Introduction
Gastric cancer is one of the most prominent causes
of cancer-associated mortalities worldwide (1). At present, surgical resection and
chemotherapy are the two major means of treating gastric cancer.
Despite improvements in treatment, the prognosis of patients with
advanced gastric cancer following curative resection remains poor,
mainly as a result of local or distant metastasis (2). Therefore, the elucidation of novel drugs
or combination chemotherapies for gastric cancer treatment are
urgently required (1,2). As a standard anticancer drug, paclitaxel
(PTX) has an important role in the treatment of a variety of
tumors, including gastric cancer. As previously reported, the
efficiency of the single anticancer drug paclitaxel was 11–23% for
treating advanced-stage gastric cancer, while that of drug
combination therapy reached 50–60% (3). Peganum harmala had been used for
numerous years in traditional medicine in China and other parts of
the world. Harmine (HM), a tricyclic compound found in Peganum
harmala and belonging to the β-carboline alkaloids, has been
proven to possess antitumor properties and was previously examined
for its potential use for cancer therapy (4,5). It was
reported that HM effectively inhibited tumor migration and invasion
in vitro (6). However, the
synergistic antitumor effect of a combination of PTX and HM on
migration and invasion of human gastric cancer cells remains to be
elucidated.
Cyclooxygenase (COX), also termed prostaglandin
synthase, is the rate-limiting enzyme which enables the conversion
of arachidonic acid to prostaglandins. COX exists as two isoforms,
including the constitutive COX-1 and the mitogen-inducible COX-2.
In gastric cancer, COX-2 is continuously expressed and was reported
to be closely associated with tumor invasiveness and metastasis
(7,8).
Previous studies have demonstrated that the inhibition of COX-2 by
selective COX-2 inhibitors or small interfering RNA (siRNA) may
attenuate proliferation and induce apoptosis in human gastric
cancer cells (9,10). It has been reported that HM inhibited
gastric cancer cell migration and invasion through the
downregulation of COX-2 expression.
The matrix metalloproteinase (MMP) family consists
of a group of closely associated enzymes involved in the cleavage
of structural components of the extracellular matrix (ECM); these
enzymes are considered to be essential factors involved in tumor
invasion and metastasis (11,12). In gastric cancer, MMP-9 and MMP-2 were
reported to be overexpressed and associated with tumor
aggressiveness (11,12). In addition, a previous study
demonstrated that MMP-9 expression was significantly correlated
with COX-2 expression in human gastric cancer.
The aim of the present study was to evaluate whether
the combination of PTX and HM exerted synergistic antitumor effects
on the migration and invasion of two human gastric cancer cell
lines, SGC-7901 and MKN-45, in which COX-2 is known to be expressed
(13). In addition, the present study
aimed to explore the possible mechanism by which this effect may
proceed.
Materials and methods
Reagents
PTX, HM (purity, >98%), dimethyl sulfoxide (DMSO)
and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
(MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). The
chemical structures of PTX and HM are shown in Fig. 1. Stock solutions of PTX and HM were
diluted in DMSO (≤0.1%) and sterilized by passage through a 0.22-µm
pore size filter (Immobilon®; EMD Millipore Corp., Bedford, MA,
USA), then diluted with culture media prior to use. RPMI-1640
medium, fetal bovine serum (FBS) and penicillin/streptomycin were
obtained from Gibco BRL (Grand Island, NY, USA). All other
chemicals were of analytical grade and used without further
purification.
Cell lines and culture
Human poorly-differentiated MKN-45 and
moderately-differentiated SGC-7901 gastric cancer cell line were
purchased from the Shanghai Institute of Cell Biology (Shanghai,
China). Cells were cultured in RPMI-1640 medium supplemented with
10% FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin at 37°C
in a humidified incubator with 5% CO2.
