Introduction
Tumor immune evasion has a critical role in
tumorigenesis and progression (1).
Major histocompatibility complex (MHC) class I molecules are
important for tumor immune evasion due to their function in antigen
presentation to T-lymphocytes and cytotoxic T-lymphocytes (CTL)
function (2). In addition, the loss
of MHC class I is a common mechanism of experimental and
spontaneous tumors, which allows them to evade recognition and
destruction by CTLs (3,4). In MHC class I downregulated or deficient
tumor cells, tumor-associated antigens are not presented to the
CTLs, which results in tumor immune evasion and affects the
prognosis of tumor patients (3).
However, whether MHC class I molecules have a positive or negative
role in tumor patients' survival remains controversial. Certain
studies have reported that MHC class I loss has poor outcomes due
to its impact on antigen presentation to T-lymphocytes and CTLs
(5,6).
By contrast, other reports have revealed that MHC class I loss may
improve patients' survival through activating natural killer (NK)
cell function (7,8). These results vary between different
types of tumors and the occurrence of lymph node metastasis
(5,7).
It has been reported that NLRC5 may have a positive
role in the regulation of MHC class I expression in human cell
lines and mice models. NLRC5 function was examined in
NLRC5-deficient mice, the results of which revealed reduced MHC
class I expression in lymphocytes, including T, NK and NKT
lymphocytes (9,10). NLRC5 localizes to the nucleus of
lymphocytes, where it promotes MHC class I gene expression via
stimulation of the H-2D and H-2K gene promoters (11,12). Human
NLRC5 is predominantly expressed in hematopoietic cells as well as
the spleen, lymph nodes, bone marrow and thymus; in addition, NLRC5
is expressed abundantly in the lungs and intestines (10,11,13–18).
Staehli et al (10) reported
that HeLa cells express NLRC5 when induced by interferon-γ. MHC
class I molecules have a critical role in tumor immune evasion and
are associated with cancer prognosis; since, NLRC5 was reported to
act as a MHC class I transactivator, it may therefore affect tumor
patients' survival through regulating tumor immune evasion through
MHC class I. Thus, evaluating the expression of NLRC5 in human
solid tumors and exploring its association with MHC class I
expression and patients' survival may offer a novel method for
predicting patient prognosis and provide potential novel
therapeutic targets.
The present study aimed to examine NLRC5 expression
in human tumor tissues and its association with clinical outcomes
of non-small-cell lung cancer (NSCLC) stage III patients. The
expression of NLRC5 and MHC class I was determined in
non-small-cell lung cancer (NSCLC) stage III tissues and the
correlation between NLRC5 (cytoplasmic and nuclear) and MHC class I
was analyzed. In addition, the clinical data of NSCLC patients was
collected in order to study the association between clinical
outcomes and the expression of NLRC5 and MHC class I. Furthermore,
the present study aimed to examine the expression of NLRC5 and MHC
class I in seven different types of common human solid tumors in
tissue microarrays (TMAs) using immunohistochemistry (IHC).
Materials and methods
NSCLC patients
A total of 62 NSCLC patients who underwent radical
resection of stage III-node involvement (N)2, without
any preoperative therapy, were included in the present
retrospective study. All patients were diagnosed and underwent
surgery at West China Hospital (Chengdu, China) between January
2001 and September 2003. Histological diagnosis was established
according to the guidelines of the World Health Organization
(19). Pathological findings,
including tumor size, location and lymph node status, were
described in the reports of board-certified pathologists. Out of
the 62 NSCLC patients, the pathological diagnoses included 31 with
adenocarcinoma cell cancer, 24 with squamous cell cancer and 7 with
other pathology types. Patients' age at the time of surgery ranged
from 26 to 75 years, with a median age of 58 years. Long-term
outcome was determined from hospital records and information from
follow-up appointments. Overall survival (OS) was measured from the
date of surgery to either mortality or the final follow-up visit.
Progression-free survival was calculated from the date of surgery
to the time of the first local or distant recurrence, or mortality
from any cause. Local recurrence was defined as tumor regrowth in
hilar, mediastinal, supraclavicular lymph nodes or at the bronchial
margin of resection, as visualized using computed tomography (CT)
scans. Recurrences beyond those sites were deemed as distant
metastases. The present study was approved by the Ethics Committee
of Sichuan University (Chengdu, China). Written informed consent
was obtained from all patients and all clinical investigations were
performed according to the principles of the Declaration of
Helsinki.
