Introduction
Cancer is a disease that is characterized by the
proliferation of abnormal cells. Mutations in specific proteins and
enzymes that are associated with the regulation of cell growth
results in uncontrolled proliferation and thus malignancy. In
recent decades, the variety of cancer treatment methods (surgery,
radiotherapy, chemotherapy and endocrine therapy) have been
markedly improved, however, cancer remains the leading cause of
mortality in China. Approximately 98% of individuals who undergo
health screening exhibit diseases or illness of different types,
50% of which are linked to the development of premalignancies or
malignancies (1,2), indicating that health screening is an
important element for improving the quality of life.
Thymidine kinase 1 (TK1) is an enzyme that is
important for the regulation of the intracellular thymidine pool
throughout the cell cycle. The TK1 enzyme is worthy of note as its
level is highly dependent on the growth stage of the cell. In
proliferating normal and tumor cells, the level of TK1 starts to
increases at late G1 phase, and reaches a maximum in
late S-phase/early G2 phase of the cell cycle, but in
quiescent cells, TK1 is almost completely absent. This singles out
TK1 as a useful indicator of cellular proliferation, and hence for
malignancy (3–6). Serum TK1 protein (STK1p) level,
determined by non-invasive serological methods, was found to be an
emerging potential cell proliferation biomarker for the prognosis
of cancer patients (7–9), for monitoring tumor treatments, relapse,
follow-up and survival, and particularly for the early detection of
cancer development risk (10,11). A sensitive chemiluminescence dot blot
assay of STK1p was developed by the Swedish TK1 Research Team in
2000 (7,9), which has become a commercial kit
(Sino-Swed Biotech Ltd., Shenzhen, China) for the early detection
of pre/early risk of developing cancer in health screening
(1,2,12). Using
this kit, a health screening study on 35,365 individuals showed a
receiver operating characteristic value of 0.96 for the STK1p
assay. At a cut-off STK1p value of 2.0 pM, the likelihood (+) value
was 236.5, and the sensitivity and the specificity were 0.78 and
0.99 (1 out of 300 false-positives), respectively, indicating that
the STK1p assay is a reliable test for the risk assessment of the
pre/early cancerous progression of individuals in health screening
(13).
The current study presents the case of a patient
that exhibited a higher risk of developing malignancies for three
types of diseases. This patient was followed up for 139 months
using the STK1p assay (last 83 months) in combination with imaging,
histological and immunological techniques, and routine clinical
laboratory tests.
Case report
Patient
The patient was a 52-year-old premenopausal female
with regular menstruation. The patient works at a medical hospital
as a nurse in a health-screening center, is married with one
daughter, and has undergone an abortion twice. No family history of
cancer and no history of exposure to any environmental risk factors
were recorded. The patient provided written informed consent for
participation in the present study.
Three types of proliferating
diseases
The case study was performed between January 2003
and July 2014 (139 months). At least three types of proliferating
diseases linked to cancer development risk were found: i) Gastric
diseases detected by gastroscopy and biopsy; ii) cervical disease
detected by cervical human papilloma virus (HPV)-DNA detection,
thinprep cytological test, colposcopy and biopsy; and iii) breast
proliferative tissue detected by automated breast volume scan
(ABVS) and color Doppler ultrasound scan.
Health screening tests
The health-screening included an ear, nose and
throat physical examination, blood pressure tests, liver,
gallbladder, spleen, pancreas, double kidney and thyroid analysis
by ultrasound, blood and biochemical tests, analysis of urine
routine, thyroid function and sex hormone levels, a oral glucose
tolerance test, and analysis of rheumatoid and tumor markers
(cancer antigen (CA)125, CA153, α-fetoprotein and carcinoembryonic
antigen). These types of tests were performed annually between 2007
and 2014 (83 months).
STK1 assay
The STK1p test was included in the annual health
screening of the patient in 2008 and used until 2014 (83
months).
The STK1p assay was performed using a commercial
kit, based on an enhanced chemiluminescence dot blot assay (SSTK
Biotech Ltd., Shenzhen, China) (13).