MTT cell proliferation assay
SGC-7901 and MKN-45 cells (200 µl/well) were seeded
at a density of 1×104 cells in 96-well plates and
incubated overnight in 10% FBS medium. Cells were then administered
various concentrations of PTX or HM, alone or in combination, in
serum-free conditions. The control group consisted of cells
incubated in serum-free medium. Following 48 h of incubation at
37°C, 20 µl MTT solution [5 mg/ml in phosphate buffered saline
(PBS)] was added to each well and incubated for a further 4 h at
37°C. Subsequently, 100 µl DMSO was added into each well and
incubated at 37°C for 2 h. Optical density (OD) values were
measured using a spectrophotometer (FLx800; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 570 nm. All experiments were performed
in triplicate and the results are presented as the percentages
relative to the controls.
In vitro Transwell® migration
assays
Migration assays were performed in 24-well
Transwell® chambers (Corning Life Sciences, Inc., Tewksbury, MA,
USA) without Matrigel®. In brief, SGC-7901 and MKN-45 cells were
allowed to grow to 80% confluence and were serum-starved for 48 h
at 37°C in a humidified incubator with an atmosphere of 5%
CO2. Following detachment using trypsin (Sigma-Aldrich),
the cells in different groups were washed with PBS, resuspended in
serum-free medium and 1×105 cells/group were added to
the upper chamber. Complete medium was added to the bottom chamber
as a chemoattractant. Unmigrated cells on the upper surface of the
filter were mechanically removed using a cotton swab and the
invasive cells on the bottom membrane surface were then fixed with
methanol (Grand Island, NY, USA) and stained with 0.1% crystal
violet (Immobilon, Millipore Corp., Bedford, MA, USA). The
migrating cells were counted and images were captured using a
microscope (magnification, x100; Eclipse E800; Nikon Corporation,
Tokyo, Japan). All experiments were performed in triplicate and
nine fields of vision were counted per filter in each group.
In vitro Transwell® invasion
assays
Invasion assays were performed in 24-well Transwell®
chambers containing polycarbonate filters with 8-µm pores coated
with Matrigel® (BD Biosciences, Bedford, MA, USA). The remaining
steps were identical to those described for the Transwell®
migration assays.
Western blot analysis
Cells were treated with 2 ng/ml PTX, 4 µg/ml HM or a
combination of these two drugs for 48 h at 37°C in a humidified
incubator with an atmosphere of 5% CO2. Cells were then
washed twice with ice-cold PBS and protein extraction was performed
by lysis in RIPA buffer [150 mM NaCl, 1% (v/v) NP-40, 0.5% (w/v)
sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS), 50 mM
Tris HCl (pH 8), 10 mM EDTA and 1 mM phenylmethylsulfonyl fluoride;
Sigma-Aldrich] for 30 min at 4°C, followed by centrifugation for 15
min at 12,000 × g. Protein concentrations were determined using a
bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL,
USA) according to the manufacturer's instructions. Subsequently,
proteins (60 µg) were loaded onto a 10% (w/v) SDS-polyacrylamide
gel, electrophoresed and transferred onto a polyvinylidene fluoride
membranes (EMD Millipore Corp., Billerica, MA, USA) which was then
blocked for 2 h at room temperature with blocking buffer
[Tris-buffered saline containing 0.1% (v/v) Tween 20
(Sigma-Aldrich) and 5% (w/v) milk powder]. The following primary
antibodies (1:1,000) were applied for 1 h at room temperature or
overnight at 4°C: Polyoclonal rabbit anti-mouse COX-2 (cat. no.
13314; Cell Signaling Technology, Inc., Beverly, MA, USA),
polyclonal rabbit anti-mouse MMP-9 (cat. no. sc-21733; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and polyclonal rabbit
anti-mouse GAPDH (cat. no. G5262; Sigma-Aldrich). Membranes were
then incubated for 2 h with polyclonal goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1:20,000; cat. no.
BA-1000; Vector Laboratories, Inc., Burlingame, CA, USA) at 37°C in
a humidified incubator with an atmosphere of 5% CO2.
