Introduction
Hepatocellular carcinoma (HCC) is the third most
common cause of cancer-associated mortality worldwide. The
prognosis for patients with HCC is poor, due to the high
possibility of recurrence, and intrahepatic and extrahepatic
metastasis following surgical resection (1). Extrahepatic metastasis of HCC occurs in
~30–50% of patients (2–5), and the most common metastatic sites are
the lungs, abdominal lymph nodes and bones (2–5).
Metastasis to the gingiva is rare and, to the best of our
knowledge, only 13 such cases have been reported in English
literature thus far (6–17). The age range of these patients was
46–78 years and the average age was 61 years. The presentation
typically imitates other conditions, such as pyogenic granuloma
affecting the oral cavity (16).
Gingival tumor may be the first and only manifestation of HCC
(17). Furthermore, the disease has a
high incidence rate in male patients in Asia, possibly due to the
incidence of HCC (15). A final
diagnosis of HCC with metastasis to the gingiva primarily depends
on the histopathological characteristics and immunohistochemical
features of the tumor (18).
Microscopically, the tumor cells may be trabecular, solid or
tubular. Intranuclear pseudoinclusions caused by cytoplasmic
invaginations may be present, and the cytoplasm may contain
Mallory's hyaline, copper, pale bodies or bile pigment (19). In addition, an important diagnostic
feature is the network of sinusoidal vessels that surrounds the
tumor cells. Immunohistochemically, the tumor cells are positive
for α-fetoprotein (AFP), cytokeratin (CK)18, glypican 3 (GPC3) and
hepatocyte paraffin 1 (HepPar-1), and of these, GPC3, AFP and/or
HepPar-1 are relatively specific to the diagnosis of HCC.
Patient histories must be considered when
establishing a diagnosis. The prognosis is poor for patients with
extrahepatic metastases and predominantly depends on the metastatic
site at the time of diagnosis. It has been reported that the time
between appearance of the gingival metastasis and mortality is no
more than a few weeks. However, the survival of individual patients
may reach 8 months (15). The current
study presented a case of metastatic HCC to the gingival, and