TMAs
Seven different types of human tumor
paraffin-tissues were purchased from the company of Ailina
Biotechnology Co., Ltd (Xi'an, China), including 69 renal carcinoma
cases (BC070140), 30 cervical carcinoma cases (CR602), 37 rectal
cancer cases (RE482), 67 gastric adenocarcinoma cases (BS01012), 40
liver cancer cases (LV483), 40 malignant melanoma cases (ME418a)
and 40 prostate cancer cases (PR483a). The TMAs were composed of
normal tissue, adjacent tissue and different types of tumor
tissues.
IHC analysis
NSCLC tissues were fixed in 10% buffered formalin
(Beyotime Institute of Biotechnology, Shanghai, China), embedded in
paraffin (Beyotime Institute of Biotechnology) and cut into 5-µm
sections. TMAs and NSCLC sections were deparaffinized in xylene
(Beyotime Institute of Biotechnology), rehydrated in a series of
descending ethanol (Beyotime Institute of Biotechnology)
concentrations and incubated in 0.03% hydrogen peroxide (Beyotime
Institute of Biotechnology), then stored in dark place for 10 min.
Antigen retrieval was performed in 10 mM sodium citrate buffer (pH
6.0; Beyotime Institute of Biotechnology) for 10 min at room
temperature. The tissue sections and TMAs were then incubated with
antibodies at room temperature for 45 min. Commercial antibodies
employed were rabbit monoclonal antibodies: Anti-NLRC5 (1:200;
ab117624; Abcam, Cambridge, UK), anti-human leukocyte antigen
(HLA)-ABC (1:100; ab70328; Abcam). Following incubation, the
specimens were washed with Tris-buffered saline with Tween (TBS-T;
0.5% Tween, 0.1 M Tris-base, 0.9% NaCl; pH 7.6; Beyotime Institute
of Biotechnology) and incubated with peroxidase-labeled polymer
(Beyotime Institute of Biotechnology) at room temperature for 30
min. The samples were then washed with TBS-T buffer and incubated
with freshly prepared 3,3′-diaminobenzidine tetrahydrochloride
(DAB; Zhongshan Jinqiao Biological Technology Ltd., Beijing, China)
and substrate-chromogen buffer (Beyotime Institute of
Biotechnology) at room temperature for 7 min. Immunohistochemical
reactions were developed in freshly prepared DAB at room
temperature for 7 min, then lightly counterstained with hematoxylin
(Beyotime Institute of Biotechnology) prior to mounting. The
intensity of staining and the percentage of positive cells were
assessed in a semi-quantitative manner. Images were captured using
an Olympus BX51 fluorescence microscope (Olympus Corp., Tokyo,
Japan) equipped with an Olympus Micro DP 72 camera (Olympus Corp.).
The distribution of positive cells was scored as follows: Not
stained, 0; <1/3 cells stained, 1; <2/3 cells stained, 2; and
>2/3 cells stained, 3. The intensity of staining was graded as
follows: Not stained, 0; mild stained, 1; and strong stained, 2.
The scores for distribution and intensity were added and graded as
follows: 0–2, negative; and 3–5, positive (20).