Membranes were then visualized using an enhanced chemiluminescence
kit and signals were quantified by scanning densitometry (Quantity
One software; Bio-Rad Laboratories, Inc.). The relative expression
levels of COX-2 and MMP-9 were normalized to that of GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS
version 11.0 software (SPSS, Inc., Chicago, IL, USA). Values are
presented as the mean ± standard deviation and were analyzed using
two-tailed Student's t-test or one-way analysis of variance with
Dennett's multiple comparison tests. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Effects of PTX and HM on the
proliferation of SGC-7901 and MKN-45 cells
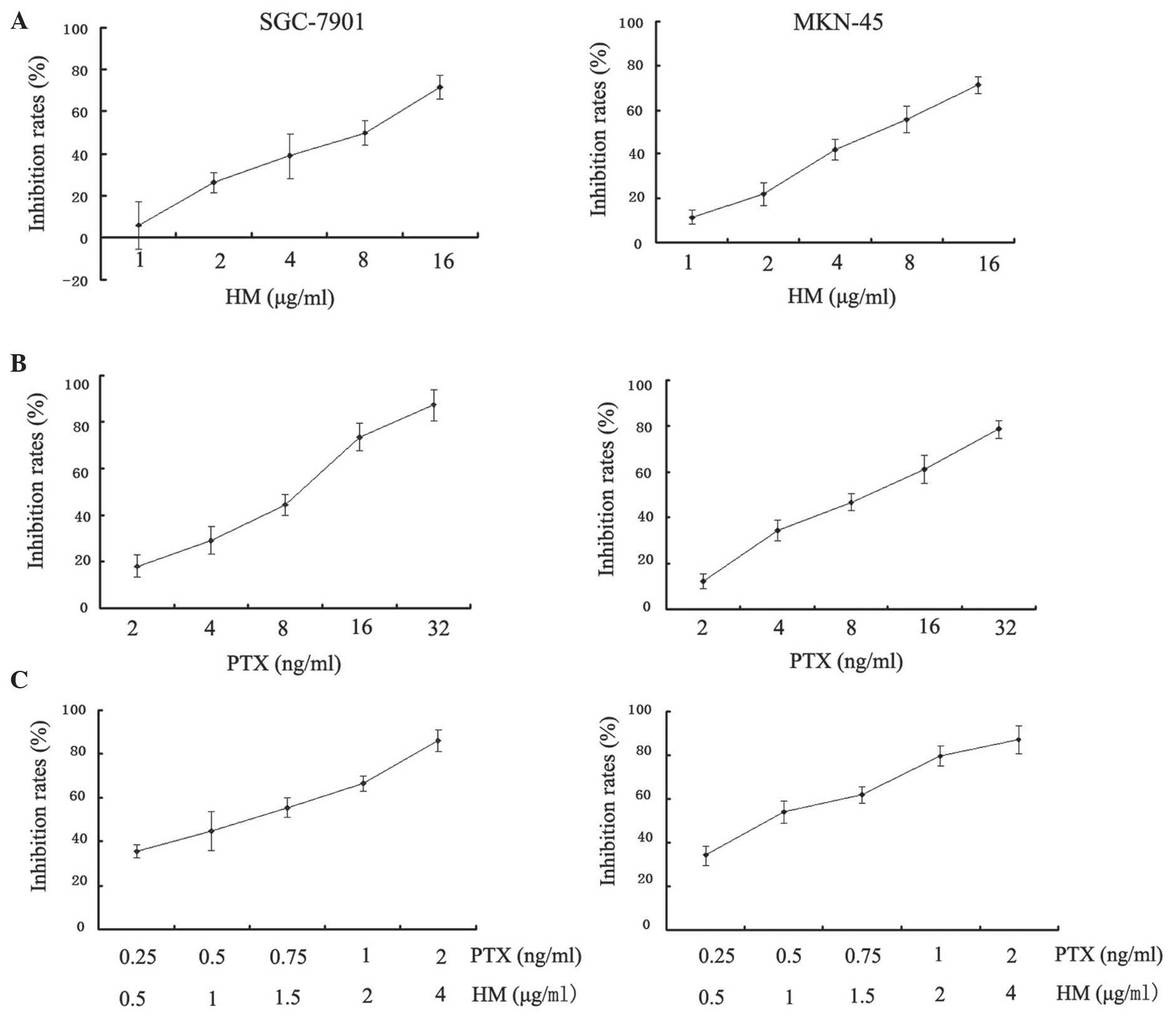
MTT assays were performed in order to analyze the
metabolic activity of proliferating cells. The results demonstrated
that PTX and HM inhibited the cell proliferation in a
dose-dependent manner when administered individually (Fig. 2A and B). In addition, the inhibitory
effects of combined treatment with PTX and HM on cell proliferation
were significantly enhanced and produced marked inhibition at lower
concentrations in SGC-7901 and MKN-45 cells (P<0.05; Fig. 2C).
