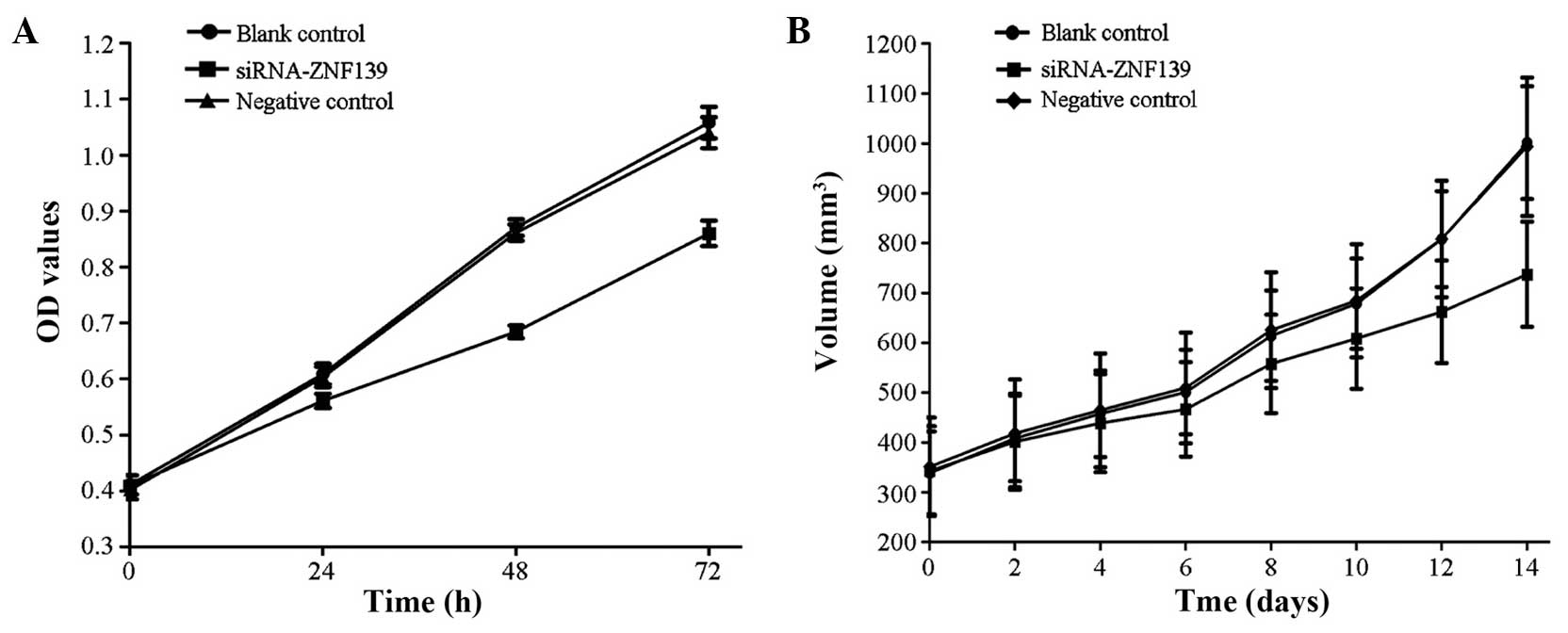
The growth of the SGC7901 human GC cells of the
siRNA-ZNF139 group slowed down significantly compared with that of
the control and negative control groups (P<0.05) (Fig. 2A).
RNAi-mediated inhibition of ZNF139 in
vivo
The size of the human in situ grafted GC
tumor of the siRNA-ZNF139 group was significantly decreased
compared with that of the control and negative control groups
(P<0.05) (Fig. 2B).
Chemosensitivity changes
The average IR of the cells of the siRNA-ZNF139
group was significantly higher (P<0.05) compared with that of
the control and negative control groups (Table I).
 | Table I.Alterations in cell chemosensitivity
following RNA interference. |
Table I.