Statistical analysis
Statistical analysis of the study data was performed
using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). The
significance of associations was determined using the Pearson
χ2 test. For OS analysis, Kaplan-Meier curves were
derived and the statistical significance of differences between the
survival of groups with different MHC class I and NLRC5 expression
was determined using the log-rank test. The results were censored
if the patients remained alive, had died from any other causes or
had withdrawn from the study. Cox regression analysis was used for
multivariate analysis to allow for comparison of the effects of
several different factors on survival. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Correlation of NLRC5 and MHC class I
expression in NSCLC patients
NLRC5 and MHC class I expression were detected in 62
cases, including 31 with adenocarcinoma cell cancer, 24 with
squamous cell cancer and 7 with other pathology types. IHC staining
and analysis revealed that 83.9% of tissues exhibited MHC class
I-positive expression, 67.7% of tissues demonstrated cytoplasmic
NLRC5-positive expression and 88.7% were nuclear NLRC5-positive
(Table I). The association study
revealed that the expression of MHC class I was significantly
correlated with the expression of nuclear NLRC5 (P=0.008); in
addition, nuclear NLRC5 was found to be associated with the
expression of NLRC5 in the cytoplasm (P=0.002). However, no
correlation was observed between MHC class I and cytoplasmic NLRC5
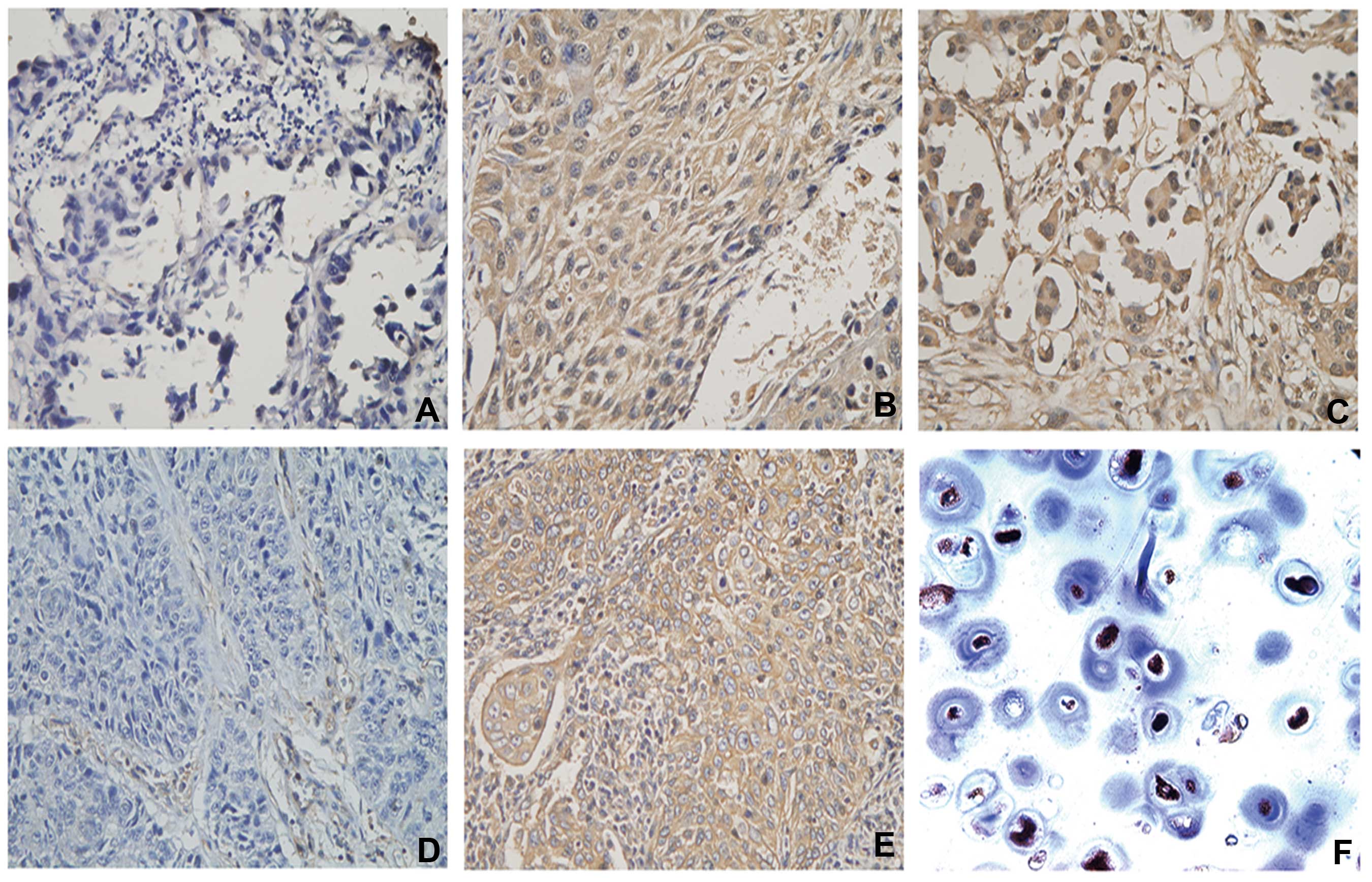
(P=0.570) (Table II). Fig. 1 shows representative IHC images of
HLA-ABC, cytoplasmic and nuclear NLRC5 staining in NSCLC tissues,
including negative cytoplasmic and nuclear NLRC5 staining, positive
expression of NLRC5 in the cytoplasm and nucleus, HLA-ABC lose and
HLA-ABC expression in the tumor cell membrane.