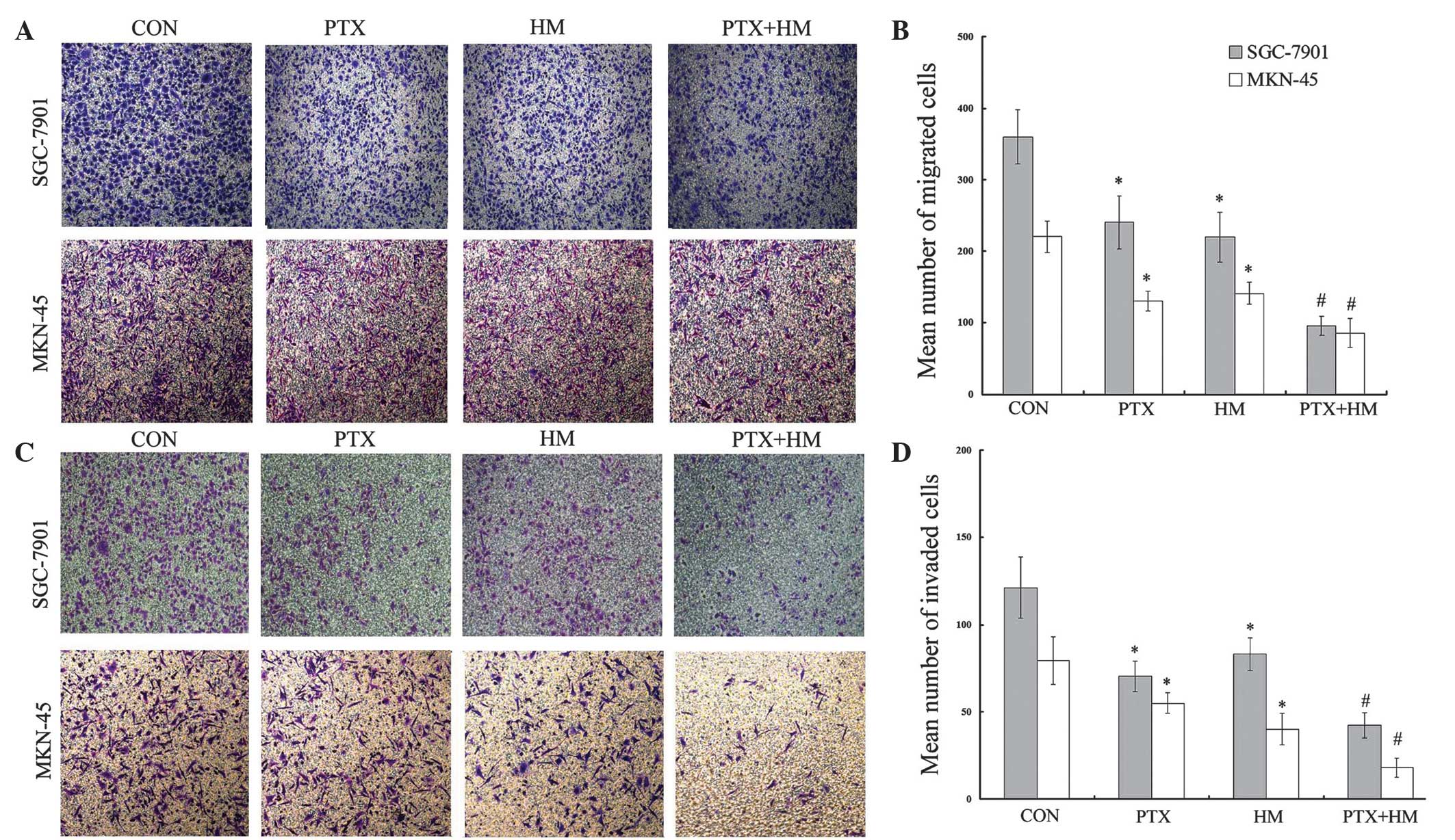
Effects of PTX and HM on the migration
of SGC-7901 and MKN-45 cells
In order to evaluate the effects of PTX and HM on
the invasion and metastasis of MKN-45 and SGC-7901 cells,
Transwell® migration assays were performed. As shown in Fig. 3A and B, the number of migrated cells
in the individually-treated PTX and HM groups were significantly
reduced compared with the control group (P<0.05). In addition,
the number of migrated cells in the combination group was further
attenuated compared with the single drug groups (P<0.05).
Effects of PTX and HM on the invasion
of SGC-7901 and MKN-45 cells
The effects of PTX and HM on gastric cancer cell
invasion were evaluated using Matrigel®-coated Transwell® invasion
assays. The results demonstrated that, when treated individually,
PTX and HM significantly inhibited the invasion ability of gastric
cancer cells compared with the control group (P<0.05). In
addition, the results clearly revealed that the combined treatment
of PTX and HM resulted in a significant decrease in the number of
invaded cells compared with the effects of either PTX or HM alone
(P<0.05; Fig. 3C and D).