Alterations in cell chemosensitivity
following RNA interference.
|
| SGC7901 human GC
cells | Human in situ
grafted GC cells |
|---|
|
|
|
|
|---|
| Experimental
groups | 5-FU | CDDP | MMC | 5-FU | CDDP | MMC |
|---|
| Blank control | 29.18±3.01 | 30.67±1.55 | 19.58±2.17 | 31.25±2.86 | 32.64±1.69 | 21.29±2.23 |
| siRNA-ZNF139 |
40.08±1.97a |
39.76±1.24a |
33.91±1.39a |
42.28±2.13a |
41.94±1.53a |
35.70±1.66a |
| Negative control | 28.98±3.21 | 30.50±1.84 | 19.52±1.77 | 31.47±2.75 | 32.48±1.91 | 21.48±1.94 |
2D-DIGE
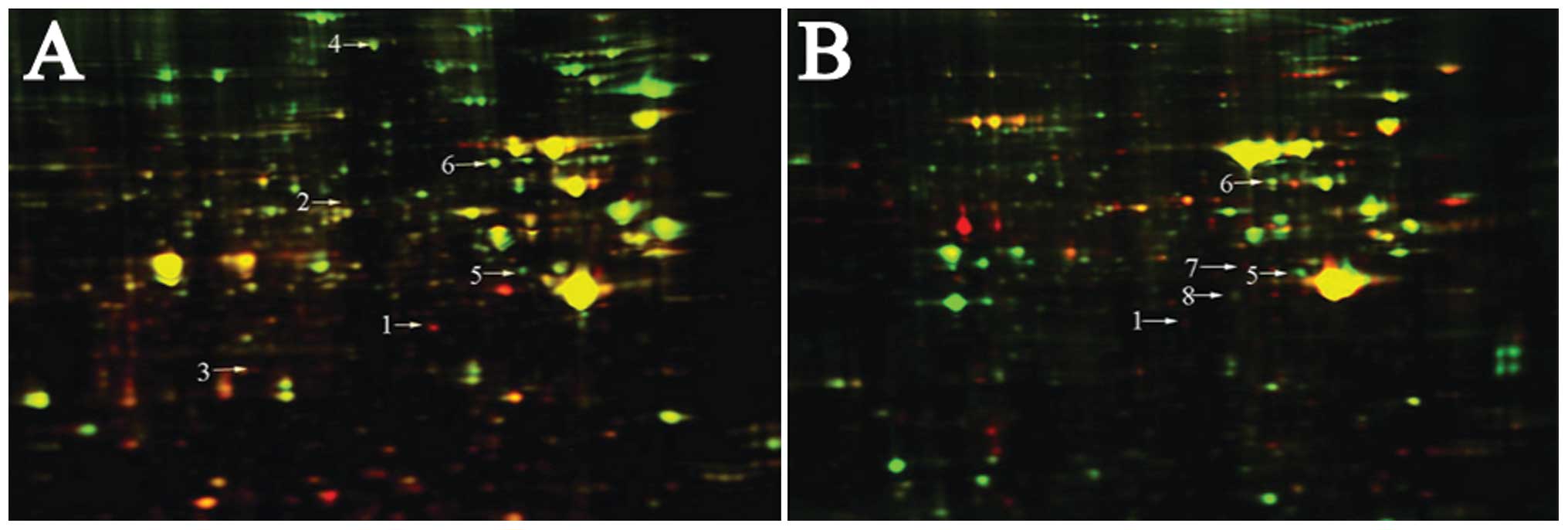
According to the 2D-DIGE spectrum of the SGC7901
human GC cells and in situ grafted GC tumors, the protein points
were 1,958±67 with 90.5% matching rate and 5,227±59 with 88.7%
matching rate, respectively. DeCyder difference analysis software
was used to select the eight and six distinct protein points in the
SGC7901 human GC cells and in situ grafted GC tumors, respectively.
The experiment was performed thrice.
