Introduction
Prostate sarcoma is a rare malignancy accounting for
<1% of all primary prostate malignancies in adults (1). It has an extremely poor prognosis, with
a median overall survival time of 23 months, partly due to the
difficulty of early detection. The early clinical symptoms, such as
dysuria or abdomen pain, are unspecific and there is no specific
serum marker for the entity, thus it is always firstly detected by
imaging (2). Imaging-guided biopsy is
the standard diagnostic technique used to identify prostate sarcoma
(3). In recent years,
contrast-enhanced ultrasound (CEUS), which can depict the micro-
and macro-vascularity of prostate, has been proved effective in
detecting prostate adenocarcinoma (4,5). However,
to the best of our knowledge, the CEUS features of prostate sarcoma
remain unknown. Thus, the current study presents a case of prostate
rhabdomyosarcoma, with emphasis on the CEUS findings. The
associated literature on prostate sarcoma is also reviewed. Written
informed consent was obtained from the patient.
Case report
A 33-year-old male was referred to the Department of
Ultrasound (Shanghai Tenth People's Hospital, Shanghai, China) in
March 2014 due to frequent micturition, accompanied with a
low-grade fever (37.5°C) and lower abdomen pain. Prior to this, the
patient had been treated for prostatitis in a community hospital
for 3 months, but without evident remission. No abnormal laboratory
test findings were recorded, with the exception of a grade of 1+
for urinary occult blood upon urinalysis. The prostate-specific
antigen (PSA) level (1.26 ng/ml) was within normal limits. Other
symptoms, such as frequent micturition and a poor urinary stream
were present occasionally. Local stenosis of the rectum was
suspected upon digital rectal examination. There was no family
history of genitourinary cancer.
Transrectal US (TRUS) was performed with a LOGIQ E9
scanner (GE Healthcare, Milwaukee, WI, USA), which was equipped
with a transrectal transducer (E8C; 5–9 MHz). The patient was
examined in the left recumbent position, with slightly bent knees.
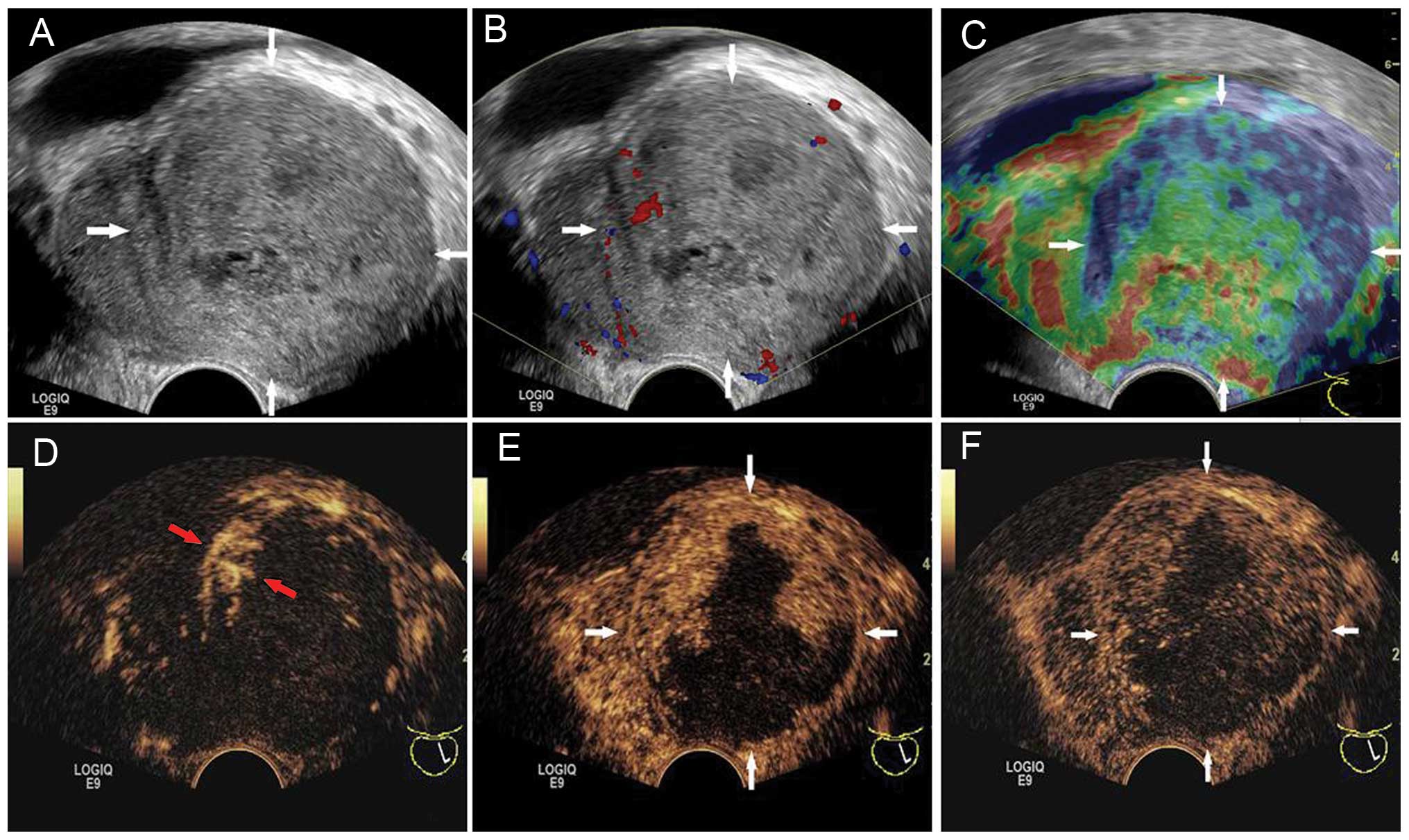
The prostate was enlarged asymmetrically on gray-scale US imaging,
measuring ~7.0×5.1×5.2 cm in size. The volume (V) of the prostate
was computed to be 97 ml when using the following formula: V = π ×
