Introduction
Esophageal squamous cell carcinoma (ESCC) is ranked
as the sixth leading cause of cancer-associated mortality
worldwide, and is prevalent and particularly common in Asia
(1). Although significant progress
has been made in the development of surgical and adjuvant
chemoradiotherapeutic techniques, the diagnosis and prognosis of
patients with ESCC has remained poor. Investigations into
alterations in the levels of specific proteins that occur in this
cancer may provide clues to indicate novel biomarkers, required in
order to improve diagnosis and guide targeted therapy.
Angiogenesis is required for tumor growth, and can
be quantified as the microvessel density (MVD), which has become
the morphological gold standard used to assess the neovasculature
of human tumors (2). Newly formed
microvessels are able to be visualized by cluster of
differentiation 31 (CD31) staining in endothelial cells (3). However, there are disadvantages to this
technique of angiogenesis detection, including potential
cross-reactions with plasma cells and loss of antigens due to
fixatives. Results using anti-CD31 antibody alone may erroneously
indicate MVD in the diagnosis and prognosis of human tumors
(2).
Vascular endothelial growth factor (VEGF) is an
angiogenic factor that promotes the proliferation and migration of
endothelial cells, enhances the permeability of blood vessels,
reduces apoptosis of endothelial cells and promotes stromal
proteolysis (4). VEGF therefore has a
critical role in angiogenesis in numerous solid malignancies. The
effect of VEGF on progression and recurrence of esophageal cancer
has previously been investigated (5);
however, the association between VEGF overexpression and the
prognosis of patients with ESCC remains controversial.
Phosphate and tensin homology (PTEN) is a
phosphatase with dual-specificity for proteins and lipids, which
inhibits cell migration, spreading and focal adhesions (6). Studies of certain cancer cell lines have
suggested that PTEN dysregulation may be involved in almost all
types of cancer, including solid tumors and hematological
malignancies (7–9). A study revealed that decreased levels of
PTEN were correlated with tumor differentiation, infiltration depth
and pTNM staging (10). The 5-year
survival rate of patients with PTEN-positive ESCC was significantly
higher than that of patients with PTEN-negative ESCC (10). This suggested that PTEN may have a
significant role in carcinogenesis and the progression of ESCC, and
therefore, may have significant clinical implications.
Analysis of a single protein biomarker is
insufficient for accurate prediction of clinical outcome. CD31,
PTEN and VEGF are functionally distinct molecules and may underlie
various mechanisms in the pathogenesis and progression of specific
types of cancer. The objective of the present study was therefore
to investigate the clinical implications of PTEN and VEGF
expression status and MVD, as well as their associations in ESCC,
in order to provide insight into the development of techniques for
the diagnosis and prognosis of ESCC. The PTEN and VEGF levels and
MVD were determined in ESCC tumor and normal specimens from
patients using immunohistological staining.
Materials and methods
Patients and specimens
The Institutional Review Board of Rizhao People's
Hospital (Rizhao, China) approved the present study, and all
participants provided written informed consent.
The study included 50 patients with ESCC (31 male,
19 female; aged 57.45±7.53 years; age range, 30–75 years) who
underwent esophagectomy at the First Affiliated Hospital of
Southern Medical University (Guangzhou, China) or Rizhao People's
Hospital between January 2009 and January 2014. None of the
patients had received radiotherapy or chemotherapy prior to
surgery. ESCC specimens and adjacent normal mucosa were collected
via gastrointestinal endoscopic biopsy.
ESCC specimens were confirmed by tumor
histopathology, with screening and evaluation conducted by two
experienced pathologists blinded to the clinical information.
Normal specimens were collected from the distal normal mucosa.
Within 30 min of surgery, each specimen was divided into two
sections: One was flash-frozen in liquid nitrogen and stored at
−80°C, while the other was stored in 4% buffered formamide (Laiyang
Fine Chemical Factory, Laiyang, China). Specimens stored in 4%
buffered formamide were used for standard clinicopathological
diagnosis and immunohistological staining.