Identification of LC-MS
The Ettan spot picker was used to assess the
different protein spots. Following in-gel digestion, LC-MS was used
for the analysis. Among the different proteins of the SGC7901 human
GC cells, six were identified by LC-MS, namely PDXK, desmin,
nuclear phosphoprotein (NPM), heat-shock protein 70 (HSP70), ANXA2
and fascin. In the siRNA-ZNF139-transfected SGC7901 cells, PDXK,
desmin and NPM were upregulated, whereas HSP70, ANXA2 and fascin
were downregulated. Five proteins were identified by LC-MS among
the different proteins in the in situ grafted human GC tumors,
namely ANXA1, PDXK, fascin, ANXA2 and heterogeneous nuclear
ribonucleoprotein (hnRNP). Following transfection, ANXA1 and PDXK
were upregulated, whereas fascin, ANXA2 and hnRNP were
downregulated (Table II and Fig. 3).
 | Table II.Identification results of difference
proteins. |
Table II.
Identification results of difference
proteins.
| Number | Proteins | Version | MW | Score | Coverage | pI | Expression |
|---|
| 1 | PDXK | gi|4505701 | 35079.90 | 30.14 | 11.20 | 5.75 | U |
| 2 | Desmin | gi|55749932 | 53503.23 | 30.17 | 6.60 | 5.21 | U |
| 3 | NPM | gi|10835063 | 32554.90 | 10.18 | 3.70 | 4.64 | U |
| 4 | HSP70 | gi|5123454 | 69995.16 | 30.16 | 4.70 | 5.47 | D |
| 5 | ANXA2 | gi|50845386 | 38579.82 | 40.23 | 2.90 | 7.57 | D |
| 6 | Fascin | gi|4507115 | 54496.09 | 10.21 |
2.80 | 6.84 | D |
| 7 | ANXA1 | gi|4502101 | 38690.00 | 60.20 | 17.30 | 6.57 | U |
| 8 | hnRNP | gi|55956919 | 35945.22 | 40.21 | 12.70 | 6.49 | D |
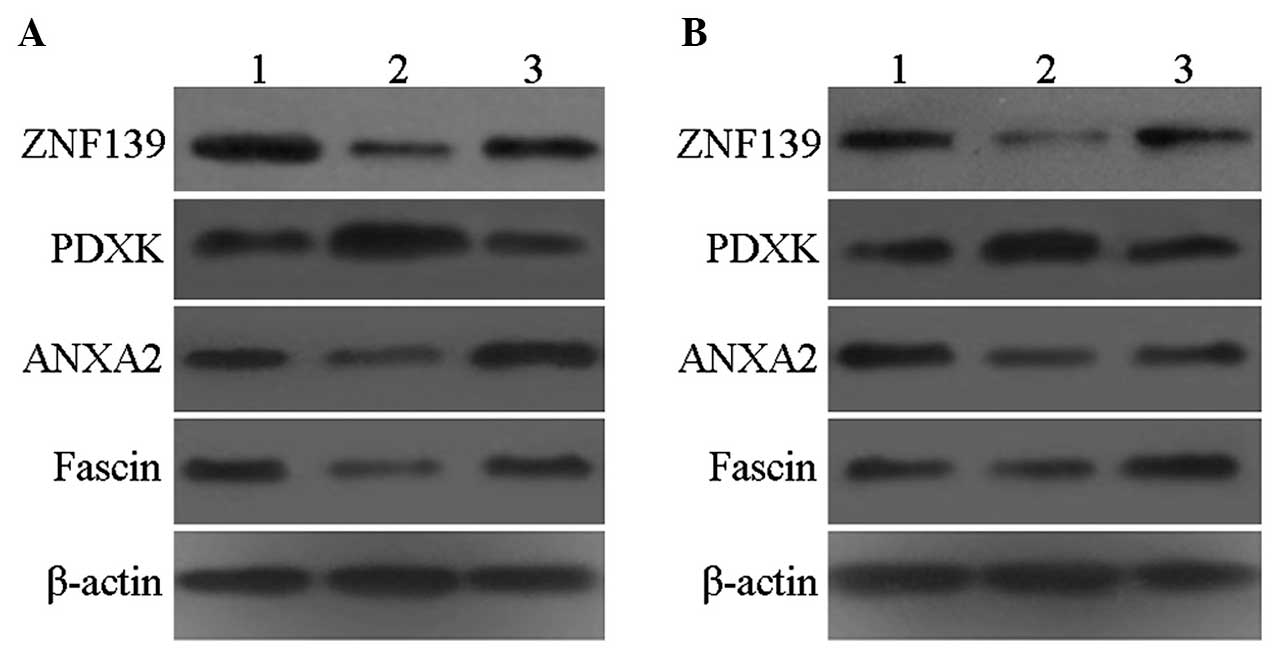
Western blot analysis
The expression of ZNF139 was significantly decreased
in the siRNA-ZNF139 group compared with that in the control and
negative control groups. PDXK expression was significantly
increased, whereas the expression of ANXA2 and fascin was
significantly decreased, in accordance with the proteomics results
(Fig. 4).