L × W × H / 6, where L is the length, W is the width and H is the
height of the prostate. The left lobe of the prostate was
protruding with a well-delineated margin, and the left lobe was
markedly larger (volume, 78 ml) than the right lobe (volume, 18
ml). The prostatic urethra and ejaculatory ducts were pushed to the
right and were not clearly shown (Fig.
1A). The left lobe was heterogeneous in echogenicity on US,
with irregular small hypoechoic areas. Color Doppler imaging showed
dotted blood flow within the left lobe (Fig. 1B).
Transrectal elastography was performed with the same
scanner to provide information on stiffness. The peripheral zone of
the left lobe was displayed in blue, indicating tissue components
with relatively hard stiffness. The central zone of the left lobe
was mainly displayed green (i.e., intermediate stiffness), with red
or yellow patch-like areas (i.e., soft or low stiffness). The right
lobe was mainly displayed in green (Fig.
1C).
CEUS was performed subsequent to the injection of
2.4 ml contrast agent (SonoVue, Bracco, Milan, Italy) followed by
10 ml of normal saline flush through the antecubital vein. Early
arterial enhancement started from the edge of the left lobe at 11
sec after contrast injection. There was rim-like hyper-enhancement
surrounding the left lobe (Fig. 1D).
Following this, the contrast agent extended immediately inward and
formed a number of hyper-enhancement zones that were irregular, but
not isolated (Fig. 1E). The contrast
agent washed out slowly and the hyper-enhancement lasted during the
whole arterial (<30 sec), venous (31–120 sec) and late (121–180
sec) phases. Adversely, contrast agent never extended into the
other region of the left lobe (Fig.
1F). The non-enhancement area was mainly at the center and
posterior of the left lobe, with an irregular shape, which
corresponded to the intermediate and low elasticity areas on
elastography examination, and the irregular hypoechoic areas on
baseline US.
Based on the results of CEUS, the ‘left lobe’ was
supposed to be a rare malignant neoplasm with high confidence.
Hence, the patient was admitted to hospital immediately and a
biopsy was performed. Only 4 samples were obtained (2 from the
upper pole and 2 from the lower pole), which were pale and fragile,
with high moisture contents.