Briefly, 3 µl serum were directly applied onto a nitrocellulose
membrane. The serum samples were probed with human anti-TK1 chicken
immunoglobulin Y antibody. Varying concentrations of TK1-pepetide
(20, 6.6 and 2.2 pM) were used as an extrapolation standard. The
spot intensities on the membrane were determined by a CIS-l Imaging
System (SSTK Biotech Ltd.). From the intensities of the TK1
standard of known concentrations, the STK1 concentration was
calculated and expressed in pM. The coefficient of variation was
<10%. The threshold value of STK1 was set to 2.0 pM. STK1p
values of <2.0 pM were denoted as normal, considered as a lower
risk for developing malignancy. STK1p values >2.0 pM were
denoted as elevated, likely representing individuals with an
increased risk of pre-malignancy/malignancy progression.
TK1 immunohistochemical staining
TK1 immunohistochemical staining was performed using
the EnVision System according to the manufacturer's instructions
(Maxin Biotech, Fuzhou, China), as previously described (10,14). In
brief, two serial sections were used for the staining of the breast
tissue with human TK1 monoclonal antibody (dilution, 1:800 in PBS;
stock concentration, 1 mg/ml; SSTK Biotech. Ltd., Shenzhen, China).
The quality of the TK1 monoclonal antibody used in this study was
also confirmed by independent research groups. At least 100 cells
were counted in ~10 light microscopic fields at x400
magnification.
The present study was approved by the Committee of