investigated its histopathological characteristics and
immunohistochemical features. In addition, the current study
highlighted the importance of obtaining a comprehensive patient
history following the diagnosis of a metastatic malignant tumor of
the gingival. Written informed consent was obtained from the
patient's family.
Case report
A 43-year-old male was admitted to Tangdu Hospital
(Xi'an, China) on April 26, 2014 due to the presence of a lesion in
the gingiva, which had been identified >1 year prior to
admission, but the patient did not seek medical advice. Examination
of the patient's medical history revealed that a solid mass,
measuring 1.5 cm in diameter, was identified in the right liver 2
years prior to presentation at the hospital; however, no biopsy was
performed as the patient did not agree to perform the biopsy. An
oral examination confirmed the presence of a 2×1.5×1-cm irregular
mass located in the right upper gingival soft tissue. The mass was
subsequently resected, fixed in 10% neutral formaldehyde and
embedded in paraffin. Serial sections were cut at a thickness of
4-µm and stained with hematoxylin and eosin. Microscopically, the
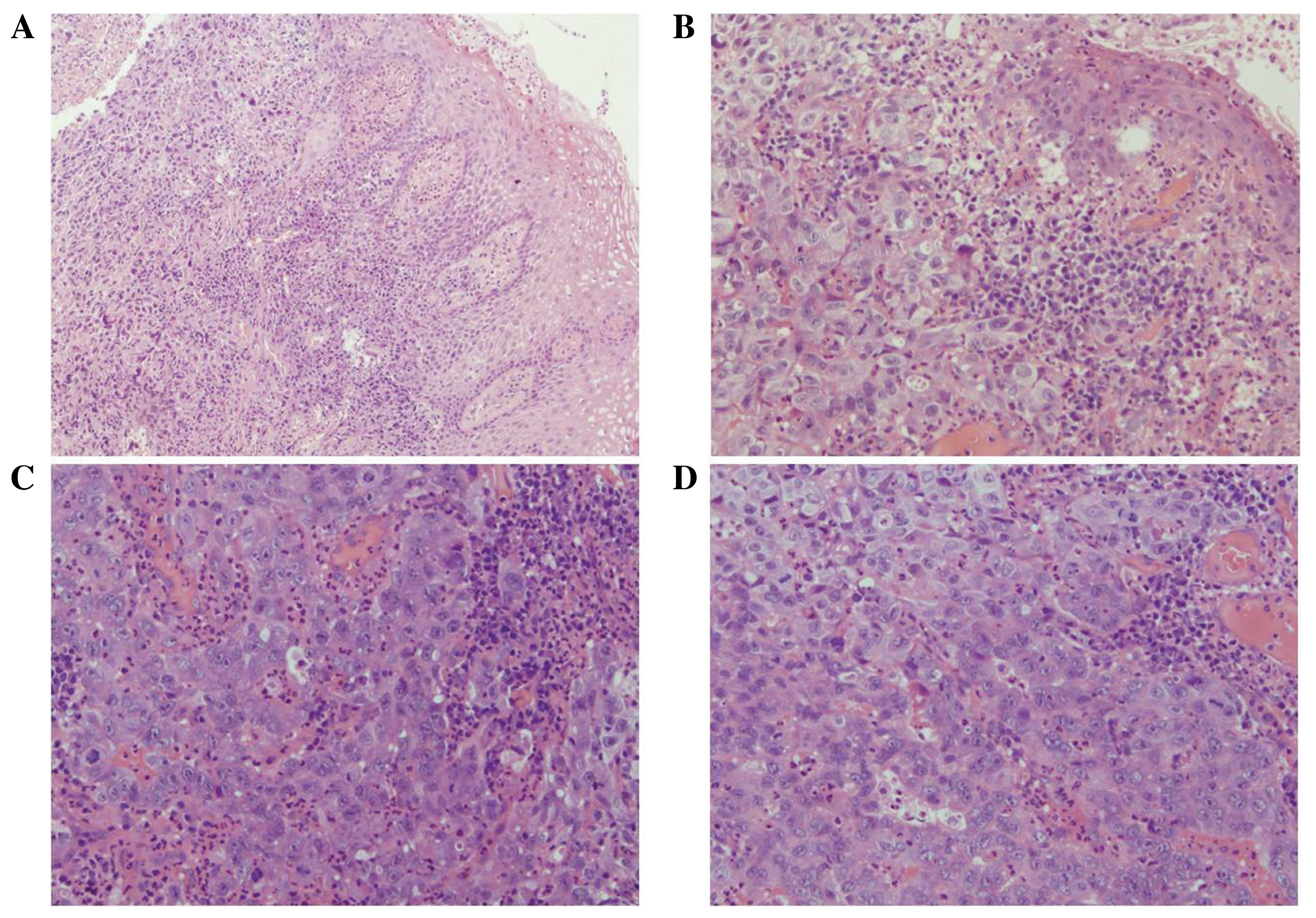
tumor cells were located in the submucosa (Fig. 1A) and predominantly arranged in a
solid, trabecular growth pattern (Fig.
1B). Furthermore, the cells were arranged in pseudoglandular
patterns in the focal area (Fig. 1C)
and were uniform in size with prominent nuclei (Fig. 1D). Inflammatory exudates and necrosis
were observed in the surface of the mucosa (Fig. 1C). Initial observations indicated that
the histological morphology was similar to an atypical squamous
cell carcinoma. Thus, immunostaining was performed to clarify this
diagnosis using a streptavidin-labeled peroxidase kit (Maixin
Biotech. Co., Ltd, Fuzhou, China), according to the manufacturer's
instructions. The following primary rat anti-human monoclonal
antibodies were used in the current study: Anti-high molecular
weight CK, anti-epithelial membrane antigen (EMA), anti-p63,
anti-CK5/6 and anti-vimentin. All reagents were supplied by Fuzhou
Maxin Biotechnology Co., Ltd. (Fuzhou, China).