Prior to the biopsy, a series of pre-operative
examinations, including abdominal and pelvic magnetic resonance
imaging (MRI), chest radiographs, bone scintigraphy and certain
serum tests, had been performed to evaluate the tumor staging. The
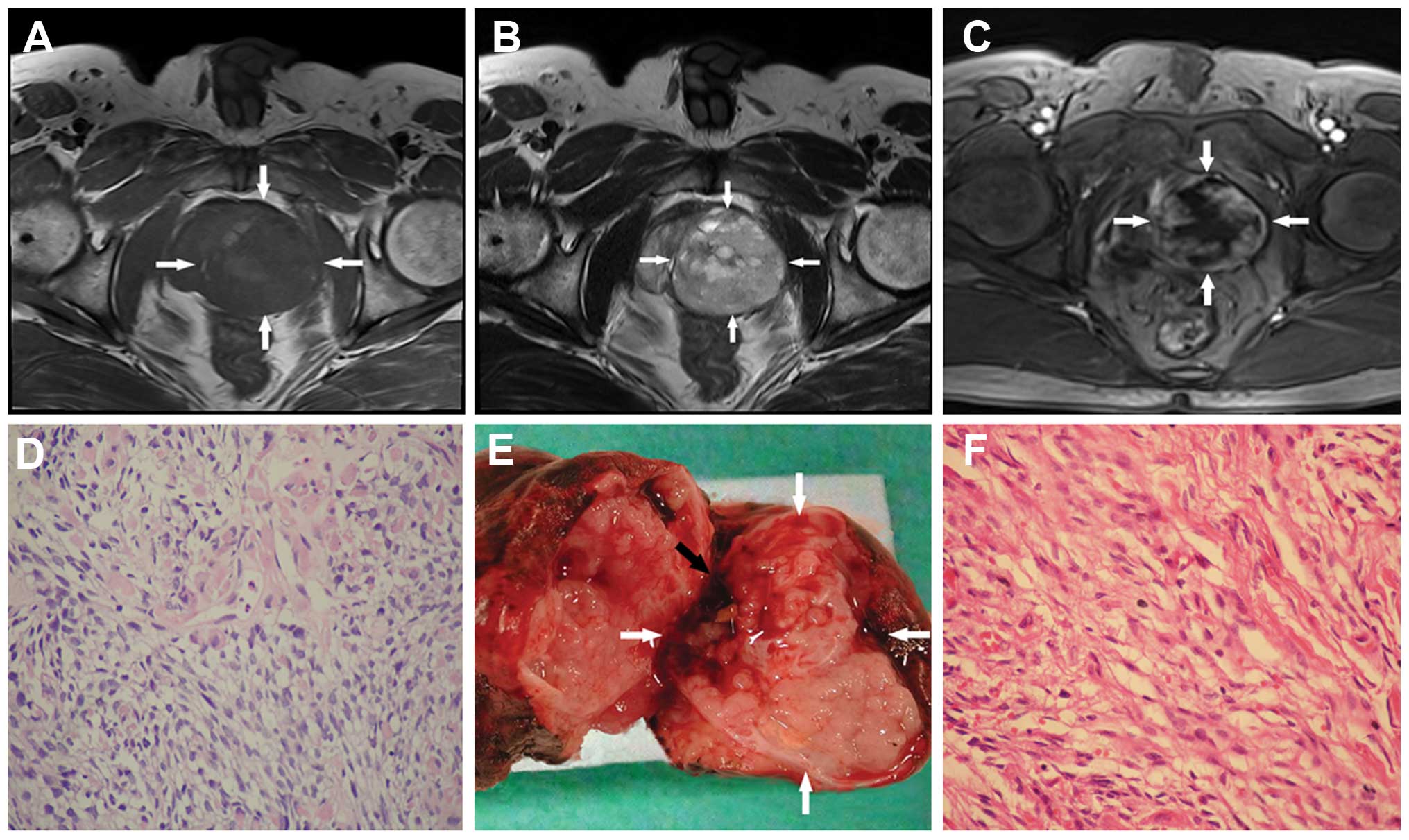
MRI depicted a pelvic mass measuring ~5.4×5.6×5 cm in size, which
was well defined, pushed the urethra to the right and compressed
the left seminal vesicle observably. The tumor revealed isointense
signals on T1-weighted imaging (Fig. 2A) and increased signal intensity on
T2-weighted imaging, with heterogeneity (Fig. 2B). Multiple small high signal
intensity areas distributed inside the mass were considered to be
cysts. Following intravenous gadolinium administration,
heterogeneous hyper-enhancement was noted (Fig. 2C). The diagnosis was a malignant
lesion in the pelvic cavity, without a precise location, and no
abnormal iliac lymph nodes were noted. The remaining scans and
serum tests negative.