Research Ethics at the Third Xiangya Hospital (Central South
University, Changsha, Hunan, China). The patient provided written
informed consent to participate in this study, which was conducted
in accordance with the Helsinki Declaration of 1983.
Test results
Health screening tests
The physical examination and the biochemical routine
tests, excluding the STK1p assay, showed normal values.
Gastric diseases
The patient had experienced a gastrointestinal
problem with stool shapeless for >20 years. In January 2003,
using gastroscopy and a biopsy of the gastric tissue, an
adenomatous polyp was detected, but no gastric ulcer. In June 2014,
the gastric adenomatous polyp developed into a small foci with flat
gastric erosive and inflammation of the cardia (Fig. 1). No treatment for these changes was
performed.
Cervix diseases
The patient had been affected by chronic follicular
cervicitis since August 2007. The pathological changes were found
based on several tests with cervical HPV-DNA detection and TCT.
However, in July 2014, aside from the chronic follicular cervicitis
previously found in 2007, small foci with squamous cell hyperplasia
and suspected ulcerated cervix were found using colposcopy and
biopsy of the cervix (Fig. 1). No
treatment was performed for this type of changes.
Breast diseases
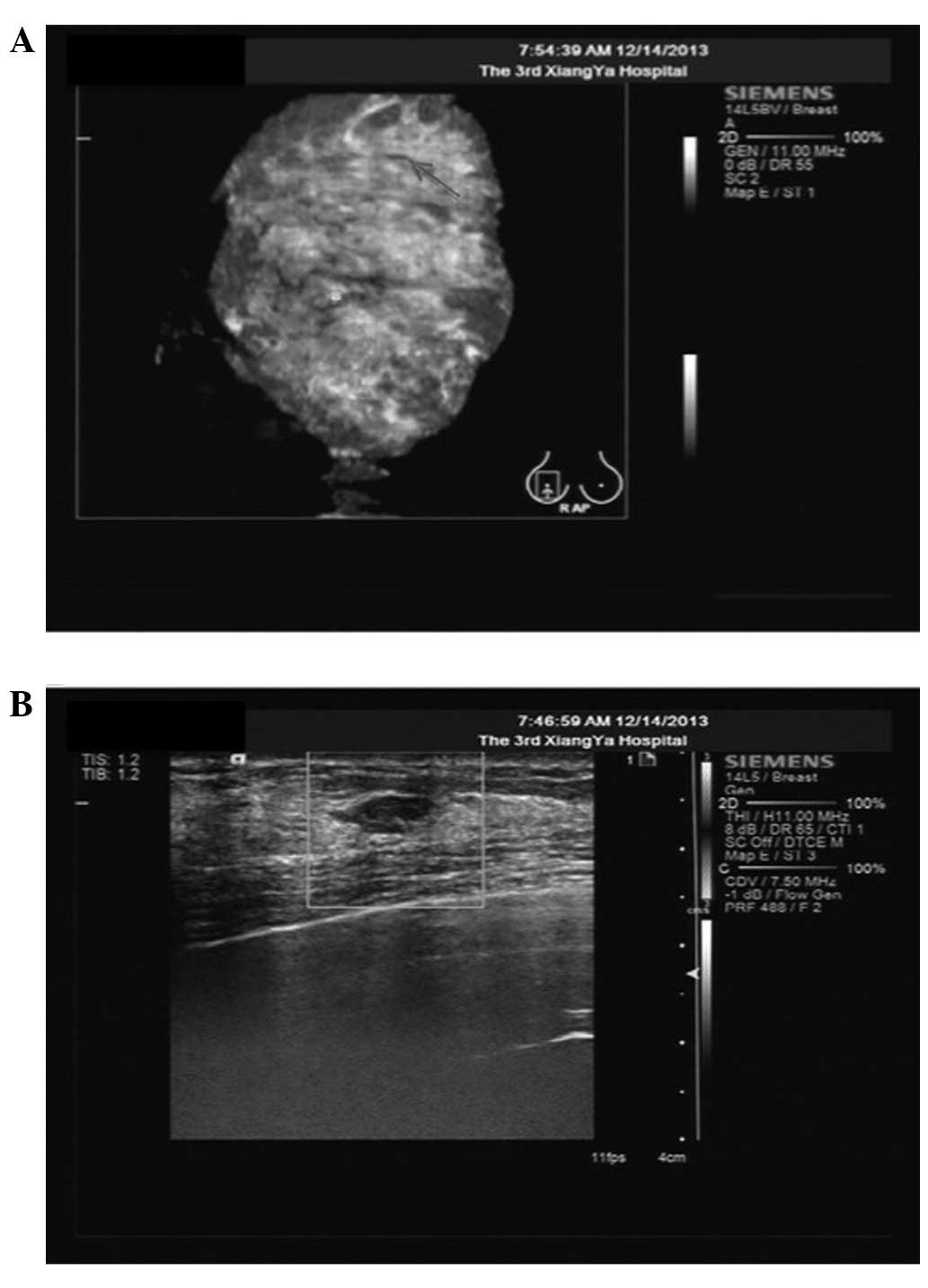
In March 2010, using the ABVS and color Doppler
ultrasound scan, a breast hypoechoic nodule was found in the right
breast (4×5 mm) (Fig. 1). The patient