Discussion
A previous study (8)
demonstrated that siRNA may guide the RNA-induced silencing complex
(RISC) to cut the homologous single-stranded mRNA and function as
the primer to bind the target RNA. A number of secondary dsRNAs are
synthesized under RNA polymerase, and the newly synthesized dsRNA
may be sliced by the Dicer enzyme to generate a large number of
secondary siRNAs. Therefore, the RNAi effect increases further,
resulting in the completion of the degradation of the target mRNA.
The results of the present study demonstrated that siRNA-ZNF139 was
able to inhibit the expression of ZNF139 and the growth of SGC7901
human GC cells and in situ grafted GC tumors in nude mice.
These findings may provide the theoretical basis for the
involvement of the ZNF139 gene in the occurrence, development and
chemosensitivity of human GC.
The multidrug resistance (MDR) phenomenon of GC is a
major problem that currently complicates clinical treatment, and
one of the main reasons for the poor therapeutic effect of
chemotherapy (9). MDR occurs when the
tumor cells resist a specific anticancer drug and then exhibit
cross-resistance to other, previously unencountered anticancer
drugs, which may be structurally unrelated and have different
targets and mechanisms of action (10). The fundamental solution for MDR is
dependent on in-depth studies on its cause and underlying
mechanism; such studies may provide a method for its complete
reversion (11). Based on the present
study, the application of siRNA to inhibit the expression of ZNF139
in SGC7901 human GC cells and in situ grafted GC tumors may
increase the sensitivity of tumor cells to 5-FU, CDDP and MMC. The
inhibition of ZNF139 expression in GC cells may improve the
efficiency of chemotherapy in GC, thus providing a basis for future
research on novel GC treatments.
Wasinger et al conducted a study applying
proteomics (12); this technology has
been widely used in various fields of biology due to its high
sensitivity, accuracy and throughput. Franco et al (13) applied the combination of 2D-DIGE and
LC-MS, and observed that the occurrence of GC was associated with
the type of Helicobacter pylori strain. Xin et al
(14) applied proteomics technology
to compare the differences in SGC7901 human GC cells when cultured
with and without methionine and observed that the lack of
methionine may inhibit the proteins that are associated with the
regulation of the apoptosis of GC cells. Yang et al
(15) compared the proteome
differences between the SGC7901 human GC cell line and a
vincristine-resistant SGC7901 cell line, and identified a variety
of MDR-related proteins. In the present study, siRNA-ZNF139 was
applied to inhibit the expression levels of ZNF139 in SGC7901 human
GC cells and in situ grafted GC tumors. Proteomics was used
to identify the different proteins expressed prior to and following
inhibition. Among the different identified proteins, PDXK, ANXA2
and fascin exhibited similar changes. Following transfection, PDXK
was upregulated, whereas ANXA2 and fascin were downregulated.
Therefore, ZNF139 may regulate the expression of PDXK, ANXA2 and
fascin, thus participating in the occurrence, development and
chemosensitivity of GC.
