Introduction
Primary hepatocellular carcinoma (HCC) is a highly
malignant tumor with the characteristics of rapid progression and a
poor prognosis. It is one of the most commonly observed malignant
tumors in China, with ~130,000 associated mortalities annually,
accounting for ~40% of liver cancer-associated mortality (1,2). The
physical symptoms of HCC are largely dependent on the stage of
disease. Early stage HCC is often asymptomatic, whereas patients
with advanced stage HCC often present with symptoms such as,
weakness, malaise, anorexia, upper abdominal pain and weight loss.
In addition, hepatomegaly is identified in the majority of HCC
patients (3). A number of treatment
modalities exist for HCC, including hepatic resection, liver
transplantation, percutaneous ethanol injection (PEI),
radiofrequency ablation (RFA), and transcatheter arterial
chemoembolization (TACE) (4–7). Treatment choice is dependent on various
factors, which include tumor stage, liver function reserve and
patient performance status [Barcelona Clinic Liver Cancer stage
(8)] and thus, a multidisciplinary
approach is required for optimal treatment. Hepatic resection
(5,9,10) and
liver transplanatation (11–13) are the only curative options for
patients with early stage HCC. However, recently, there have been
significant advances in local ablative and transarterial therapies
(14–16). For patients with small HCC (nodular
diameters <2 cm), RFA has been shown to exhibit the same
efficacy as surgical resection, with five-year survival rates
ranging between 40 and 70% (17) and
has replaced PEI as the locoregional therapy of choice. Previous
studies have demonstrated that RFA treatment provides better local
control and survival outcomes when compared with PEI (18–20). TACE
is considered as the conventional treatment method for patients
with unresectable HCC (21,22). The majority of hepatic lesions are
identified by B-mode ultrasound, computed tomography (CT), magnetic
resonance imaging (MRI) or digital subtraction angiography (DSA).
However, such examinations are often unable to detect smaller
lesions, particulary those in the liver. Thus, a number of issues
must be overcome to enable the early detection and treatment of
liver cancer. The present study reports the case of an HCC patient
with an elevated α-fetoprotein (AFP) level, with lesions that went
undetected by B-mode ultrasound, CT and DSA, but were finally
detected by TACE. The diagnosis and treatment through TACE for such
an HCC patient provides novel insights into clinical and basic
research. To the best of our knowledge, the current study is the
first to report the use of TACE as an examination technique in any
disease. In the majority of cases, TACE was considered to be an