Immunohistological staining
All materials were purchased from Fuzhou Maixin
Biotechnology Development Co., Ltd. (Fuzhou, China), unless
otherwise stated. The PTEN and VEGF levels, as well as the presence
of CD31 was detected by immunohistochemistry using an
Ultrasensitive S-P kit, in accordance with the manufacturer's
instructions. Briefly, specimen slices were dewaxed and then
incubated in 3% hydrogen peroxide for 30 min at room temperature to
block endogenous peroxidase activity. Following rinsing with
phosphate-buffered saline (PBS; batch no. 14122303) and incubation
in citrate buffer (10 mM, pH 6.0; batch no. 14073001) at 95°C for
20 min, slices were blocked with rabbit serum for 30 min. The
slices were then incubated with biotin-conjugated primary
antibodies [monoclonal anti-PTEN (cat. no. MAB-0369), anti-VEGF
(cat. no. MAB-0243) or anti-CD31 (cat. no. MAB-0031), all 1:100) at
4°C overnight, prior to incubation with horseradish
peroxidase-labeled streptavidin (1:50) at 37°C for 30 min. After
rinsing with PBS, diaminobenzidine (batch no. 1409030031) was added
and the sections were washed with tap water. Subsequently, the
slices were processed sequentially with hematoxylin (Shanghai Ruji
Biotechnology Development Co., Ltd., Shanghai, China), alcohol
dehydration and xylene (Shanghai Aibi Chemistry Preparation Co.,
Ltd, Shanghai, China). Finally, the slices were mounted on slides
and sealed with neutral balsam resin (Genetech Co., Ltd., Shanghai,
China).
Breast cancer tissues known to express PTEN and
colon cancer tissues known to express VEGF were prepared by
histologists in the Department of Histology, Rizhao People's
Hospital. These were used as references for PTEN and VEGF
expression, respectively.
Determination of PTEN-and
VEGF-positivity, and MVD
Following immunohistological staining with anti-PTEN
antibody, brownish-yellow or light yellow granules in the cytoplasm
or nuclei of cells were considered to indicate PTEN expression.
Using an Olympus CH30 microscope (Olympus Corporation, Tokyo,
Japan), ten microscopic fields (magnification, x400) were randomly
selected and >1,000 cells were counted for each specimen. If
≤10% of cells expressed PTEN, the specimen was scored as
PTEN-negative. If 11–100% of the cells expressed PTEN, the specimen
was scored as PTEN-positive.
For specimens stained with anti-VEGF antibody,
brownish-yellow granules in the cytoplasm or cytoplasmic membrane
of cells were considered indicative of VEGF expression. If ≤50% of
cells contained VEGF, the specimen was scored as VEGF-negative,
whereas if 51–100% of cells contained VEGF, the specimen was scored
as VEGF-positive.
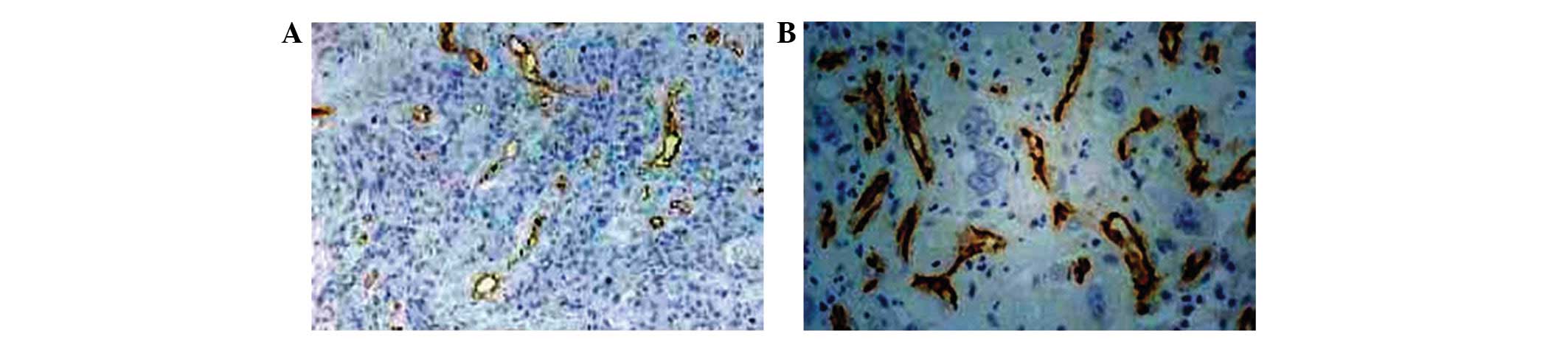
MVD was determined in accordance with a previously
published method (4). The
microvessels were identified by immunohistological staining for
CD31. The number of microvessels was counted in three randomly
selected microscopic fields in the vascular areas (magnification,
x400), and the mean was calculated.