was then followed up with ultrasound several times. The size of the
breast hypoechoic nodule increased to 8×7 mm after 45 months
(December 2013). The ABVS and color Doppler ultrasound scan showed
inhomogeneous breast proliferative tissues (Fig. 2). A pixel pitch of 8×7 mm showed a
hypoechoic focus with a circumscribed and irregular shape, 7 mm
under the skin surface, as evaluated as Breast Imaging Reporting
and Data System category 3 (BI-RADS 3, American College of
Radiology, 1998; Fig. 2). The color
Doppler ultrasound scan of the right breast revealed that the
mammary glands were coarsely heterogeneous (Fig. 2), indicating a suspicious
malignancy.
Pre-operative STK1p
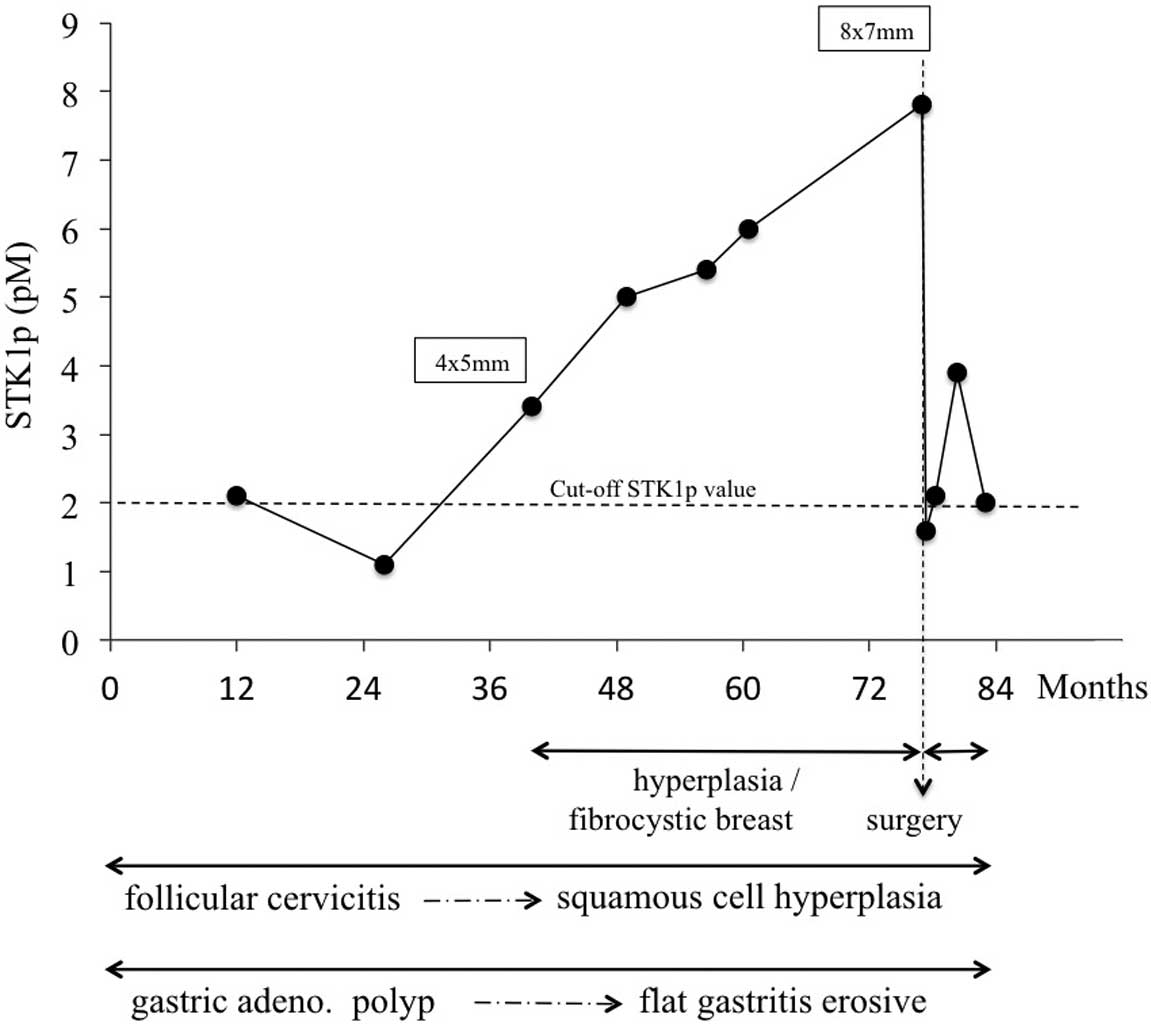
The changes in the levels of STK1p are summarized in
Fig. 1. In January 2008, STK1p
analysis was included in the annual health screening, revealing a
fluctuating STK1p value of between 1.2 and 2.1 pM. The STK1p value
of healthy individuals without any known diseases or illness is
<1.0 pM (1,2,11,12).
In March 2010, the STK1p value was elevated (3.4 pM)
and continued to increase up to 7.8 pM in December 2013 (Fig. 1). The increase in the STK1p value was
parallel with the discovery of hypoechoic nodules in the right
breast, as determined by ultrasound examination (Fig. 2). Based on the hypoechoic nodules and
the high STK1p value (7.8 pM), surgery to remove the nodules was
performed on December 20, 2013, using minimally invasive surgery
with the Mammotome® Biopsy System (Mammotome, Cincinnati, OH, USA).