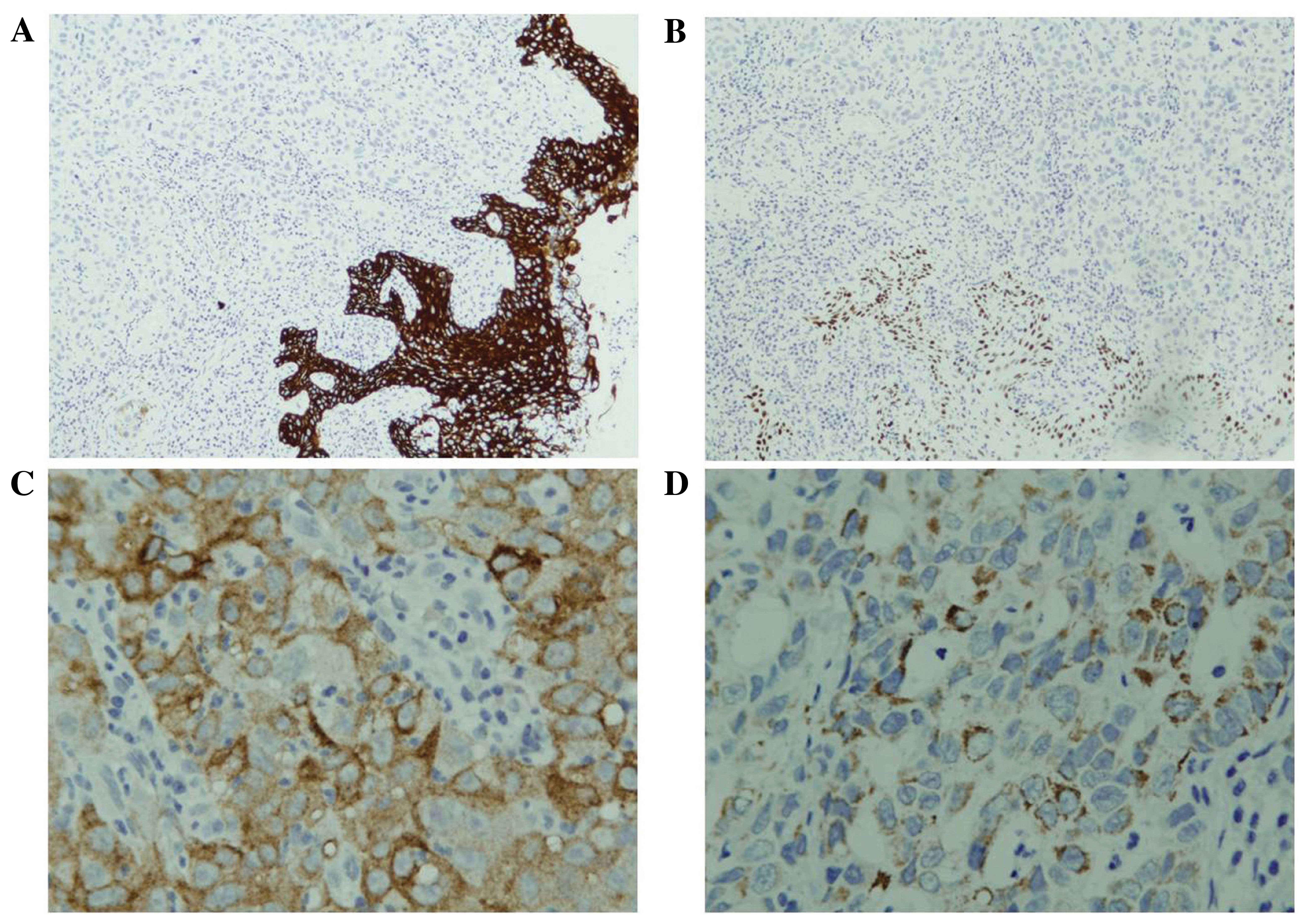
Immunohistochemical analysis identified that the
tumor cells were negative for all the aforementioned antibodies
(Fig. 2). Combining the
immunohistochemical analysis results and the patient history, a
diagnosis of metastatic HCC was considered. Further
immunohistochemical analysis for additional markers was performed
to confirm this diagnosis, using rat anti-human monoclonal
antibodies, such as GPC3 (MX005), CK18 (MX004), hepatocyte
(OCH1E5), AFP, CD56 (56C04), and chromogranin A (LK2H10+PHE5), and
a rabbit anti-human polyclonal antibody against synaptophysin. In
addition, a physical examination on the liver was advised. The
results demonstrated that the tumor cells were positive for GPC3,
hepatocytes, CK and CK18, and negative for AFP, EMA, chromogranin
A, synaptophysin and CD56 (Fig. 2).