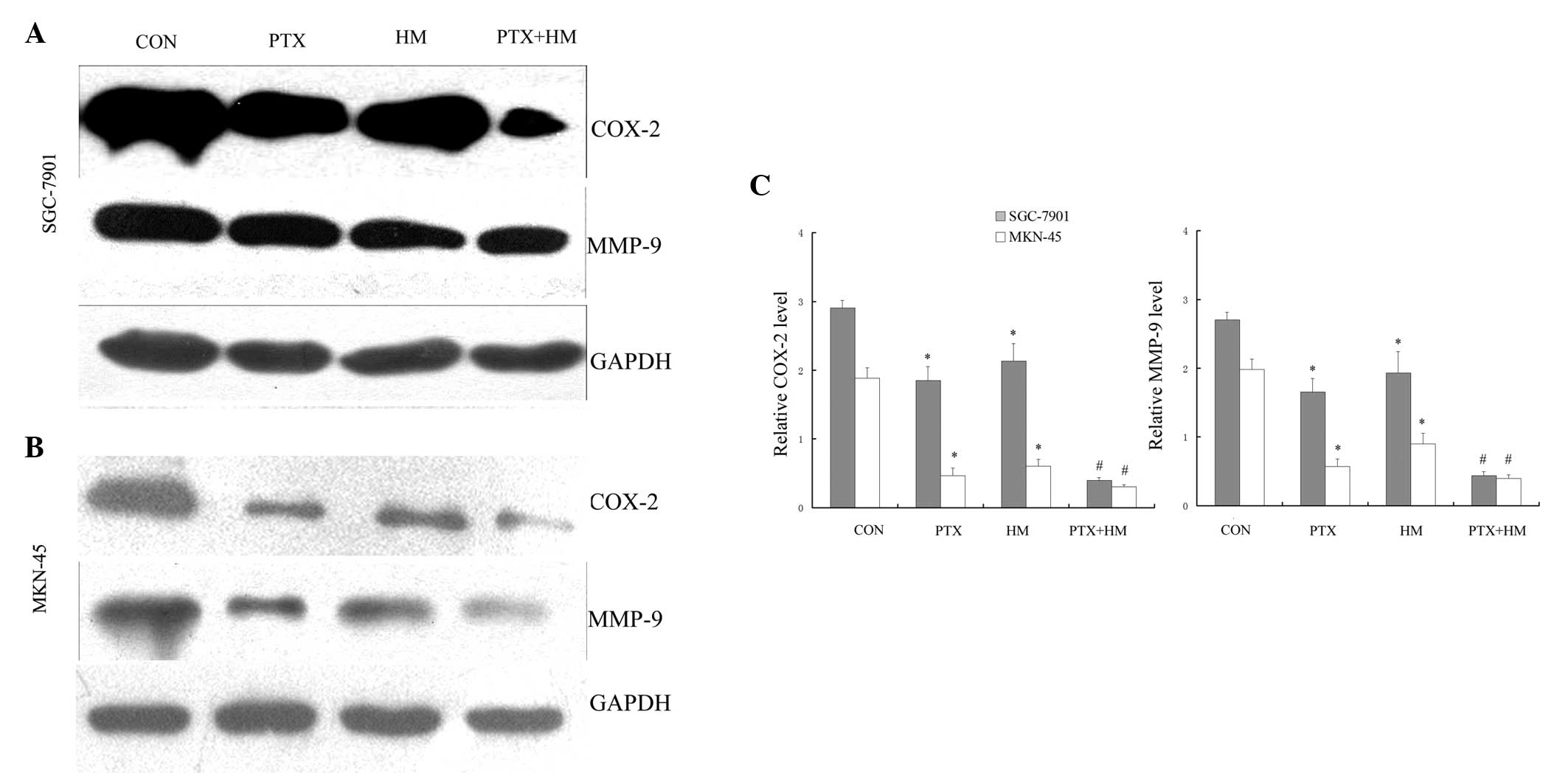
Effects of PTX and HM on COX-2 and
MMP-9 expression in SGC-7901 and MKN-45 cells
As determined using western blot analysis, the
exposure of SGC-7901 and MKN-45 gastric cancer cells to PTX or HM,
individually, markedly decreased the expression levels of COX-2 and
MMP-9 (P<0.05; Fig. 4A and B). In
addition, the combined application of PTX and HM resulted in a
further reduction in COX-2 and MMP-9 expression compared with the
effects of either of drugs alone (P<0.05; Fig. 4C).
Discussion
Gastric cancer, one of the most prevalent types of
malignant tumors, is a major cause of cancer-associated mortality.
Invasion and metastasis are important biological behaviors of
malignant tumors which affect the prognosis and effective treatment
of gastric cancer (14,15). Invasion and metastasis are a complex
and continuous multi-step process, for which degradation of the ECM
is critical. It is therefore of great significance for reducing the
metastasis of gastric cancer to take effective preventive measures
against these processes. A previous study reported that the use of
Traditional Chinese Medicine against invasion and metastasis in
cancer is increasingly popular. Numerous studies have demonstrated
that certain Traditional Chinese Medicines and their extracts may
effectively inhibit tumor invasion and metastasis (5,6).
Paclitaxel has been reported to be an important drug
for treating gastric cancer, as it was shown to effectively prolong
the survival time of gastric cancer patients and improve their
quality of life (16). However, a
number of clinical and experimental studies in recent years have
discovered that several types of malignant tumors including breast
cancer, lung cancer, ovarian cancer and gastric cancer exhibited
primary or secondary drug resistance to paclitaxel (17). Therefore, it is important to explore a
novel methods of enhancing the anticancer effect of PTX and to
elucidate novel therapies that may reverse the resistance to PTX in
cancer patients. Harmine (HM), a tricyclic compound belonging to
the β-carboline alkaloids, has been demonstrated to inhibit
migration and invasion in gastric cancer in vitro (6). However, the synergistic antitumor effect
of a combined PTX and HM treatment on migration and invasion in
human gastric cancer cells remained to be elucidated.