PDXK, a member of the ribose kinase superfamily, is
widely present in biological organisms. PDXK is a key metabolic
enzyme of vitamin B6 and catalyses its phosphorylation. The
catalysed phosphorylation enables transfer into active
pyridoxal-5′-phosphate (PLP), which has an important function in
the synthesis and catabolism of amino acids. Matsubara et al
(16) demonstrated that PLP is able
to inhibit the proliferation of endothelial cells. Furthermore,
Komatsu et al (17)
demonstrated the inhibition of tumor cell growth by PLP.
ANXs are mainly expressed inside the nucleus and
cytoplasm and under the cell membrane; they are a class of
structurally-related calcium-dependent phospholipid-binding
proteins divided into five subfamilies, namely A, B, C, D and E.
The A family of ANXs includes 13 members (A1-13) found inside
vertebrate cells (18). ANXA2 was
first observed inside Rous sarcoma virus-transformed chicken embryo
fibroblasts, which are located in the human chromosome 15q21-q22.
ANXA2 is composed of 12 introns and 13 exons, with 339 amino acids
and a molecular weight of 36 kDa (19). ANXA2 is mainly expressed inside
endothelial cells, macrophages, mononuclear cells, nerve cells and
certain tumor cells. This protein may be highly expressed in
proliferating and transforming cells but its expression is low in
terminally differentiated cells. It was recently demonstrated that
ANXA2 is associated with various cytokines, including vascular
endothelial growth factor (VEGF); this protein promotes VEGF
expression on the tumor cell surface and the formation of new blood
vessels, thus promoting the growth, invasion and metastasis of
tumor cells (20). Zhang et al
(21) demonstrated that ANXA2 is
associated with MDR. Meng et al (22) applied proteomics technology to compare
the parental human bladder cancer cell line (pumc-91) with the
respective doxorubicin-resistant cell line (pumc-91/ADM) and
observed that ANXA2 was highly expressed in pumc-91/ADM, which may
indicate it is a resistance factor.
Fascin is an evolutionarily conserved cytoskeletal
protein with a molecular weight of 55 kDa. The main function of
fascin is to bind F-actin and assemble it in the parallel actin
bundle. Fascin has an important function in cell migration,
adhesion and exchange of intracellular information. During the
occurrence and development of tumors, fascin protein expression
increases, resulting in increased cell surface projections and
decreased cell differentiation. Therefore, cell proliferation may
be promoted, the actin cytoskeleton reconstructed and cell motility
enhanced. Tumor cells exhibit enhanced invasiveness and migration
ability, enabling them to penetrate the basement membrane and
spread to the surrounding tissues (23).
In the present study, western blot analysis detected
a significant increase in PDXK protein levels following
siRNA-ZNF139 transfection. However, the expression levels of ANXA2
and fascin were significantly decreased. Therefore, ZNF139 may be
involved in the occurrence, development and chemotherapeutic
sensitivity of GC by promoting the expression of ANXA2 and
inhibiting the expression of fascin and PDXK.
It was previously reported that the ZNF139 gene is
closely associated with GC (4). With
the aim to investigate novel GC treatments and governed by the
principles of RNAi, we herein constructed an siRNA plasmid to the
ZNF139 gene. Based on the analysis of the protein differences prior
to and following siRNA-ZNF139 transfection, it was concluded that
ZNF139 may promote the expression of ANXA2 and fascin, while
suppressing the expression of PDXK. Therefore, ZNF139 may be
significantly involved in the occurrence, development and
chemosensitivity of GC. These findings are consistent with those of
Komatsu et al (17), Zhang
et al (21) and Meng et
al (22). In conclusion, ZNF139
may inhibit the occurrence and development of GC, while increasing
its sensitivity to chemotherapeutic agents, thus providing novel
targets for the treatment of GC patients.
References
|
1
|
Are C, Rajaram S, Are M, et al: A review
of global cancer burden: trends, challenges, strategies and a role
for surgeons. J S urg Oncol. 107:221–226. 2013. View Article : Google Scholar
|
|
2
|
Bora RS, Gupta D, Mukkur TK and Saini KS:
RNA interference therapeutics for cancer: challenges and
opportunities (review). Mol Med Rep. 6:9–15. 2012.PubMed/NCBI
|
|
3
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clin Chim Acta. 424:53–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Tan BB, Zhao Q, Fan LQ, Liu Y and
Wang D: Regulatory mechanism of ZNF139 in multi-drug resistance of
gastric cancer cells. Mol Biol Rep. 41:3603–3610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parguiña AF and García A: Platelet
proteomics in transfusion medicine: a reality with a challenging
but promising future. Blood Transfus. 10:(Suppl 2). 113–114.