appropriate treatment method for HCC in which the lesions are
clearly detected.
Case report
A 41-year-old male presented to the Jiangxi Province
Cancer Hospital (Nanchang, Jiangxi, China) in December 2013 with an
elevated AFP level that was indicative of HCC. Upon admission, the
patient was examined by B-mode ultrasound and CT, but no suspicious
tumor lesions were found in the liver. The AFP level was 3,635
ng/ml (normal range, 0–7 ng/ml), and the patient was hepatitis B
virus (HBV) surface antigen-positive, with an HBV DNA level of
246.2 upon quantitative examination (normal value, <100), and
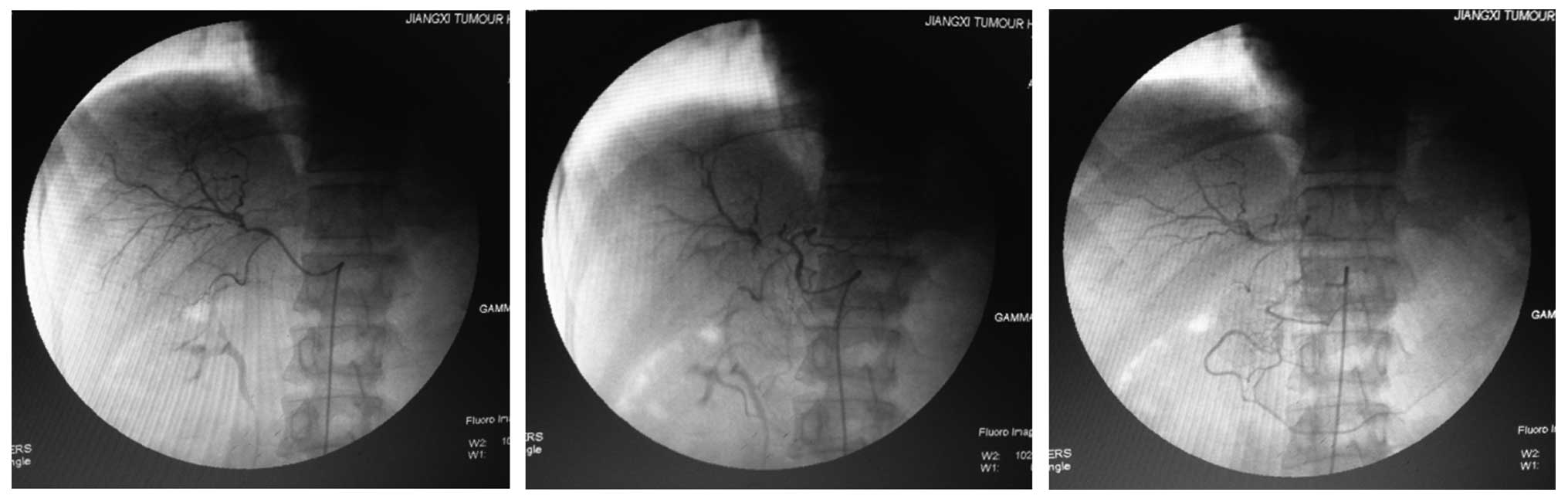
Child-Pugh grade A liver function (23). Other diseases were excluded and DSA
was recommended for examination of the patient. However, DSA did
not locate any suspicious lesions in the liver (Fig. 1). Next, TACE (10 mg Adriamycin + 5 ml
Lipiodol) was performed upon the assumption that lesions would be
found during the procedure, due to the raised AFP level in this
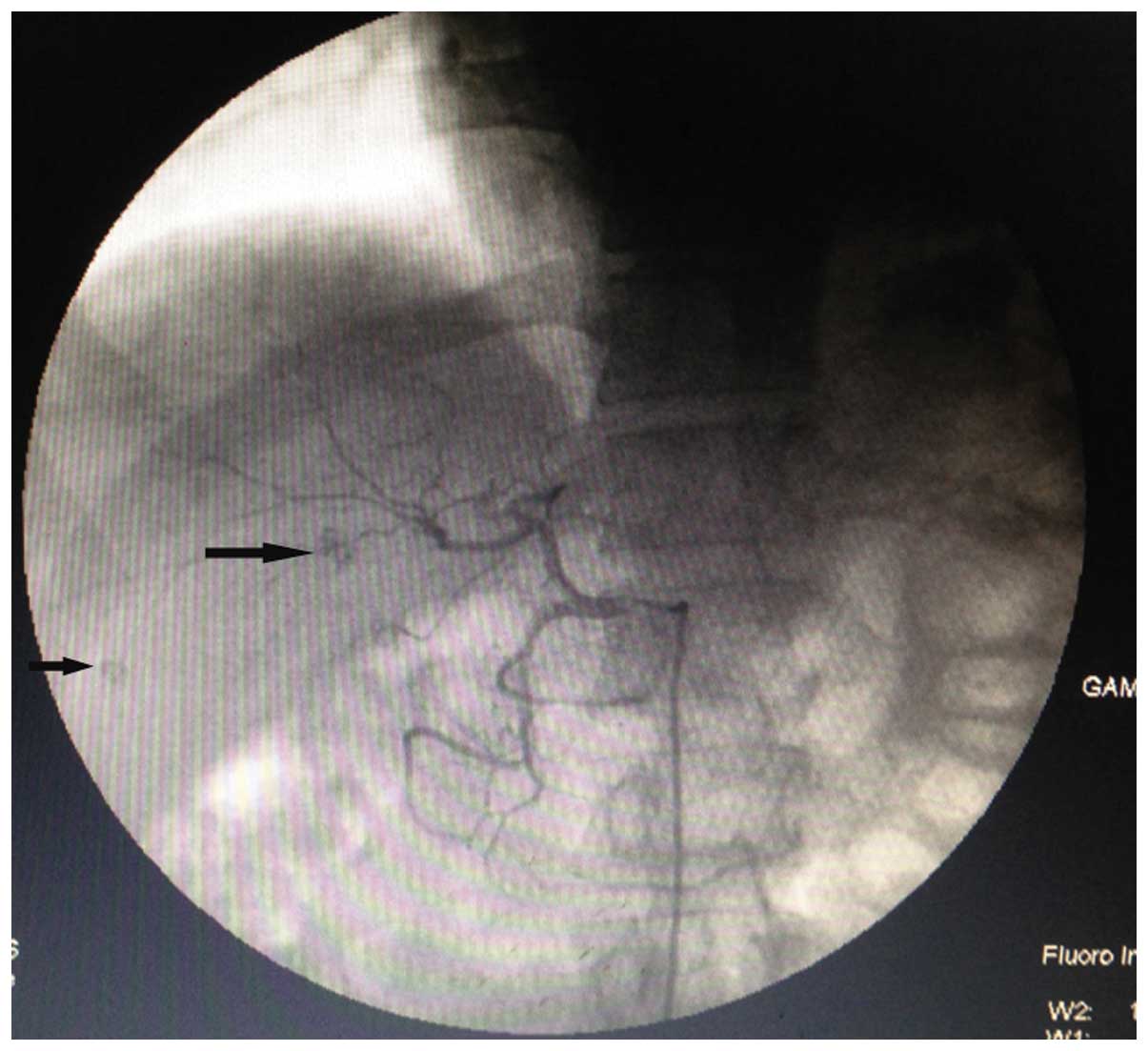
patient. Lipiodol was injected into the left and right hepatic
arteries, respectively. During surgery, two lesions with Lipiodol
deposition (~0.5 and 0.8 cm in diameter) were located in the right
liver lobe, while no Lipiodol deposition was found in the left
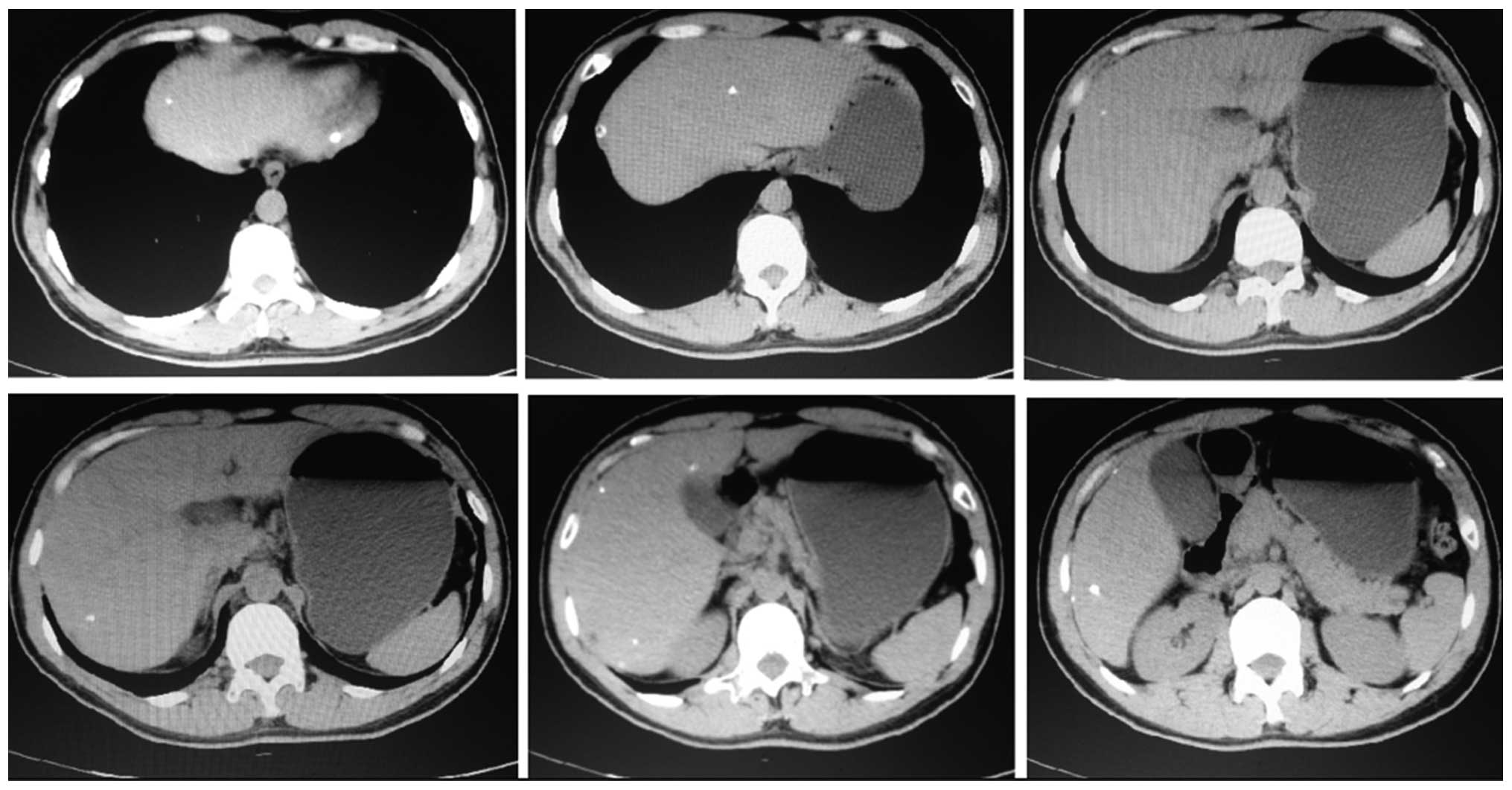
liver lobe (Fig. 2). A month after
TACE, liver CT scanning found 11 lesions with Lipiodol deposition,
including 2 lesions previously detected during the surgery
(Fig. 3). Among them, 8 lesions were
located in the right liver lobe and 3 were located in the left
liver lobe, and the diameter of the majority of the lesions was
<0.3 cm. A follow-up was conducted every 3 months and six months
after surgery, the patient's AFP level had decreased to almost
normal (20.32 ng/ml). Therefore, no further treatment was provided
for the patient and thus far no tumor recurrence within the liver
or distant metastasis have been observed.
Discussion
There are a number of examination methods for
finding small HCC lesions, such as B-mode ultrasound, CT, C-arm CT,
MRI, DSA and positron emission tomography-CT (24,25),
however, each examination has its own advantages and disadvantages.
HCC detection by B-mode ultrasound is considered to be relatively
inaccurate, whereas CT and MRI have been used to establish a
typical imaging profile for HCC (26). Previous studies have reported that
iodinated-enhanced C-arm CT improved the detection rate of small
HCC lesions during TACE (27,28), and the results also demonstrated that
PET provided better accuracy in investigating patients with HCC
compared with CT or MRI (29,30). However, the aformentioned examination
methods are unable to detect all small hepatic tumor lesions.