COX-2 is a molecular target for cancer prevention
and treatment that has been extensively studied over the past
decade (10). COX-2 overexpression
was reported to be involved in the genesis and development of
gastric cancer and was suggested to be closely associated with the
invasiveness and metastasis of gastric cancer (6). It has been well-established that
prostaglandins are synthesized from arachidonate under the
catalytic effects of COX-2 and these prostaglandins may enhance the
activity of several MMPs, strengthen CD44 expression and reduce the
expression of epithelial cadherin, resulting in the reinforced
invasiveness of tumors (18–20). It was previously demonstrated that HM
significantly inhibited COX-2 expression in BGC-823 and SGC-7901
cells (6). The western blot analysis
results of the current study revealed that the combined application
of PTX and HM resulted in a marked decrease in the expression of
COX-2 compared with the effects of either drug alone.
Tumor invasion and metastasis are known to be the
major causes of morbidity and mortality in gastric cancer patients.
Tumor cell-induced ECM and basement membrane degradation is a
critical step in the processes of tumor invasion and metastasis
(12). MMP-9, an important isoform in
the MMP family, is considered to be closely correlated with tumor
invasion and metastasis, which may be due to the degradation of
type IV collagen (11). Numerous
in vivo and in vitro data have demonstrated that
inducing MMP-9 expression may be one of the mechanisms by which
COX-2 promotes the development and metastasis of gastric cancer
(6,7).
The present study has demonstrated that PTX combined with HM
resulted in a marked decrease in COX-2 and MMP-9 expression
compared with the effects of either of drugs alone in SGC-7901 and
MKN-45 cells. Furthermore, in vitro migration and invasion
assays have confirmed that PTX combined with HM exerted an enhanced
inhibitory effect on the migration and invasion of gastric cancer
cells compared with that of individual treatments.
In conclusion, the present study demonstrated that
PTX combined with HM inhibited the migration and invasion of human
gastric cancer cells, the mechanism of which may proceed via the
downregulation of COX-2 and MMP-9. It has been previously reported
that overexpression of COX-2 was involved in apoptosis resistance,
angiogenesis, decreased host immunity as well as enhanced invasion
and metastasis. Further studies are required to elucidate whether
the synergistic effect of PTX and HM also exerts in the gastric
cancer in vivo.
References
|
1
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006.PubMed/NCBI
|
|
2
|
Lee JH, Kim KM, Cheong JH and Noh SH:
Current management and future strategies of gastric cancer. Yonsei
Med J. 53:248–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakamoto J, Matsui T and Kodera Y:
Paclitaxel chemotherapy for the treatment of gastric cancer.
Gastric Cancer. 12:69–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao MR, Li Q, Liu ZL, Liu HH, Wang W, Liao
XL, Pan YL and Jiang JW: Harmine induces apoptosis in HepG2 cells
via mitochondrial signaling pathway. Hepatobiliary Pancreat Dis
Int. 10:599–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao X, Che X, Zhao W, Zhang D, Bi T and
Wang G: The β -adrenoceptor antagonist, propranolol, induces human
gastric cancer cell apoptosis and cell cycle arrest via inhibiting
nuclear factor κB signaling. Oncol Rep. 24:1669–1676.
2010.PubMed/NCBI
|
|
6
|
Zhang H, Sun K, Ding J, Xu H, Zhu L, Zhang
K, Li X and Sun W: Harmine induces apoptosis and inhibits tumor
cell proliferation, migration and invasion through down-regulation
of cyclooxygenase-2 expression in gastric cancer. Phytomedicine.