Serum AFP levels were high (5,860.00 ng/ml; normal range <20
ng/ml). Considering the observed immunohistochemical
characteristics and the clinical history of the patient, metastatic
HCC was diagnosed, despite the unconfirmed primary HCC. Thus,
regular treatment for HCC was recommended, including chemotherapy
and transcatheter arterial chemoembolization (TACE). However, the
patient chose to end his own treatment and was lost in
follow-up.
Discussion
HCC is one of the most common types of cancer
worldwide, with high prevalence and mortality rates. High mortality
rates are in part attributed to rapid recurrence and the
development of intra- and extrahepatic metastasis following
surgical intervention (1).
Extrahepatic metastasis occurs in 30–50% of HCC cases (2–5), and the
most common metastatic sites are the lungs, regional lymph nodes
and bones (2–5). However, metastatic HCC to the oral
cavity, particularly the gingival soft tissues, is rare. To the
best of our knowledge, only 13 such cases have been reported in the
English literature to date (6–17).
The pathogenesis of the metastasis of malignant
tumors to the gingiva is unclear. Following a comprehensive review
of the literature, numerous studies were identified that discussed
an association between HCC metastasis in particular and chronic
periodontitis (20–23). The patient in the present study had
chronic periodontitis before the gingival tumor was observed. From
these studies, circulating reactive oxygen species (ROS), which are
produced by host inflammatory cells upon stimulation by bacterial
pathogens (24), were identified as a
fundamental attributable factor to the association between HCC
metastasis and chronic periodontitis. Furthermore, Severi et
al identified that ROS were involved in the transcriptional
activation of a large series of cytokines and growth factors that
ultimately lead to malignant transformation (25). The results indicated that
periodontitis may affect HCC by increasing the number of
circulating ROS. Tamaki et al (23) recruited 64 patients with HCC,
including 31 patients with chronic periodontitis and 33
periodontally healthy controls, and recorded the Japan Integrated
Stage (JIS), which combines the tumor, lymph node, metastasis and
Child-Turcotte-Pugh systems, and serum levels of reactive oxygen
metabolites (ROM) in all patients. The results demonstrated that
patients with HCC and periodontitis had higher JIS scores and
circulating ROS levels compared with patients with HCC but without
periodontitis (23). Therefore, the
authors concluded that the stage of HCC may be associated with
periodontitis, and increased ROS serum levels caused by
periodontitis may be detrimental to hepatic health in patients with
HCC (23). Another study proposed
that inflammation may result in the migration of metastatic cells
to the gingival soft tissues, as well as affecting where they
invade, multiply and form a new tumor (26). However, metastatic spread to the
gingiva from primary tumors is considered to primarily occur via
the hematogenous route (26), the
mechanism for which has yet to be fully determined. The ROM serum
levels of the patient in the present study were not examined;
therefore, an association with metastasis of HCC was not
conclusive.