 | Table I.MHC class I and NLRC5 expression and
association with OS in non-small-cell lung cancer. |
Table I.
MHC class I and NLRC5 expression and
association with OS in non-small-cell lung cancer.
| Marker | Expression | n (%) | Median OS
(months) | 95% CI | P |
|---|
| MHC class I | Negative | 10(16.1) | 28.0 | 19.5–36.5 | 0.032 |
|
| Positive | 52(83.9) | 19.0 | 16.0–20.0 |
|
| CNLRC5 | Negative | 7(11.3) | 32.0 | 0.0–75.0 | 0.086 |
|
| Positive | 55 (88.7) | 19.4 | 16.7–22.2 |
|
| NNLRC5 | Negative | 20(32.3) | 30.0 | 24.5–35.5 | 0.039 |
|
| Positive | 42(67.7) | 17.0 | 14.0–20.0 |
|
 | Table II.Correlation of major
histocompatibility complex class I and NLRC5 in seven common human
solid tumors. |
Table II.
Correlation of major
histocompatibility complex class I and NLRC5 in seven common human
solid tumors.
|
|
| HLA-ABC &
NNLRC5 | NNLRC5 &
CNLRC5 | HLA-ABC &
CNLRC5 |
|---|
| Tumor type | n | P-value | P-value | P-value |
|---|
| Renal
carcinoma | 69 | 0.926 | 0.942 | 0.703 |
| Gastric
adenocarcinoma | 67 | 0.014 | 0.010 | 0.045 |
| Cervical
carcinoma | 30 | 0.295 | 0.363 | 0.885 |
| Prostate
cancer | 39 | 0.018 | 0.746 | 0.342 |
| Malignant
melanoma | 40 | 0.015 | 0.057 | 0.031 |
| Liver cancer | 40 | 0.008 | 0.002 | 0.570 |
| Rectal cancer | 37 | 0.019 | 0.812 | 0.354 |
| All tumor
cases | 384 | <0.001 | 0.003 | <0.001 |
MHC class I and nuclear NLRC5 indicate
the prognosis of NSCLC patients
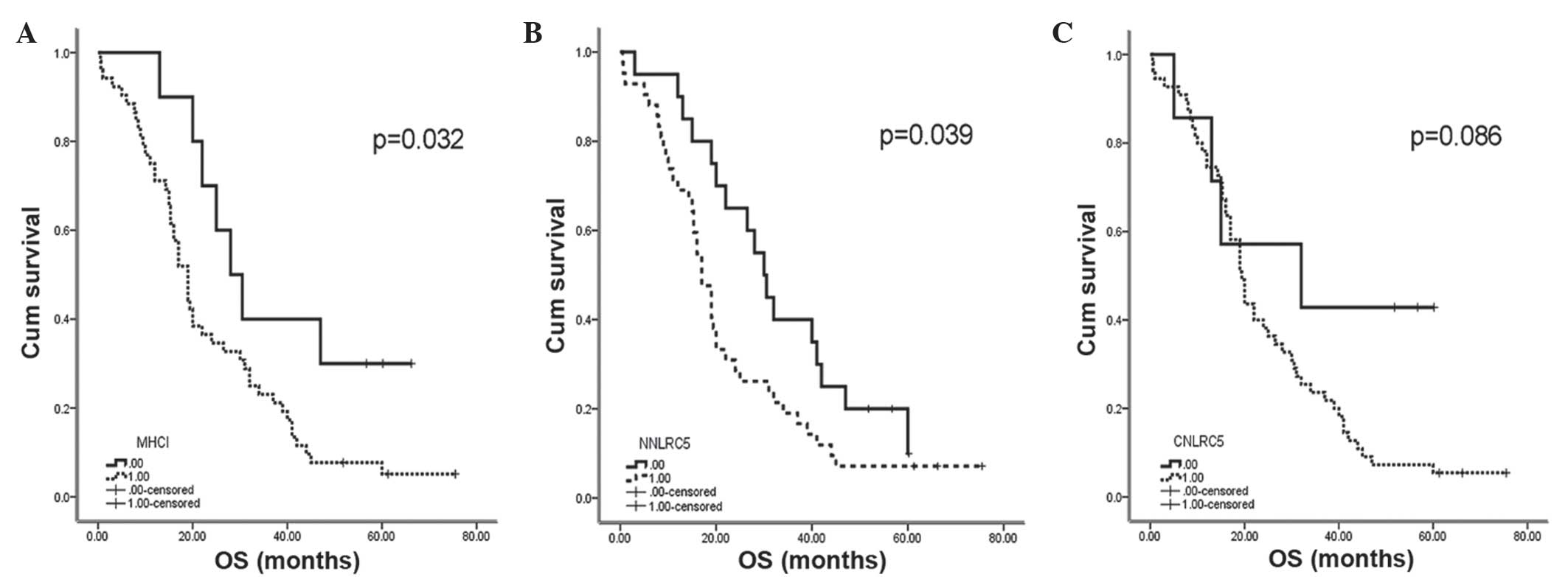
Nuclear NLRC5 and MHC class I positive expression
were revealed to be correlated with a reduced OS rate in NSCLC
stage III patients (log-rank, P=0.039 and P=0.032, respectively)
(Table I). The median OS of MHC class
I-positive patients was 19 months, while the OS for MHC class
I-negative patients was 28 months (P=0.032). The median OS of
nuclear NLRC5-positive patients was 17 months, while the negative
group was 30 months (P=0.039) (Fig.
2). For the cytoplasmic NLRC5-positive and -negative patients,