Previous studies have demonstrated that B-mode ultrasound, CT,
C-arm CT and DSA are able to detect for hepatocellular carcinoma
lesions 1–3 cm in diameter (31,32). In
the majority of cases, TACE is considered as a treatment method for
HCC where lesions have been clearly detected. The present study
reports a case of HCC in which the lesions remained undetected by
B-mode ultrasound, CT and DSA, and where 2 HCC lesions were
revealed during the TACE procedure. Moreover, a month after TACE,
liver CT scanning located 9 additional lesions in the liver. The
majority of the lesions were <0.3 cm in diameter. These results
suggested that TACE was a better examination method than DSA in the
detection of small HCC lesions, and at the same time, that liver CT
scans can identify more and smaller tumor lesions a month after
TACE treatment; however, whether these lesions were newly
identified because they were not present at the time of the
original TACE procedure or because they were too small remains
unclear. In view of its therapeutic role and the advantage in early
detection, we believe that TACE should be a preferred inspection
option for multiple and scattered microlesions that are difficult
to find in HCC patients.
In general, the therapeutic effects of surgical
resection, liver transplantation, percutaneous ethanol injection
and radiofrequency ablation for primary liver carcinoma are better
than those of TACE (20,33–38).
However, in the present patient treated with TACE, liver CT scans
found complete necrosis in all lesions and the AFP level had
decreased to almost normal. Surgical resection, liver
transplantation, percutaneous ethanol injection and radiofrequency
ablation are not suitable for such a patient with HCC. Therefore,
it is promising that TACE may be used as a radical treatment method
under certain conditions, particularly for primary liver cancer,
such as in the present case. Nevertheless, further studies are
required to support this.
In the present study, in order to avoid missing
intrahepatic lesions, a catheter was inserted into the left and
right hepatic arteries and Lipiodol was injected through each.
However, during the surgery, only 2 suspicious lesions were located
in the right liver lobe, with no other lesions found in the left
liver robe. Significantly, a month after TACE therapy 3 lesions
were located in the left liver lobe and 8 were located in the right
liver lobe. In fact, according to our previous treatment
experience, when the anatomical orientation of HCC is clear, TACE
treatment is performed only for a focal hepatic lobe lesion or for
segments with detected hepatic lesions, while conventional TACE is
rarely performed for focal hepatic lobe lesions or for segments
where no small lesions have been found by B-mode ultrasound, CT and
DSA. In conclusion, TACE therapy in the patient with HCC in the
current study must be conducted with certain considerations: To
prevent the omission of small hepatic tumor lesions, even when the
anatomical orientation of HCC is clear, TACE treatment must be
performed for focal hepatic lobe lesions or for segments in which
no small lesions have been identified by B-mode ultrasound, CT and
DSA, particularly on the initial treatment.
From the aforementioned clinical data, it can be
observed that among the small hepatic lesions found by TACE
examination, the majority are <0.5 cm, and can even be <0.3
cm. Following hepatic arterial embolization, Lipiodol is well
deposited in these tumors. For this clinical phenomenon, there are
two possible explanations. One explanation is that tumor lesions
<0.5 or 0.3 cm have an arterial blood supply. Another
explanation is that Lipiodol can diffuse into small intrahepatic
tumors. In accordance with previous theories, tumors <0.3 cm
rarely have been observed with an arterial blood supply. Therefore,
we infer that Lipiodol can diffuse into small intrahepatic tumors.
However, in the clinic, it was not difficult to find that certain
hepatic tumors that lacked an arterial blood supply had no or poor
Lipiodol deposition following TACE treatment. These paradoxical
clinical findings do not support the aforementioned explanation
that Lipiodol can diffuse into intrahepatic tumors. These results
result in reconsideration of the association between tumor
angiogenesis and tumor size, and the effect of tumor cell
heterogeneity and the microenvironment of liver on the differences
in biological behavior between HCC lesions with arterial an blood
supply and those without.