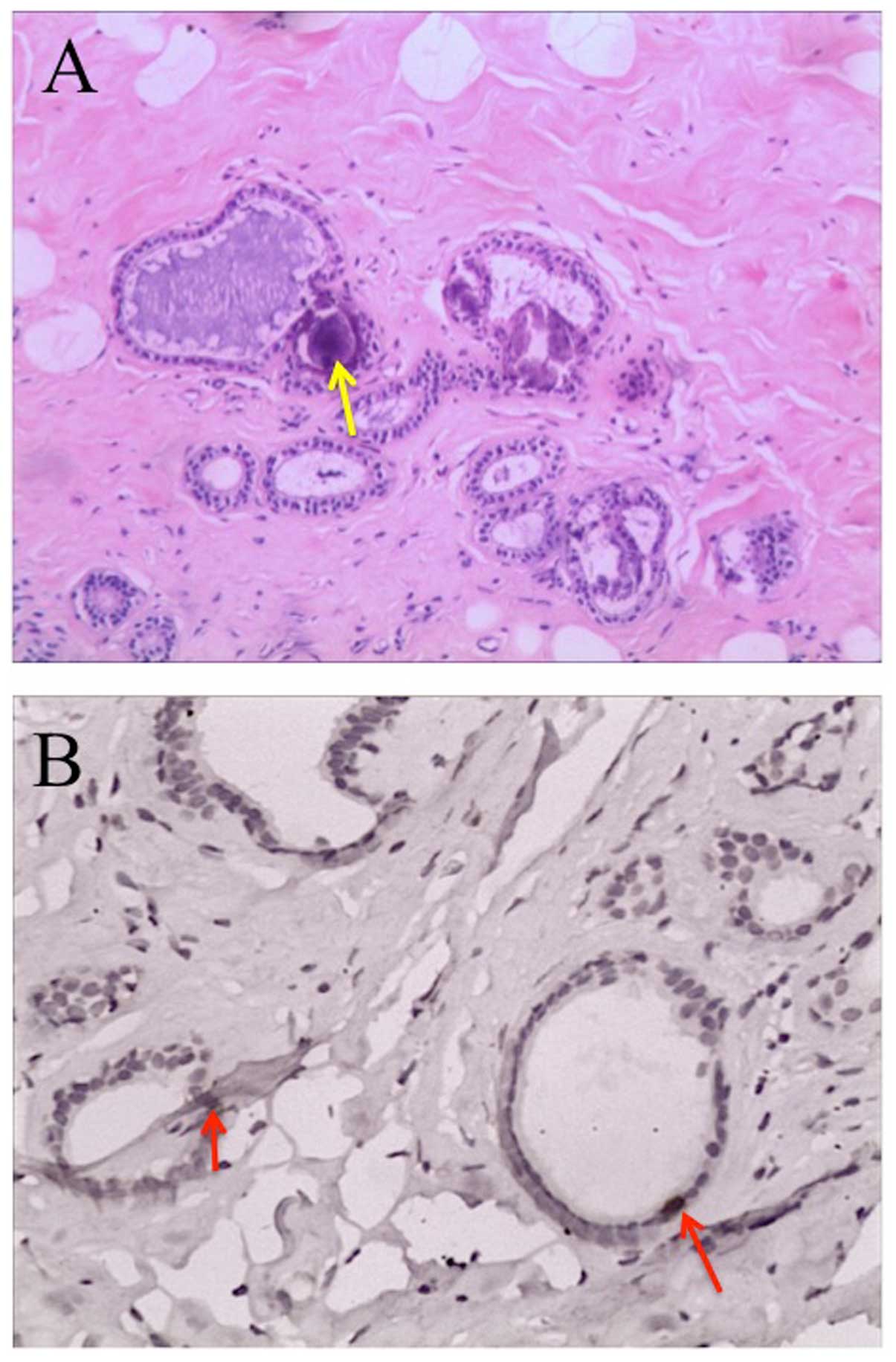
Histopathological investigations on the hypoechoic nodules showed
an expanding fibrocyst type, but no evidence of cancer (Fig. 3A). In addition, TK1
immunohistochemistry showed strong positive staining in the ductal
epithelial cells (Fig. 3B).
Post-operative STK1p
Following surgery, the BI-RADS scan did not show any
significant mass in the breast. The patient did not undergo any
type of additional treatment. The STK1p values declined to nearly
normal values (1.6 pM) one week after surgery. The STK1p levels,
however, followed a somewhat increasing during the following 7
months, fluctuating around 2.0 pM (Fig.
1).
Discussion
Premalignancies and long-term chronic diseases are
linked to the risk of the development of malignant diseases.
Cancer-related lifestyle risk factors, such as physical activity,
smoking and eating habits, play a primary role in the etiology of
cancer (15,16). Premalignancies are easier to treat
than malignancies and therefore, early screening is a
cost-effective cancer prevention strategy. Premalignancy treatment
is also responsible for the most marked reduction in cancer-related
mortality attributable to any medical intervention (17,18). The
detection of premalignancies with routine screening could prevent
the occurrence of the majority of human cancers (11). However, the majority of tumor
biomarkers are often insufficient to diagnose specific types of
malignancies and as a result, are not recommended for the screening
of early cancer diseases (19).