Metastatic malignant tumors of the oral cavity are
rare. The most common malignancies are lung, breast and renal cell
carcinomas (27,28). However, HCC metastases to the oral
cavity are rarely observed. Therefore, it is difficult to diagnose
gingival metastatic HCC without a history of primary HCC,
particularly in cases of atypical histological morphology, such as
the current patient. The patient's final diagnosis was primarily
dependent on medical history, immunohistochemical analysis results
and a physical examination of the liver. If a history of HCC was
noted in advance in the current patient, the typical
histopathological characteristics of HCC could have been identified
according to the criteria described by Edmondson and Steiner
(18,29). For instance, certain tumor cells were
uniform in size with prominent nuclei and were arranged in
pseudoglandular patterns, characteristic of HCC.
At present, in addition to surgical resection, the
treatment strategies selected for patients with extrahepatic
metastasis are typically TACE and/or administration of the targeted
agent sorafenib, which is an inhibitor of tyrosine protein kinase.
Furthermore, in patients with advanced HCC, concurrent treatment
with sorafenib and TACE may increase the time to progression
compared with TACE monotherapy (30).
The prognosis is poor for patients with extrahepatic metastases and
predominantly depends on the metastatic site at the time of
diagnosis. In the present case, the gingival lesion was initially
identified >1 year prior to presentation at the hospital;
however, the patient refused the proposed treatment regime due to
poor economic conditions. Follow-up was not performed.
In conclusion, the present case illustrated the
difficulties in diagnosing metastatic HCC without a prior history
of primary HCC. The final diagnosis appears to predominantly depend
on the pathological characteristics and immunohistochemical
features of the lesion.
Acknowledgements
The present study was supported by Shaanxi Province
Natural Science basic research projects (grant no. 2015JM8426), the
National Natural Science Foundation of China (grant no. 30800417
and No. 81372226) and the National Basic Research Program (973
Program) of China (grant no. 2015CB553703).
References
|
1
|
International Agency for Research on
Cancer, . Liver cancer: Estimated incidence, mortality and
prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxDecember
12–2013
|
|
2
|
Sawabe M, Nakamura T, Kanno J and Kasuga
T: Analysis of morphological factors of hepatocellular carcinoma in
98 autopsy cases with respect to pulmonary metastasis. Acta Pathol
Jpn. 37:1389–1404. 1987.PubMed/NCBI
|
|
3
|
Katyal S, Oliver JH III, Peterson MS,
Ferris JV, Carr BS and Baron RL: Extrahepatic metastases of
hepatocellular carcinoma. Radiology. 216:698–703. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Natsuizaka M, Omura T, Akaike T, et al:
Clinical features of hepatocellular carcinoma with extrahepatic
metastases. J Gastroenterol Hepatol. 20:1781–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe J, Nakashima O and Kojiro M:
Clinicopathologic study on lymph node metastasis of hepatocellular
carcinoma: A retrospective study of 660 consecutive autopsy cases.
Jpn J Clin Oncol. 24:37–41. 1994.PubMed/NCBI
|
|
6
|
Lapeyrolerie FM and Manhold JH Jr:
Hepatoma metastasic to the gingiva. Oral Surg Oral Med Oral Pathol.
18:365–367. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lund BA, Soule EH and Moertel CG:
Hepatocellular carcinoma with metastasis to gingival mucosa: Report
of case. J Oral Surg. 28:604–607. 1970.PubMed/NCBI
|
|
8
|
Llanes F, Sanz-Ortega J, Suarez B and
Sanz-Esponera J: Hepatocellular carcinomas diagnosed following
metastasis to the oral cavity. Report of 2 cases. J Periodontol.
67:717–719. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radden BF and Reade PC: Gingival
metastasis from a hepatoma. Oral Surg Oral Med Oral Pathol.
21:621–625. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wedgwood D, Rusen D and Balk S: Gingival
metastasis from primary hepatocellular carcinoma. Report of a case.