In conclusion, the clinical data from the present
unusual case not only provides another choice of technique for
finding small hepatic tumor lesions that are difficult to detect by
B-mode ultrasound, CT and DSA, but also raise new questions with
regard to the basic theory behind liver cancer. These issues are
worthy of further in-depth study.
References
|
1
|
Villanueva A, Minguez B, Forner A, Reig M
and Llovet JM: Hepatocellular carcinoma: Novel molecular approaches
for diagnosis, prognosis, and therapy. Annu Rev Med. 61:317–328.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meyers WC: Neoplasms of the liverTextbook
of Surgery: The Biological Basis of Modern Surgical Practice.
Sabiston DC Jr: 1. 15th. W.B. Saunders Co.; Philadelphia: pp.
1069–1072. 1999
|
|
4
|
Bruix J and Sherman MAmerican Association
for the Study of Liver Diseases: Management of hepatocellular
carcinoma: An update. Hepatology. 53:1020–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer,
. EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lencioni R, Chen XP, Dagher L and Venook
AP: Treatment of intermediate/advanced hepatocellular carcinoma in
the clinic: How can outcomes be improved? Oncologist. 15 (Suppl
4):S42–S52. 2010. View Article : Google Scholar
|
|
7
|
Yamashita T and Kaneko S: Treatment
strategies for hepatocellular carcinoma in Japan. Hepatol Res.
43:44–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikai I, Kudo M, Arii S, et al: Report of
the 18th follow-up survey of primary liver cancer in
Japan. Hepatol Res. 40:1043–1059. 2010. View Article : Google Scholar
|
|
10
|
Cucchetti A, Ercolani G, Vivarelli M, et
al: Is portal hypertension a contraindication to hepatic resection?
Ann Surg. 250:922–928. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mazzaferro V, Regalia E, Doci R, et al:
Liver transplantation for the treatment of small hepatocellular
carcinomas in patients with cirrhosis. N Engl J Med. 334:693–699.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mazzaferro V: Results of liver
transplantation: With or without Milan criteria? Liver Transpl. 13
(11 Suppl 2):S44–S47. –2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzaferro V, Bhoori S, Sposito C, et al:
Milan criteria in liver transplantation for hepatocellular
carcinoma: An evidence-based analysis of 15 years of experience.
Liver Transpl. 17 (Suppl 2):S44–S57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishikawa H, Kimura T, Kita R and Osaki Y:
Radiofrequency ablation for hepatocellular carcinoma. Int J
Hyperthermia. 29:558–568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lammer J, Malagari K, Vogl T, et al: Role:
PRECISION V InvestigatorsProspective randomized study of
doxorubicin-eluting-bead embolization in the treatment of
hepatocellular carcinoma: Results of the PRECISION V study.
Cardiovasc Intervent Radiol. 33:41–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nicolini D, Svegliati-Baroni G, Candelari
R, et al: Doxorubicin-eluting bead vs conventional transcatheter
arterial chemoembolization for hepatocellular carcinoma before
liver transplantation. World J Gastroenterol. 19:5622–5632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng K, Yan J, Li X, et al: A randomized
controlled trial of radiofrequency ablation and surgical resection
in the treatment of small hepatocellular carcinoma. J Hepatol.