Statistical analysis
The χ2 test, one-way analysis of variance
and Student's t-test were used to evaluate data. P<0.05
was considered to indicate a statistically significant difference
for all tests. All statistical analyses were performed using SPSS
18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Histopathological characteristics of
ESCC
The ESCC tumors were classified based on
pathological characteristics. According to the differentiation
status (11), each ESCC specimen was
assigned one of three grades: 19 cases were well differentiated
(Grade I), 17 cases were moderately differentiated (Grade II) and
14 cases were poorly differentiated (Grade III). ESCC specimens
were also divided by depth of invasion: 15 cases were classified as
level I, in which the tumor was found in the mucosa, submucosa or
superficial muscle; while 35 cases were level II, in which the
tumor was in the deep muscular or outer layer. A total of 19 cases
were positive for metastasis, where tumors were identified in the
esophageal lymph nodes of the ESCC specimens. No tumors were
detected in the esophageal lymph nodes of the remaining 31
specimens (the metastasis-negative group).
MVD and PTEN/VEGF expression in ESCC
and normal specimens
To determine differences in the MVD, as well as PTEN
and VEGF expression status between ESCC and normal tissue
specimens, an immunohistological staining assay using anti-CD31,
anti-PTEN and anti-VEGF antibodies, respectively, was performed
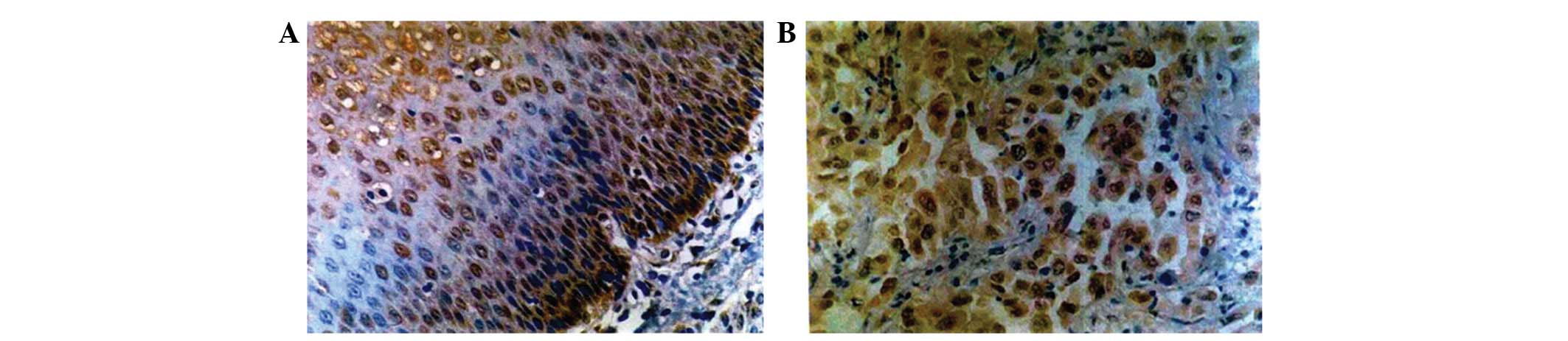
(Figs. 1–3).
The positive or negative status of PTEN and VEGF
expression in the specimens was determined by the percentage of
cells that were positively stained (Table
I). Of the 50 ESCC specimens, 27 and 23 cases were
PTEN-positive and -negative, respectively, while all normal healthy
specimens were PTEN-positive.
 | Table I.Association between PTEN status, VEGF
status, MVD and ESCC. |
Table I.