Tumors of a small size are now identified by in vivo imaging
techniques, but these techniques are expensive. Furthermore, such
imaging techniques cannot differentiate premalignant lesions from
malignant lesions that well. The imaging technique is also limited
regarding a premalignancy or extremely small malignant tumors.
Premalignancies are the earliest morphological discernible lesions
that precede the development of carcinoma in situ. Numerous
premalignancies can be identified by pathology (17,20).
However, it is not easy to assess the proliferation rate. In the
present study, STK1p combined with TK1 immunohistochemical staining
was shown to be a reliable biomarker for the assessment of abnormal
proliferation in the lesion, indicating the presence of
physiological changes associated with the development of
premalignancies.
A previous study on healthy individuals without any
tumor diseases showed that 99% exhibited a STK1p level of <1.0
pM (1). In cancer patients, the STK1p
values correlate to tumor cell proliferating rates of different
levels (5,9,11).
Patients are considered to be tumor-free when the STK1p values were
normalized (<1.0 pM) within 1–3 months after the end of the
treatment. Thus, a close correlation has been found between the
STK1p values and clinical parameters, including the outcome of
tumor treatments (10,11).
In the present study, a close correlation was found
between the development of breast nodes and the STK1p value, and a
decline was observed in the STK1p values from high to almost normal
values following minimally invasive surgery using the Mammotome
Biopsy System (Fig. 1). These results
confirmed that STK1p is a useful serum proliferation biomarker in
health screening and in monitoring the treatment.
Beside the usefulness of STK1p, TK1
immunohistochemical staining in combination with histology is also
a useful tool for the assessment of the risk of developing cancer.
As shown in Fig. 3, the fibrocystic
breast disease developed into cystic expansion of proliferating
cells in parallel with strong TK1 staining of ductal epithelial
cells, indicating a risk of developing malignancy (20).
Subsequent to minimally invasive surgery, the STK1
values tended to increase, fluctuating around 2.0 pM (Fig. 1). The reason for this could be that
the changes in the adenomatous gastric polyps and the chronic
follicular cervicitis over time into more advanced types were not
treated. These changes also indicate that premalignancies were
developing (20). The patient is
currently being followed up by STK1p combined with imaging and
pathology in order to begin therapeutic intervention as early as
possible to avoid the risk of developing cancer.
In conclusion, STK1p and TK1 immunohistochemistry in
combination with imaging and pathology are useful for the early
detection of premalignancy processes and for the early risk warning
of invisible tumors.
Acknowledgements
This study was made possible by grants from the
Health Management Centre of the Third Xiangya Hospital and the
Department of Pathology (Shenzhen Second Hospital, Shenzhen,
Guangdong, China). The authors would also like to thank Sino-Swed
Tongkang Biotech. Ltd., for providing technical support.
References
|
1
|
Chen Z, Zhou H, Li S, He E, Hu J, Zhou J
and Skog S: Serological thymidine kinase 1 (STK1) indicates an
elevated risk for development of malignant tumours. Anticancer Res.
28:3897–3907. 2008.PubMed/NCBI
|
|
2
|
Huang S, Lin J, Guo N, Zhang M, Yun X, Liu
S, Zhou J, He E and Skog S: Elevated serum thymidine kinase 1
predicts risk of pre/early cancerous progression. Asian Pac J
Cancer Prev. 12:497–505. 2011.PubMed/NCBI
|
|
3
|
Sherley JL and Kelly TJ: Regulation of
human thymidine kinase during the cell cycle. J Biol Chem.
263:8350–8358. 1988.PubMed/NCBI
|
|
4
|
Kauffman MG and Kelly TJ: Cell cycle
regulation of thymidine kinase: Residues near the carboxyl terminus
are essential for the specific degradation of the enzyme at
mitosis. Mol Cell Biol. 11:2538–2546. 1991.PubMed/NCBI
|
|
5
|
He Q, Zhang P, Li Z, Li H, Wang X, Zou S,
Fornander T and Skog S: Concentration of thymidine kinase 1 in
serum (S-TK1) is a more sensitive proliferation marker in human
solid tumors than its activity. Oncol Rep. 14:1013–1019.