57:794–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orlando A, Leandro G, Olivo M, et al:
Radiofrequency thermal ablation vs. percutaneous ethanol injection
for small hepatocellular carcinoma in cirrhosis: Meta-analysis of
randomized controlled trials. Am J Gastroenterol. 104:514–524.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho YK, Kim JK, Kim MY, et al: Systematic
review of randomized trials for hepatocellular carcinoma treated
with percutaneous ablation therapies. Hepatology. 49:453–459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen A, Zhang H, Tang C, et al: Systematic
review of radiofrequency ablation versus percutaneous ethanol
injection for small hepatocellular carcinoma up to 3 cm. J
Gastroenterol Hepatol. 28:793–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takayasu K, Arii S, Kudo M, et al:
Superselective transarterial chemoembolization for hepatocellular
carcinoma. Validation of treatment algorithm proposed by Japanese
guidelines. J Hepatol. 56:886–892. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takayasu K: Chemoembolization for
unresectable hepatocellular carcinoma in Japan. Oncology. 78 (Suppl
1):S135–S141. 2010. View Article : Google Scholar
|
|
23
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease - should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bieze M, Klümpen HJ, Verheij J, et al:
Diagnostic accuracy of (18) F-methylcholine positron emission
tomography/computed tomography for intra- and extrahepatic
hepatocellular carcinoma. Hepatology. 59:996–1006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tenley N, Corn DJ, Yuan L and Lee Z: The
effect of fasting on PET imaging of hepatocellular carcinoma. J
Cancer Ther. 4:561–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu NC, Chaudhari V, Raman SS, et al: CT
and MRI improve detection of hepatocellular carcinoma, compared
with ultrasound alone, in patients with cirrhosis. Clin
Gastroenterol Hepatol. 9:161–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JJ, Zheng JS, Cui SC, et al: C-arm
Lipiodol CT in transcatheter arterial chemoembolization for small
hepatocellular carcinoma. World J Gastroenterol. 21:3035–3040.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tognolini A, Louie JD, Hwang GL, et al:
Utility of C-arm CT in patients with hepatocellular carcinoma
undergoing transhepatic arterial chemoembolization. J Vasc Interv
Radiol. 21:339–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim MJ, Kim YS, Cho YH, et al: Use of
(18)F-FDG PET to predict tumor progression and survival in patients
with intermediate hepatocellular carcinoma treated by transarterial
chemoembolization. Korean J Intern Med. 30:308–315. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho Y, Lee DH, Lee YB, et al: Does 18F-FDG
positron emission tomography-computed tomography have a role in
initial staging of hepatocellular carcinoma? PLoS One.
9:e105679–2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng J, Li J, Cui X, Ye H and Ye L:
Comparison of diagnostic sensitivity of C-arm CT, DSA and CT in
detecting small HCC. Hepatogastroenterology. 60:1509–1512.
2013.PubMed/NCBI
|
|
32
|
Bartolozzi C, Lencioni R, Caramella D,
Palla A, Bassi AM and Di Candio G: Small hepatocellular carcinoma.
Detection with US, CT, MR imaging, DSA, and Lipiodal-CT. Acta
Radio. 37:69–74. 1996. View Article : Google Scholar
|
|
33
|
Peng ZW, Zhang YJ, Chen MS, et al:
Radiofrequency ablation with or without transcatheter arterial
chemoembolization in the treatment of hepatocellular carcinoma: A
prospective randomized trial. J Clin Oncol. 31:426–432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang HJ, Lee JH, Lee DH, et al: Small
single-nodule hepatocellular carcinoma: Comparison of transarterial
chemoembolization, radiofrequency ablation, and hepatic resection
by using inverse probability weighting. Radiology. 271:909–918.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scheuermann U, Kaths JM, Heise M, et al:
Comparison of resection and transarterial chemoembolisation in the
treatment of advanced intrahepatic cholangiocarcinoma - a
single-center experience. Eur J Surg Oncol. 39:593–600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao M, Huang J, Zhang T and Wu H:
Transarterial chemoembolization in combination with local therapies
for hepatocellular carcinoma: A meta-analysis. PLoS One.
8:e684532013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salhab M and Canelo R: An overview of
evidence-based management of hepatocellular carcinoma: A
meta-analysis. J Cancer Res Ther. 7:463–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin ZZ, Shau WY, Hsu C, et al:
Radiofrequency ablation is superior to ethanol injection in
early-stage hepatocellular carcinoma irrespective of tumor size.
PLoS One. 8:e802762013. View Article : Google Scholar : PubMed/NCBI
|