the data were 32 months and 19.4 months, respectively; these
results were not significantly associated with prognosis (P=0.086).
By contrast, there was no significant association between the
expression of MHC class I or NLRC5 and progression free survival
(data not shown). Cox regression analysis was used for multivariate
analysis to compare the effects of several different factors on
survival, including gender, age, pathological type and
tumor-node-metastasis values; however, no significant positive
correlations were identified (Table
III).
 | Table III.Association between MHC class I,
NLRC5 expressions and clinicopathologic factors in non-small-cell
lung cancer patients. |
Table III.
Association between MHC class I,
NLRC5 expressions and clinicopathologic factors in non-small-cell
lung cancer patients.
| Characteristic | n | HLA-ABC+ (%) | P | NNLRC5+ (%) | P | CNLRC5+ (%) | P |
|---|
| Gender |
|
| 0.681 |
| 0.349 |
| 0.651 |
| Male | 48 | 41(85.4) |
| 34(70.8) |
| 43(89.6) |
|
| Female | 14 | 11(78.6) |
| 8(57.1) |
| 12(85.7) |
|
| Age |
|
| 0.493 |
| 1.00 |
| 0.432 |
| >60 | 33 | 29(87.9) |
| 22(66.7) |
| 28(84.8) |
|
| <60 | 29 | 23(79.3) |
| 20(69.0) |
| 27(93.1) |
|
| Histological |
|
|
|
|
|
|
|
| AC | 31 | 24(77.4) |
| 21(67.7) |
| 26(83.9) |
|
| Classification |
|
| 0.359 |
|
|
| 0.401 |
| SC | 24 | 22(91.7) |
| 16(66.7) |
| 22(91.7) |
|
| Others | 7 | 6(85.7) |
| 5(71.4) |
| 7(100.0) |
|
| TNM |
|
| 0.228 |
| 0.625 |
| 0.406 |
| T1N2M0 | 3 | 2(66.7) |
| 1(33.3) |
| 2(66.7) |
|
| T2N2M0 | 25 | 23(92.0) |
| 17(68.0) |
| 23(92.0) |
|
| T3N2M0 | 17 | 15(88.2) |
| 12(70.6) |
| 14(82.4) |
|
| T4N2M0 | 17 | 12(70.6) |
| 12(70.6) |
| 16(94.1) |
|
NLRC5 and MHC class I are expressed in
different tumor tissues
The expression of NLRC5 and MHC class I were
examined in 323 cases of tumor tissue in TMAs by IHC; the tumors
investigated were the seven most common types of human solid