21:348–355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun WH, Sun YL, Fang RN, Shao Y, Xu HC,
Xue QP, Ding GX and Cheng YL: Expression of cyclooxygenase-2 and
matrix metalloproteinase-9 in gastric carcinoma and its correlation
with angiogenesis. Jpn J Clin Oncol. 35:707–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang F, Lin C, Shi YH and Kuerban G:
MicroRNA-101 inhibits cell proliferation, invasion, and promotes
apoptosis by regulating cyclooxygenase-2 in Hela cervical carcinoma
cells. Asian Pac J Cancer Prev. 14:5915–5920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan MW, Wong CY, Cheng AS, Chan VY, Chan
KK, To KF, Chan FK, Sung JJ and Leung WK: Targeted inhibition of
COX-2 expression by RNA interference suppresses tumor growth and
potentiates chemosensitivity to cisplatin in human gastric cancer
cells. Oncol Rep. 18:1557–1562. 2007.PubMed/NCBI
|
|
10
|
Sun WH, Zhu F, Chen GS, Su H, Luo C, Zhao
QS, Zhang Y, Shao Y, Sun J, Zhou SM, et al: Blockade of
cholecystokinin-2 receptor and cyclooxygenase-2 synergistically
induces cell apoptosis, and inhibits the proliferation of human
gastric cancer cells in vitro. Cancer Lett. 263:302–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ram M, Sherer Y and Shoenfeld Y: Matrix
metalloproteinase-9 and autoimmune diseases. J Clin Immunol.
26:299–307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Velinov N, Poptodorov G, Gabrovski N and
Gabrovski S: The role of matrixmetalloproteinases in the tumor
growth and metastasis. Khirurgiia (Sofiia). 1:44–49. 2010.(In
Bulgarian). PubMed/NCBI
|
|
13
|
He XP, Shao Y, Li XL, Xu W, Chen GS, Sun
HH, Xu HC, Xu X, Tang D, Zheng XF, et al: Downregulation of miR-101
in gastric cancer correlates with cyclooxygenase-2 overexpression
and tumor growth. FEBS J. 279:4201–4212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Yang Q, Liu B and Zhu Z: Serum
proteomics for gastric cancer. Clin Chim Acta. 431:179–184. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Neugut AI, Hayek M and Howe G:
Epidemiology of gastric cancer. Semin Oncol. 23:281–291.
1996.PubMed/NCBI
|
|
16
|
Tuan TF, Tsai ML, Yeh KC, Huang HC, Chung
CT, Huang CL, Han CH, Chen CP, Wang MH, Shen CC, et al: Intravenous
paclitaxel against metastasis of human gastric tumors of diffuse
type. Cancer Chemother Pharmacol. 66:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Papadaki C, Mavroudis D, Trypaki M,
Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki E,
Georgoulias V and Souglakos J: Tumoral expression of TXR1 and TSP1
predicts overall survival of patients with lung adenocarcinoma
treated with first-line docetaxel-gemcitabine regimen. Clin Cancer
Res. 15:3827–3833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams CS, Smalley W and DuBois RN:
Aspirin use and potential mechanisms for colorectal cancer
prevention. J Clin Invest. 100:1325–1329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Mahony CA, Beauchamp RD, Albo D, Tsujii
M, Sheng HM, Shao J, Dubois RN and Berger DH: Cyclooxygenase-2
alters transforming growth factor-beta 1 response during intestinal
tumorigenesis. Surgery. 126:364–370. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu XH, Kirschenbaum A, Yao S, Stearns ME,
Holland JF, Claffey K and Levine AC: Upregulation of vascular
endothelial growth factor by cobalt chloride-simulated hypoxia is
mediated by persistent induction of cyclooxygenase-2 in a
metastatic human prostate cancer cell line. Clin Exp Metastasis.
17:687–694. 1999. View Article : Google Scholar : PubMed/NCBI
|