The biopsy samples were analyzed by an experienced
pathologist and showed mixed bundles of primitive, undifferentiated
and diffusely distributed cells. Cytological atypia was moderate,
but the mitotic activity was high. Necrotic tissues were noted in
these samples (Fig. 2D).
Immunohistochemical studies showed that the neoplastic cells were
positive for muscle-specific actin, fast myosin and vimentin,
whereas the cells were negative for desmin, keratin AE1/AE3,
cytokeratin 5/6, PSA and prostate-specific acid phosphatase. All
pathological manifestations proved that the patient had a subtype
of prostate sarcoma.
The patient underwent a radical prostatectomy. The
whole prostate, seminal vesicles, urethral bulb and soft tissue
adjacent to the prostate were totally removed. Macroscopically, the
complete tumor was soft, fragile, moist with internal hemorrhage
and encased by a pseudo-capsule (Fig.
2E). The final pathological diagnosis was of a rhabdomyosarcoma
(Fig. 2F) with large necrotic areas.
At 12 days post-surgery, the patient recovered well and was
discharged in a stable condition. To date, the patient has finished
8 courses of chemotherapy (cisplatin and bleomycin were
administered using bladder irrigation) and is alive with no signs
of recurrence or metastases.
Discussion
Adult prostate sarcoma is extremely rare, with a
morbidity rate of 0.1–0.2% in all prostate tumors (6), which is far below the morbidity rate of
prostate adenocarcinoma (7). The main
subtypes of prostate sarcoma consist of leiomyosarcoma,
rhabdomyosarcoma, synovial sarcoma, fibrosarcoma, spindle cell
sarcoma, prostatic stromal sarcoma and undifferentiated sarcoma
(6). Rhabdomyosarcoma usually occurs
in young men, particularly in children as the most common subtype
(8). This high-grade malignant tumor
with a dismal prognosis grows fast and invasively (6), and is prone to bleeding, necrosis or
cystic degeneration. Sarcomas often metastasize hematogenously in
the early stage due to the nutrient-rich blood supply (9). Early diagnosis and surgical resection
with a curative intent offers patients the best chance of survival
(10).
However, for the majority of urologists or
radiologists, their experience of this diagnosis is limited. The
initial clinical symptoms are usually unspecific unless urinary
obstruction may increase the clinician's vigilance (11). Additionally, there appears to be no
specific tumor marker for sarcoma in the serum (12). Due to these issues, an early diagnosis
is extremely difficult, resulting in a dismal prognosis (2).
Ultrasound, MRI and cytology findings in the urine
are reported as methods for the detection of prostate sarcoma, On
TRUS, a markedly enlarged volume and irregular margins are
important characteristics of prostate sarcoma (13,14). In
addition, TRUS is usually used to guide a biopsy to confirm the
diagnosis (1). The imaging features
of MRI, such as a large size, irregular margins and heterogeneity,
are similar to those of TRUS (15,16).
Besides that, it has an advantage in detecting metastatic lesions
and revealing the adjacent structures (17). Cytology findings in the urine may be
useful, but are also varied, therefore their use is not indicated
as a routine procedure (18).
In the present case, the volume and margin of the
tumor on TRUS were not typical, as previously reported (3,13). Due to
the benefits of using CEUS, unusual features such as intralesional
non-enhancement areas and rim-like hyper-enhancement around the
lesion were revealed, so that timely and effective treatment was
performed. In addition, only 4 biopsy samples were obtained from
the hyper-enhancement areas on CEUS. This avoided the unnecessary
trauma to the patient and the risk of metastasis whenever possible.
In the majority of situations, at least 12 cores will be sampled
due to the large volume (19).
As aforementioned, due to the extremely low
morbidity of prostate sarcoma, it is not necessary to perform CEUS
routinely. Under certain situations, for example, a markedly
enlarged volume, an irregular and asymmetrical appearance, marked
heterogeneity internally or other abnormal signs in the prostate,
CEUS may be useful as a rapid diagnostic test, particularly in
young patients. Likewise, CEUS is useful to determine the biopsy
sites to decrease any unnecessary trauma and risks of metastasis.