tumors. The results demonstrated that MHC class I was widely
expressed in all seven types of tumor tissue examined and certain
sections of the tissues demonstrated high levels of MHC class I
expression. The percentages of MHC class I-positive TMAs were 82.6%
(57/69) in renal carcinoma, 83.6% (56/67) in gastric
adenocarcinoma, 60% (18/30) in cervical squamous carcinoma tissue,
60% (24/40) in prostate cancer, 82.5% (33/40) in malignant
melanoma, 67.5% (27/40) in liver cancer and 59.5% (22/37) in rectal
cancer. For nuclear and cytoplasmic expression of NLRC5, there
results were: 73.9% (51/69) and 88.4% (61/69) in renal carcinoma;
86.6% (58/67) and 77.6% (52/67) in gastric adenocarcinoma; 56.7%
(17/30) and 63.3% (19/30) in cervical squamous carcinoma; 85.0%
(34/40) and 87.5% (35/40) in prostate cancer; 67.5% (27/40) and
75.0% (30/40) in malignant melanoma; 60% (24/40) and 75.0% (30/40)
in liver cancer; and 83.8% (31/37) and 86.5% (32/37) in rectal
cancer, respectively (Table IV).
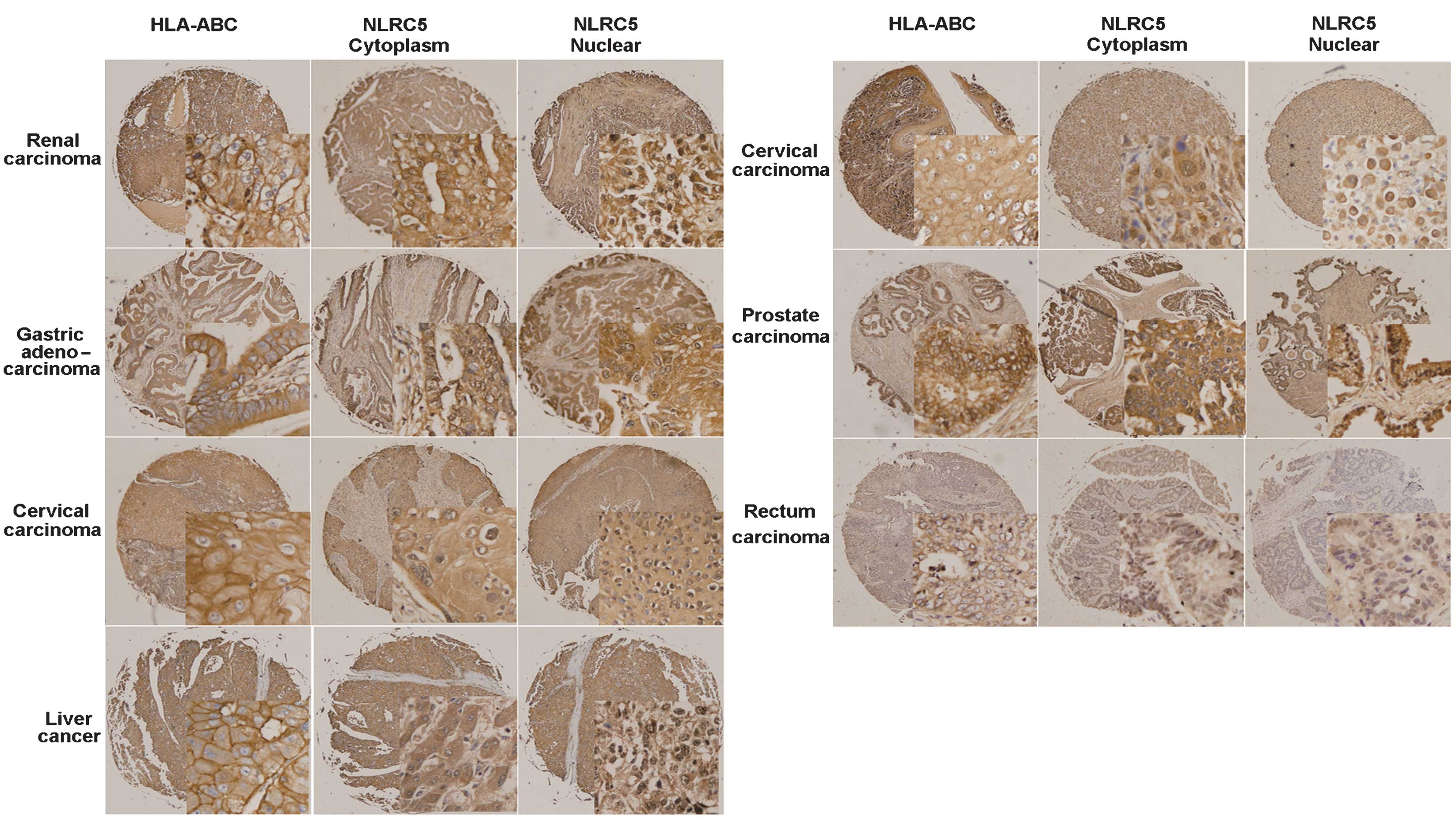
Fig. 3 shows the immunohistochemical
staining of seven human solid tumor tissue microarrays. Each panel
shows a representative example of tumor tissue exhibiting positive
HLA-ABC, cytoplasmic NLRC5 and nuclear NLRC5 staining.
 | Table IV.Expression of major
histocompatibility complex class I and NLRC5 in seven common human
solid tumors. |
Table IV.
Expression of major
histocompatibility complex class I and NLRC5 in seven common human
solid tumors.
| Tumor type | n | HLA-ABC+ (%) | CNLRC5+ (%) | NNLRC5+ (%) |
|---|
| Renal
carcinoma | 69 | 57 (87.6) | 61 (88.4) | 51 (73.9) |
| Gastric
adenocarcinoma | 67 | 56 (83.6) | 52 (77.6) | 58 (86.6) |
| Cervical
carcinoma | 30 | 18 (60.0) | 19 (63.3) | 17 (56.7) |
| Prostate
cancer | 40 | 24 (60.0) | 35 (87.5) | 34 (85.0) |
| Malignant
melanoma | 40 | 33 (82.5) | 30 (75.0) | 27 (67.5) |
| Liver cancer | 40 | 27 (67.5) | 30 (75.0) | 24 (60.0) |