Oral Surg Oral Med Oral Pathol. 47:263–266. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishimura Y, Yakata H, Kawasaki T and
Nakajima T: Metastatic tumours of the mouth and jaws. A review of
the Japanese literature. J Oral Maxillofac Surg. 10:253–258.
1982.
|
|
12
|
Morishita M and Fukuda J: Hepatocellular
carcinoma metastatic to the maxillary incisal gingiva. J Oral
Maxillofac Surg. 42:812–815. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maiorano E, Piattelli A and Favia G:
Hepatocellular carcinoma metastatic to the oral mucosa: Report of a
case with multiple gingival localizations. J Periodontol.
71:641–645. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanazawa H and Sato K: Gingival metastasis
from primary hepatocellular carcinoma: Report of a case and review
of literature. J Oral Maxillofac Surg. 47:987–990. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramón Ramirez J, Seoane J, Montero J,
Esparza Gómez GC and Cerero R: Isolated gingival metastasis from
hepatocellular carcinoma mimicking a pyogenic granuloma. J Clin
Periodontol. 30:926–929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Greenstein A, Witherspoon R, Iqbal F and
Coleman H: Hepatocellular carcinoma metastasis to the maxilla: A
rare case. Aust Dent J. 58:373–375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Terada T: Hepatocellular carcinoma
metastatic to the gingiva as a first manifestation of
hepatocellular carcinoma. J Maxillofac Oral Surg. 10:271–274. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosai J: Surgical Pathology. Ninth. Mosby;
Maryland Heights, MO: pp. 998–1001. 2004
|
|
19
|
Hamilton SR and Aaltonen LA: Pathology
& Genetics of Tumours of the Digestive SystemWorld Health
Organization Classification of Tumours. 2nd. IARC Press; Lyon,
France: pp. 161–166. 2000
|
|
20
|
Hujoel PP, Drangsholt M, Spiekerman C and
Weiss NS: An exploration of the periodontitis-cancer association.
Ann Epidemiol. 13:312–316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tezal M, Sullivan MA, Hyland A, Marshall
JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, et
al: Chronic periodontitis and the incidence of head and neck
squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
18:2406–2412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tezal M, Sullivan MA, Reid ME, Marshall
JR, Hyland A, Loree T, Lillis C, Hauck L, Wactawski-Wende J and
Scannapieco FA: Chronic periodontitis and the risk of tongue
cancer. Arch Otolaryngol Head Neck Surg. 133:450–454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamaki N, Takaki A, Tomofuji T, Endo Y,
Kasuyama K, Ekuni D, Yasunaka T, Yamamoto K and Morita M: Stage of
hepatocellular carcinoma is associated with periodontitis. J Clin
Periodontol. 38:1015–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sculley DV and Langley-Evans SC: Salivary
antioxidants and periodontal disease status. Proc Nutr Soc.
61:137–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Severi T, van Malenstein H, Verslype C and
van Pelt JF: Tumor initiation and progression in hepatocellular
carcinoma: Risk factors, classification, and therapeutic targets.
Acta Pharmacol Sin. 31:1409–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirshberg A, Leibovich P and Buchner A:
Metastases to the oral mucosa: Analysis of 157 cases. J Oral Pathol
Med. 22:385–390. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Will TA, Agarwal N and Petruzzelli GJ:
Oral cavity metastasis of renal cell carcinoma: A case report. J
Med Case Reports. 2:3132008. View Article : Google Scholar
|
|
28
|
Makos CP and Psomaderis K: A literature
review in renal carcinoma metastasis to the oral mucosa and a new
report of an epulis-like metastasis. J Oral Maxillofac Surg.
67:653–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu H, Duan Z, Long X, Hertzanu Y, Shi H,
Liu S and Yang Z: Sorafenib combined with transarterial
chemoembolization versus transarterial chemoembolization alone for
advanced-stage hepatocellular carcinoma: A propensity score
matching study. PLoS One. 9:e966202014. View Article : Google Scholar : PubMed/NCBI
|