Surgery, chemotherapy and radiotherapy are the main methods of
treatment. Among them, surgical resection is the most recommended.
In conclusion, this present case of prostate rhabdomyosarcoma
benefitted from the application of CEUS.
Acknowledgements
This study was supported in part by grants from the
Key Project of the Shanghai Health Bureau (no. 20114003) and the
Shanghai Talent Development Project of the Shanghai Human Resource
and Social Security Bureau (no. 2012045).
References
|
1
|
Janet NL, May AW and Akins RS: Sarcoma of
the prostate: A single institutional review. Am J Clin Oncol.
32:27–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sexton WJ, Lance RE, Reyes AO, Pisters PW,
Tu SM and Pisters LL: Adult prostate sarcoma: The M. D. Anderson
Cancer Center Experience. J Urol. 166:521–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Liu L, Tang H, Rao Z, Zhan W, Li
X, Zeng H, Zhang P, Wei B, Lin T, et al: Twenty-five cases of adult
prostate sarcoma treated at a high-volume institution from 1989 to
2009. Urology. 82:160–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie SW, Li HL, Du J, Xia JG, Guo YF, Xin M
and Li FH: Contrast-enhanced ultrasonography with contrast-tuned
imaging technology for the detection of prostate cancer: Comparison
with conventional ultrasonography. BJU Int. 109:1620–1626. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao HW, Luo JH, Xu HX, Wang DH, Lai YR,
Chen MN, Lv JY, Xie XY, Lu MD and Chen W: The value of
contrast-enhanced transrectal ultrasound in predicting the nature
of prostate diseases and the Gleason score of prostate cancer by a
subjective blood flow grading scale. Urol Int. 87:165–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JY, Cho YM and Ro JY: Prostatic
stromal sarcoma with rhabdoid features. Ann Diagn Pathol.
14:453–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez EA, Kassira N, Cheung MC, Koniaris
LG, Neville HL and Sola JE: Rhabdomyosarcoma in children: A SEER
population based study. J Surg Res. 170:e243–e251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dundore PA, Cheville JC, Nascimento AG,
Farrow GM and Bostwick DG: Carcinosarcoma of the prostate. Report
of 21 cases. Cancer. 76:1035–1042. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bisceglia M, Magro G, Carosi I, Cannazza V
and Ben Dor D: Primary embryonal rhabdomyosarcoma of the prostate
in adults: Report of a case and review of the literature. Int J
Surg Pathol. 19:831–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mukouyama H, Sugaya K, Ogawa Y, Koyama Y,
Hatano T and Toda T: Poorly differentiated sarcoma of the prostate
causing obstructive acute renal failure: A case report. Int J Urol.
6:615–619. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rogers CG, Parwani A, Tekes A, Schoenberg
MP and Epstein JI: Carcinosarcoma of the prostate with urothelial
and squamous components. J Urol. 173:439–440. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stilgenbauer R, Benedict M, Bamshad R and
Viduetsky A: Sarcoma of the prostate: Sonographic findings and
pathologic correlation. J Ultrasound Med. 26:1789–1793.
2007.PubMed/NCBI
|
|
14
|
Terris MK: Transrectal ultrasound
appearance of radiation-induced prostatic sarcoma. Prostate.
37:182–186. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng YC, Wang JH, Shen SH, Chang YH, Chen
PC, Pan CC and Chang CY: MRI findings of prostatic synovial
sarcoma. Br J Radiol. 80:e15–e18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren FY, Lu JP, Wang J, Ye JJ, Shao CW and
Wang MJ: Adult prostate sarcoma: Radiological-clinical correlation.
Clin Radiol. 64:171–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
ESMO/European Sarcoma Network Working
Group, . ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 23 (Suppl 7):vii92–vii99. 2012.PubMed/NCBI
|
|
18
|
Stoll LM, Johnson MW and Rosenthal DL:
High-grade prostatic sarcoma seen in a catheterized urine specimen:
Case report and differential diagnosis. Diagn Cytopathol.
39:762–766. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: European Association of Urology: EAU guidelines
on prostate cancer. Part 1: Screening, diagnosis and local
treatment with Curative Intent-Update 2013. Eur Urol. 65:124–137.
2014. View Article : Google Scholar : PubMed/NCBI
|