| Rectal cancer | 37 | 22 (59.5) | 32 (86.5) | 32 (83.8) |
Correlation of NLRC5 and MHC class I
expression in tumors
In the 385 cases (62 NSCLC and 323 TMAs) of tumor
paraffin-tissues for IHC analysis, the expression of MHC class I
(HLA-ABC), cytoplasmic NLRC5 and nuclear NLRC5 were examined and
their correlations were determined using the Pearson χ2
test. The results revealed significant correlations between MHC
class I and nuclear NLRC5 (P<0.001), MHC class I and cytoplasmic
NLRC5 (P=0.003) and between nuclear and cytoplasmic NLRC5
(P<0.001) (Table II).
Furthermore, the correlations between the three proteins expression
were analyzed in the seven TMAs separately. The results revealed
that, with the exception of renal carcinoma and cervical cancer,
all the tumor tissues demonstrated correlations between MHC class I
and nuclear NLRC5 expression (Table
II). These results indicated that, as a MHC class I
transactivitor, the expression of NLRC5 was correlated with MHC
class I in human solid tumors.
Discussion
To the best of our knowledge, the present study was
the first to examine the expression of NLRC5 in common human tumor
tissues and its association with the clinical outcomes of NSCLC
stage III patients. The results revealed that NLRC5 and MHC class I
expression were detected in NSCLC and seven other types of common
human tumor tissues. IHC staining demonstrated that NLRC5 was
expressed in the nucleus and cytoplasm of all eight common tumor
tissues. In addition, the expression of MHC class I was found to be
associated with nuclear NLRC5 in all 385 tumor cases. These results
indicated that as in normal immune situations, NLRC5 regulates MHC
class I expression in human tumors. By analyzing the clinical data
of 62 postoperative NSCLC stage III patients, it was determined
that MHC class I and nuclear NLRC5 expression were independent
predictors of poor survival, which was consistent with certain
previous studies (7,21,22).