Association between PTEN status, VEGF
status, MVD and ESCC.
| A, PTEN
expression |
|
|
|
|
|
|
|---|
|
|---|
| Specimen type | Cases, n | Negative, n | Positive, n | Positive rate, % | χ2 | P-value |
|---|
| Normal | 50 | 0 | 50 | 100 | 27.8701 | <0.0000 |
| ESCC | 50 | 23 | 27 | 54 |
|
|
|
| B, VEGF
expression |
|
|
|
|
|
|
|
| Specimen type | Cases, n | Negative, n | Positive, n | Positive rate, % | χ2 | P-value |
|
| Normal | 45 | 45 | 0 | 0 | 47.6557 | <0.0000 |
| ESCC | 50 | 16 | 34 | 68 |
|
|
|
| C, MVD |
|
|
|
|
|
|
|
| Specimen type | Cases, n |
| MVD/microscopic
field, mean±SD |
|
t-valueaa | P-value |
|
| Normal | 47 |
| 22.75±8.82 |
| 8.2205 | <0.0000 |
| ESCC | 50 |
| 37.82±9.21 |
|
|
|
A total of 34 cases of ESCC were VEGF-positive,
while none of the normal esophageal mucosa samples were
VEGF-positive. The MVD was significantly higher in ESCC tissues
(37.82±9.21/microscopic field) than that of the normal esophageal
mucosa (22.75±8.82/microscopic field; P<0.01). These results
suggested that the expression of PTEN was significantly decreased,
while VEGF expression and MVD were significantly increased in ESCC,
compared with that of the normal specimens. Therefore, PTEN and
VEGF levels and the MVD may be clinically implicated in ESCC.
To determine whether PTEN and VEGF levels and MVD
were associated with ESCC, a χ2 test was performed. The
results indicated that PTEN-negative status, VEGF-positive status
and greater MVD were significantly correlated with ESCC (Table I). These results suggested that a low
percentage of cells staining positive for PTEN, a high percentage
staining positive for VEGF and high MVD may be used in the
diagnosis of ESCC.
Association between PTEN status, VEGF
status, MVD and clinicopathological characteristics of ESCC
To determine whether PTEN and VEGF levels and MVD
were associated with histological grades of ESCC, a χ2
test was used to examine the correlation between PTEN and VEGF
expression status, the MVD, and the differentiation status of ESCC.
PTEN-positive status was found to be negatively correlated with the
histological grade of ESCC (P<0.01), while VEGF-positive status
and MVD were not correlated with the histological grade of ESCC
(P>0.05; Table II). The results
suggested that loss of PTEN expression may be an indicator of
differentiation status, carcinogenesis and progression of ESCC.
 | Table II.Associations between PTEN status,
VEGF status and MVD, and histological characteristics of ESCC. |
Table II.
Associations between PTEN status,
VEGF status and MVD, and histological characteristics of ESCC.
| A, PTEN
expression |
|
|
|
|
|
|
|---|
|
|---|
| Histological
characteristic | Cases, n | PTEN - | PTEN + | Positive rate,
% | χ2 | P-value |
|---|
| Differentiation
grade |
|
|
|
|
|
|
| I | 19 | 4 | 15 | 78.9 | 11.4693 | 0.0032 |
| II | 17 | 10 | 7 | 41.2 |
|
|
|
III | 14 | 11 | 3 | 21.4 |
|
|
| Depth of
invasion |
|
|
|
|
|
|
| Level
I | 15 | 3 | 12 | 80.0 | 6.7308 | 0.0095 |
| Level
II | 35 | 21 | 14 | 40.0 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
Positive | 19 | 13 | 10 | 31.6 | 6.2019 | 0.0128 |
|
Negative | 31 | 6 | 21 | 67.7 |
|
|
|
| B, VEGF
expression |
|
|
|
|
|
|
|
| Histological
characteristic | Cases, n | VEGF - | VEGF + | Positive rate,
% | χ2 | P-value |
|
| Differentiation
grade |
|
|
|
|
|
|
| I | 19 | 9 | 10 | 52.6 | 2.3596 | 0.3073 |
| II | 17 | 6 | 11 | 64.7 |
|
|
|
III | 14 | 3 | 11 | 78.6 |
|
|
| Depth of
invasion |
|
|
|
|
|
|
| Level
I | 15 | 7 | 8 | 53.3 | 1.0582 | 0.3036 |
| Level
II | 35 | 11 | 24 | 68.6 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
Positive | 19 | 3 | 16 | 84.2 | 5.4329 | 0.0198 |
|
Negative | 31 | 15 | 16 | 51.6 |
|
|
|
| C, MVD |
|
|
|
|
|
|
|
| Histological
characteristic | Cases, n |
| MVD/microscopic
field, mean±SD |
|
F-valueaa | P-value |
|
| Differentiation
grade |
|
|
|
|
|
|
| I | 19 |
| 32.53±9.64 |
| 2.6800 | 0.0788 |
| II | 17 |