2005.PubMed/NCBI
|
|
6
|
Welin M, Kosinska U, Mikkelsen NE, Carnrot
C, Zhu C, Wang L, Eriksson S, Munch-Petersen B and Eklund H:
Structures of thymidine kinase 1 of human and mycoplasmic origin.
Proc Natl Acad Sci USA. 101:17970–17975. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He Q, Zou L, Zhang PA, Liu JX, Skog S and
Fornander T: The clinical significance of thymidine kinase 1
measurement in serum of breast cancer patients using anti-TK1
antibody. J Biol Marker. 15:139–146. 2000.
|
|
8
|
Wu CJ, Yang R, Zhou J, Bao S, Zou L, Zhang
P, Mao Y, Wu J and He Q: Production and characterisation of a novel
chicken IgY antibody raised against C-terminal peptide from human
thymidine kinase 1. J Immuno Methods. 277:157–169. 2003. View Article : Google Scholar
|
|
9
|
He Q, Fornander T, Johansson H, Johansson
U, Hu GZ, Rutqvist LE and Skog S: Thymidine kinase 1 in serum
predicts increased risk of distant or loco-regional recurrence
following surgery in patients with early breast cancer. Anticancer
Res. 26:4753–4759. 2006.PubMed/NCBI
|
|
10
|
Aufderklamm S, Todenhöfer T, Gakis G,
Kruck S, Hennenlotter J, Stenzl A and Schwentner C: Thymidine
kinase and cancer monitoring. Cancer Lett. 316:6–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou J, He E and Skog S: The proliferation
marker thymidine kinase 1 in clinical use. Mol Clin Oncol. 1:18–28.
2013.PubMed/NCBI
|
|
12
|
Xu XH, Zhang YM, Shu XH, Shan LH, Wang ZW,
Zhou YL, Wen HK, He F, He E and Skog S: Serum thymidine kinase 1
reflects the progression of pre-malignant and malignant tumors
during therapy. Mol Med Rep. 1:705–712. 2008.PubMed/NCBI
|
|
13
|
Chen ZH, Huang SQ, Wang Y, Yang AZ, Wen J,
Xu XH, Chen Y, Chen QB, Wang YH, He E, et al: Serological thymidine
kinase 1 is a biomarker for early detection of tumours-a health
screening study on 35,365 people, using a sensitive
chemiluminescent dot blot assay. Sensors(Basel). 11:11064–11680.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guan H, Sun Y, Zan Q, Xu M, Li Y, Zhou J,
He E, Eriksson S, Wen W and Skog S: Thymidine kinase 1 expression
in atypical ductal hyperplasia significantly differs from usual
ductal hyperplasia and ductal carcinoma in situ: A useful tool in
tumour therapy management. Mol Med Reports. 2:923–929. 2009.
|
|
15
|
Ang YK, Mirnalini K and Zalilah MS: A
workplace email-linked website intervention for modifying
cancer-related dietary and lifestyle risk factors: Rationale,
design and baseline findings. Malays J Nutr. 19:37–51.
2013.PubMed/NCBI
|
|
16
|
Sulaiman S, Shahril MR, Wafa SW,
Shaharudin SH and Hussin SN: Dietary carbohydrate, fiber and sugar
and risk of breast cancer according to menopausal status in
malaysia. Asian Pac J Cancer Prev. 15:5959–5964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berman JJ and Moore GW: Part I, Limitation
of Current Approaches to Cancer Treatment; Part II, Precancer
Pathology and Biology; Part III, Eradication of Cancer By Treatment
of PrecancerPrecancer: The Beginning and the End of Cancer. Jones
and Bartlett Publishers; Sudbury, MA, USA: pp. 1–113. 2010
|
|
18
|
Pandey S and Chandravati: Breast screening
in north India: A cost-effective cancer prevention strategy. Asian
Pac J Cancer Prev. 14:853–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cigna, . Tumor markers for cancer.
http://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0172_coveragepositioncriteria_tumor_markers_for_diagnosis_mgmt_cancer.pdfAccessed
on. January 1–2015
|
|
20
|
Underwood JCE: Classification of
tumoursGeneral and Systematic Pathology. Elsevier Ltd.; pp.
227–229. pp. 240–235. pp. 374–473. pp. 4972004
|