2012.
|
|
6
|
Shi J, Wei PK, Zhang S, et al: OB glue
paste technique for establishing nude mouse human gastric cancer
orthotopic transplantation models. World J Gastroenterol.
14:4800–4804. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kruger NJ: The Bradford method for protein
quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
8
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang X, Yashiro M, Qiu H, Nishii T,
Matsuzaki T and Hirakawa K: Establishment and characterization of
multidrug-resistant gastric cancer cell lines. Anticancer Res.
30:915–921. 2010.PubMed/NCBI
|
|
10
|
Rocco A, Compare D, Liguori E, et al:
MDR1-P-glycoprotein behaves as an oncofetal protein that promotes
cell survival in gastric cancer cells. Lab Invest. 92:1407–1418.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stein U, Fleuter C, Siegel F, et al:
Impact of mutant β-catenin on ABCB1 expression and therapy response
in colon cancer cells. Br J Cancer. 106:1395–1405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wasinger VC, Cordwell SJ, Cerpa-Poljak A,
et al: Progress with gene-product mapping of the Mollicutes:
Mycoplasma genitalium. Electrophoresis. 16:1090–1094. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franco AT, Friedman DB, Nagy TA, et al:
Delineation of a carcinogenic Helicobacter pylori proteome.
Mol Cell Proteomics. 8:1947–1958. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xin L, Cao WX, Fei XF, et al: Applying
proteomic methodologies to analyze the effect of methionine
restriction on proliferation of human gastric cancer SGC7901 cells.
Clin Chim Acta. 377:206–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang YX, Xiao ZQ, Chen ZC, et al: Proteome
analysis of multidrug resistance in vincristine-resistant human
gastric cancer cell line SGC7901/VCR. Proteomics. 6:2009–2021.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsubara K, Matsumoto H, Mizushina Y, Lee
JS and Kato N: Inhibitory effect of pyridoxal 5′-phosphate on
endothelial cell proliferation, replicative DNA polymerase and DNA
topoisomerase. Int J Mol Med. 12:51–55. 2003.PubMed/NCBI
|
|
17
|
Komatsu S, Yanaka N, Matsubara K and Kato
N: Antitumor effect of vitamin B6 and its mechanisms. Biochim
Biophys Acta. 1647:127–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerke V and Moss SE: Annexins: from
structure to function. Physiol Rev. 82:331–371. 2002.PubMed/NCBI
|
|
19
|
Ozaki T and Sakiyama S: Molecular cloning
of rat calpactin I heavy-chain cDNA whose expression is induced in
v-src-transformed rat culture cell lines. Oncogene. 8:1707–1710.
1993.PubMed/NCBI
|
|
20
|
Semov A, Moreno MJ, Onichtchenko A,
Abulrob A, Ball M, Ekiel I, Pietrzynski G, Stanimirovic D and
Alakhov V: Metastasis-associated protein S100A4 induces
angiogenesis through interaction with a nnexin II and accelerated
plasmin formation. J Biol Chem. 280:20833–20841. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Zhang L, Zhang B, et al: Anxa2
plays a critical role in enhanced invasiveness of the multidrug
resistant human breast cancer cells. J Proteome Res. 8:5041–5047.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng Q, Lei T and Zhang M, Zhao J, Zhao XH
and Zhang M: Identification of proteins differentially expressed in
adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human
bladder cancer cell lines by proteome analysis. J Cancer Res Clin
Oncol. 139:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashimoto Y, Kim DJ and Adams JC: The
roles of fascins in health and disease. J Pathol. 224:289–300.
2011. View Article : Google Scholar : PubMed/NCBI
|