| 35.35±8.75 |
|
|
|
|
III | 14 |
| 40.26±10.16 |
|
|
|
| Depth of
invasion |
|
|
|
|
|
|
| Level
I | 15 |
| 30.52±8.54 |
| 3.8255 | 0.0004 |
| Level
II | 35 |
| 42.35±10.57 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
Positive | 19 |
| 46.57±5.62 |
| 7.3763 | <0.0000 |
|
Negative | 31 |
| 35.76±4.64 |
|
|
|
To determine whether levels of PTEN and VEGF and MVD
may be used to predict the depth of ESCC invasion, a χ2
test was performed to examine correlation between the levels of
PTEN and VEGF, and MVD, and the depth of ESCC invasion (Table II). The results indicated that a
PTEN-positive status was negatively correlated with depth of ESCC
invasion (P<0.01), while VEGF-positive status exhibited no
correlation and MVD was positively correlated with depth of ESCC
invasion (P<0.001). These results suggested that decreased PTEN
expression and increased MVD, but not VEGF, were indicators of ESCC
invasion.
To determine whether PTEN status, VEGF status, and
MVD were viable indicators for predicting metastasis of ESCC, a
χ2 test was used to examine the correlation between PTEN
status, VEGF status and MVD, and the lymph node metastasis status
of ESCC. The results indicated that PTEN-negative and VEGF-positive
status, and higher MVD were correlated with lymph node metastasis
of ESCC specimens (P<0.05; Table
II). These results suggested that decreased PTEN levels and
increased VEGF and MVD were indicators of metastasis in ESCC.
Association between PTEN status, VEGF
status and MVD in ESCC
To determine whether PTEN levels were associated
with VEGF levels in ESCC, a χ2 test was performed
(Table III). The results revealed
that the PTEN expression profile was negatively correlated with the
VEGF expression profile (P<0.01; Table III). Subsequently, to determine
whether MVD was associated with the levels of PTEN and VEGF in
ESCC, a t-test was conducted. The results indicated that the
MVD was significantly lower in PTEN-positive ESCC specimens than in
PTEN-negative ESCC specimens (P=0.0018), while MVD was
significantly higher in VEGF-positive ESCC specimens than in
VEGF-negative ESCC specimens (P=0.0002; Table IV). These results suggested that
decreased PTEN expression, increased VEGF expression, and high MVD
are associated in ESCC.
 | Table III.Association between PTEN and VEGF
status. |
Table III.
Association between PTEN and VEGF
status.
|
| VEGF |
|
|
|---|
|
|
|
|
|
|---|
|
| + | – | χ2 | P-value |
|---|
| PTEN |
|
|
|
|
| + | 12 | 15 | 14.9670 | 0.0001 |
| – | 22 | 1 |
|
|
 | Table IV.Association of PTEN-negative status
and VEGF-positive status with high MVD. |
Table IV.
Association of PTEN-negative status
and VEGF-positive status with high MVD.
|
| MVD | t-value | P-value |
|---|
| PTEN |
|
|
|
| + | 33.58±8.92 | 3.3145 | 0.0018 |
| – | 42.39±9.87 |
|
|
| VEGF |
|
|
|
| + | 42.53±8.57 | 4.1084 | 0.0002 |
| – | 32.25±7.51 |
|
|
Discussion
The analysis of a single protein biomarker is
insufficient for adequate and accurate diagnosis and prognosis of
ESCC. However, the examination of multiple biomarker proteins may
provide more accurate information. In the current study, the
expression profiles of PTEN and VEGF, and the MVD were investigated
in ESCC and normal specimens. Of MVD and expression statuses of
PTEN and VEGF, only PTEN expression was found to negatively
correlate with the histological grade of ESCC. PTEN expression and
MVD were correlated with the depth of ESCC invasion, while PTEN
expression, VEGF expression and MVD were correlated with the lymph
node metastasis status of ESCC specimens. Furthermore, it was
demonstrated that the expression of PTEN was negatively correlated
with the expression of VEGF, while a higher MVD was associated with
higher VEGF and lower PTEN expression. Therefore, it was concluded
that examination of MVD and the expression status of PTEN and VEGF
may provide improved information for the diagnosis and prognosis of
ESCC.
Angiogenesis is required for tumor growth and
metastasis (12), and may be
evaluated by staining endothelial cell markers CD31 and CD34
(13,14). This method has previously been applied
for the determination of the role of the neovasculature in
extrahepatic cholangiocarcinoma (4)
and Dukes' B colon cancer (15).
These studies demonstrate the significant effects of
vascularization on the survival of patients with extrahepatic
cholangiocarcinoma and Dukes B colon cancer. In the current study,
it was revealed that MVD was correlated with the depth of invasion
and lymph node metastasis of ESCC. Therefore, determination of MVD
may facilitate prediction of the depth of invasion and lymph node
metastasis of ESCC.
VEGF is an angiogenic factor, which promotes
proliferation and migration of endothelial cells, enhances
permeability of blood vessels, reduces apoptosis of endothelial
cells and increases stromal proteolysis (16). Studies have indicated that VEGF has a
crucial role in angiogenesis in various solid malignancies. VEGF-C
is lymphangiogenic, and is able to selectively induce hyperplasia
of the lymphatic vasculature. VEGF-C expression is correlated with
lymph node metastasis and poor prognosis in esophageal cancer
(17–19). Furthermore, in patients with
esophageal tumors, the expression of VEGF-C has been shown to be an
effective diagnostic factor for determining metastasis of lymph
nodes (17–19). In a meta-analysis, elevated VEGF
expression was shown to be associated with poor survival in
patients with esophageal cancer (20). Inhibition of VEGF-induced angiogenesis
suppresses tumor growth in vivo (21). The results of these studies support
the hypothesis of a role for VEGF expression as an indicator for
predicting poor survival in patients with esophageal carcinoma, and
may have implications for treatments directed at inhibiting
VEGF-mediated angiogenesis. In the current study, it was
demonstrated that upregulation of VEGF protein expression was
associated with increased MVD in ESCC. Furthermore, VEGF expression
was correlated with lymph node metastasis, but not with
differentiation status or depth of invasion. Therefore, VEGF
expression may be a predictor of risk for lymph node metastasis in
esophageal cancer.
PTEN is a phosphatase with dual-specificity for
protein and lipids. The PTEN gene has an important role in
cell proliferation, differentiation and apoptosis. Studies
conducted in certain cancer cell lines indicated that the loss of
PTEN expression may underlie almost all types of cancer, including
solid tumors and hematological malignancies. For example, the loss
of PTEN promotes tumorigenesis of breast tumors (22). In the present study, decreased PTEN
expression was found to be correlated with differentiation status
of ESCC. This is consistent with the results of several previous
studies (6–8). Therefore, PTEN is likely involved in the
tumorigenesis and progression of ESCC.
In addition to correlation with differentiation
status, decreased PTEN expression was also correlated with depth of
invasion and lymph node metastasis of ESCC. Studies have previously
suggested that PTEN may regulate interactions between cells and the
extracellular matrix by interacting with focal adhesion kinase
(FAK) and thereby reducing tyrosine phosphorylation. Therefore,
PTEN inhibits cell migration, spreading and focal adhesion. Reduced
PTEN expression may regulate integrin-mediated adhesion through
dephosphorylation of FAK and increased cell spreading (5). PTEN may suppress cell adhesion to the
extracellular matrix through its serine/threonine phosphatase role,
for example, as a negative regulator of the formation of stable
HT-29 cell adhesion to the extracellular matrix (23). Decreased PTEN expression has roles in
the tumorigenesis, progression, growth, differentiation and
angiogenesis of gastric cancer, likely via inhibition of the
expression of caspase-3 in tumor cells (24). In prostate basal cells,
conditionally-ablated PTEN enhances basal-to-luminal
differentiation and induces invasive prostate cancer in mice
(25). Hypermethylation of PTEN may
inhibit the expression of PTEN, and PTEN hypermethylation was found
to be associated with ESCC in Chinese Kazakh patients (26). Therefore, PTEN may be involved in the
regulation of cell growth, tumor invasion, metastasis and
angiogenesis.
The regulation of PTEN and VEGF expression in ESCC
appears to occur via two distinct processes. However, these factors
exert opposite effects on angiogenesis in tumors. PTEN functions as
a tumor suppressor gene and inhibits the formation of blood vessels
in tumors, while VEGF promotes tumor vascularization. Akt is a
serine/threonine kinase involved in cell growth and survival.
Activation of Akt requires phosphatidylinositol (3,4,5)-triphosphate (PIP3), a second messenger in
cells. PTEN dephosphorylates PIP3, thereby functioning in
opposition to phosphatidylinositol 3-kinase (PI3K), which has a
crucial role in the formation of PIP3. By blocking Akt activation,
PTEN regulates multiple cellular processes, including cell cycling,
translation and apoptosis (6). In
addition, PI3K has a key role in angiogenesis and regulates VEGF
expression. Overexpression of PTEN or of dominant-negative
constructs of PI3K attenuates angiogenesis in the yolk sac of
chicken embryos, indicating that PI3K and Akt signaling is required
for normal embryonal angiogenesis (27). PTEN and VEGF function in the
PI3K-PIP3-AKT network and are key regulators of cell growth in
multiple pathways (28). Studies have
demonstrated that inactivation of the PTEN gene and
overexpression of VEGF, contribute to the neovascularisation
and progression of gastric cancer (29). Angiogenesis due to decreased PTEN
expression may be attributed to the corresponding upregulation of
VEGF levels. In the current study, it was demonstrated that a
reduction or loss of PTEN expression was correlated with elevated
VEGF protein levels in ESCC. Therefore, PTEN and VEGF are
interrelated indicators of ESCC. The specific mechanisms underlying
PTEN and VEGF interaction remain to be elucidated.
In conclusion, PTEN/VEGF expression status and MVD
are differentially associated with characteristics of ESCC. The
differentiation status of ESCC may be determined by decreased PTEN
expression, while invasion of ESCC is likely associated with
decreased PTEN expression levels and higher MVD. Metastasis of ESCC
is associated with decreased PTEN expression levels, enhanced VEGF
expression levels and higher MVD. Combined detection of PTEN and
VEGF expression levels and MVD may provide information essential
for improvements in the diagnosis and prognosis of ESCC.
Acknowledgements
The present study was financially supported by the
Affiliated Hospital of Southern Medical University (Guangzhou,
China), (grant no. 2012B050600020).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nico B, Benagiano V, Mangieri D, Maruotti
N, Vacca A and Ribatti D: Evaluation of microvascular density in
tumors: Pro and contra. Histol Histopathol. 23:601–607.
2008.PubMed/NCBI
|
|
3
|
Torres C, Wang H, Turner J, Shahsafaei A
and Odze RD: Prognostic significance and effect of
chemoradiotherapy on microvessel density (angiogenesis) in
esophageal Barrett's esophagus-associated adenocarcinoma and
squamous cell carcinoma. Hum Pathol. 30:753–758. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Möbius C, Demuth C, Aigner T, Wiedmann M,
Wittekind C, Mössner J, Hauss J and Witzigmann H: Evaluation of
VEGF A expression and microvascular density as prognostic factors
in extrahepatic cholangiocarcinoma. Eur J Surg Oncol. 33:1025–1029.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kleespies A, Guba M, Jauch KW and Bruns
CJ: Vascular endothelial growth factor in esophageal cancer. J Surg
Oncol. 87:95–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamura M, Gu J, Matsumoto K, Aota S,
Parsons R and Yamada KM: Inhibition of cell migration, spreading
and focal adhesions by tumor suppressor PTEN. Science.
280:1614–1617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simpson L and Parsons R: PTEN: Life as a
tumor suppressor. Exp Cell Res. 264:29–41. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dahia PL: PTEN, a unique tumor suppressor
gene. Endocr Relat Cancer. 7:115–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chu EC and Tarnawski AS: PTEN regulatory
functions in tumor suppression and cell biology. Med Sci Monit.
10:RA235–RA241. 2004.PubMed/NCBI
|
|
10
|
Chang D, Wang TY, Li HC, Wei JC and Song
JX: Prognostic significance of PTEN expression in esophageal
squamous cell carcinoma from Linzhou City, a high incidence area of
northern China. Dis Esophagus. 20:491–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Petersen I: The new WHO classification and
recent results in soft tissue tumor pathology. Pathologe.
34:436–448. 2013.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blood CH and Zetter BR: Tumor interactions
with the vasculature: Angiogenesis and tumor metastasis. Biochim
Biophys Acta. 1032:89–118. 1990.PubMed/NCBI
|
|
13
|
Weidner N: Chapter 14. Measuring
intratumoral microvessel density. Methods Enzymol. 444:305–323.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
15
|
Sundov Z, Tomic S, Alfirevic S, Sundov A,
Capkun V, Nincevic Z, Nincevic J, Kunac N, Kontic M, Poljak N, et
al: Prognostic value of MVD, LVD and vascular invasion in lymph
node-negative colon cancer. Hepatogastroenterology. 60:432–438.
2013.PubMed/NCBI
|
|
16
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka T, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Shiozaki M, Naganawa Y, Fujii Y and Takeyama
H: Vascular endothelial growth factor C (VEGF-C) in esophageal
cancer correlates with lymph node metastasis and poor patient
prognosis. J Exp Clin Cancer Res. 29:832010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kozlowski M, Naumnik W, Niklinski J,
Milewski R, Dziegielewski P and Laudanski J: Vascular endothelial
growth factor C and D expression correlates with lymph node
metastasis and poor prognosis in patients with resected esophageal
cancer. Neoplasma. 58:311–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okazawa T, Yoshida T, Shirai Y, Shiraishi
R, Harada T, Sakaida I, Abe T and Oka M: Expression of vascular
endothelial growth factor C is a prognostic indicator in esophageal
cancer. Hepatogastroenterology. 55:1503–1508. 2008.PubMed/NCBI
|
|
20
|
Chen M, Cai E, Huang J, Yu P and Li K:
Prognostic value of vascular endothelial growth factor expression
in patients with esophageal cancer: A systematic review and
meta-analysis. Cancer Epidemiol Biomarkers Prev. 21:1126–1134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Banneau G, Guedj M, MacGrogan G, de
Mascarel I, Velasco V, Schiappa R, Bonadona V, David A, Dugast C,
Gilbert-Dussardier B, et al: Molecular apocrine differentiation is
a common feature of breast cancer in patients with germline PTEN
mutations. Breast Cancer Res. 12:R632010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haier J and Nicolson GL: PTEN regulates
tumor cell adhesion of colon carcinoma cells under dynamic
conditions of fluid flow. Oncogene. 21:1450–1460. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng HC, Li YL, Sun JM, Yang XF, Li XH,
Jiang WG, Zhang YC and Xin Y: Growth, invasion, metastasis,
differentiation, angiogenesis and apoptosis of gastric cancer
regulated by expression of PTEN encoding products. World J
Gastroenterol. 9:1662–1666. 2003.PubMed/NCBI
|
|
25
|
Lu TL, Huang YF, You LR, Chao NC, Su FY,
Chang JL and Chen CM: Conditionally ablated Pten in prostate basal
cells promotes basal-to-luminal differentiation and causes invasive
prostate cancer in mice. Am J Pathol. 182:975–991. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan QF, Li WT, Dong HC, Chen YZ, Yin L,
Liu W, Wang WW, Liu D, Li SG, Gu WY, et al: PTEN hypermethylation
profiles of Chinese Kazakh patients with esophageal squamous cell
carcinoma. Dis Esophagus. 27:396–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang BH, Zheng JZ, Aoki M and Vogt PK:
Phosphatidylinositol 3-kinase signaling mediates angiogenesis and
expression of vascular endothelial growth factor in endothelial
cells. Proc Natl Acad Sci USA. 97:1749–1753. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vanhaesebroeck B, Stephens L and Hawkins
P: PI3K signalling: The path to discovery and understanding. Nat
Rev Mol Cell Biol. 13:195–203. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou YJ, Xiong YX, Wu XT, Shi D, Fan W,
Zhou T, Li YC and Huang X: Inactivation of PTEN is associated with
increased angiogenesis and VEGF overexpression in gastric cancer.
World J Gastroenterol. 10:3225–3229. 2004.PubMed/NCBI
|