In the present study, there was a high level of
consistency among the 62 NSCLC patients, as they all underwent
radical surgery and were diagnosed with stage III-N2
NSCLC. This may reduce the effects of difference between various
tumor stages and increase credibility of the current results. In
the present study, the individual IHC results for the NSCLC, liver
cancer, gastric adenocarcinoma, rectal cancer, malignant melanoma
tissues all revealed a correlation between the expression of MHC
class I and nuclear NLRC5. However, in the renal and cervical
carcinoma cases, there was no significant correlation between MHC
class I and NLRC5 (cytoplasmic and nuclear) expression. This may be
due to the limited number of cases for cervical cancer. While in
renal carcinoma, the majority of the tumor is clear cell carcinoma,
which is adenocarcinoma of the renal tubular epithelial cell;
therefore, the tissue heterogeneity was different from the other
tumor types examined.
Due to the role of MHC class I molecules in tumor
immune surveillance and immune evasion, a number of studies have
aimed to elucidate the mechanisms by which this proceeds and its
association with patient prognosis (2,23).
Previous studies have suggested that high levels of MHC class I
expression makes tumor cells promising targets for T cells; by
contrast, weak expression prevents specific T cell recognition and
results in an increased risk of disease recurrence (3,24). For
colorectal cancer, Simpson et al (5) reported a mean survival advantage of 26.1
months in patients whose tumors had strong MHC class I expression
over those who exhibited weak MHC class I expression. Nagata et
al demonstrated that HLA class I loss was associated with
recurrence-free survival time, but not OS in NSCLC (25). However, certain studies reached
different conclusions. Madjd et al (7) reported that the total loss of MHC class
I was an independent indicator of positive prognosis in breast
cancer. In addition, Ramnath et al (8) suggested that HLA class I antigen
downregulation was associated with improved survival (8). In certain other types of tumors, the
results also varied (26–30). MHC class I loss may affect antigen
presentation to T-lymphocytes and CTL function; however, this may
increase the susceptibility of tumors to NK cells and result in an
improved prognostic outcome. Ramnath et al (8) indicated that there was a selective loss
of MHC class I heavy chain, which was associated with improved
prognosis. This may be the result of immune surveillance, which in
certain patients may select against the more aggressive tumors,
allowing for the growth of the more indolent HLA-negative tumors.
Tumor growth may then be further controlled by NK cells (8). These previous results demonstrated that
in different tumor types, T cell recognition and NK cell function
were not identical and may therefore have various effects on tumor
growth and patient survival.
The results of previous studies regarding MHC class
I as a prognostic indicator have been controversial; this may be
due to the types of samples included these studies. Certain studies
have analyzed tumor patients at different disease stages with short
clinical follow up (7,8,25).
Analysis of various tumor stages in a small group of patients may
not be sufficient to determine whether MHC class I may be used as a
prognostic indicator. The present study included 62 highly
consistent cases of NSCLC stage III patients; therefore, these
results may be more representative and indicate a novel method for
predicting the prognosis of NSCLC patients. Further studies are
required, with large-sample randomized experiments in order to
confirm the potential of MHC class I and nuclear NLRC5 as
prognostic factors for different types of cancer.
The function of NLRC5 as a MHC class I
transactivator has been studied thoroughly over the past few
decades. NLRC5 moves between the cytoplasm and nucleus, which
indicates that it may have a nuclear function (31). The expression of NLRC5-mediated MHC
class I gene requires an intact nuclear localization signal and
nuclear distribution (31).
Therefore, altered cellular localization of NLRC5 may impact MHC
class I expression as well as MHC class I-mediated antigen
presentation (9). In addition, it was
reported that NLRC5 influences histone methylation (H3K27me3) and
may therefore mediate gene expression through adjusting the
chromosome activation status of the MHC class I locus (32). In the present study, nuclear and
cytoplasmic NLRC5 expression were found to be associated with MHC
class I (P<0.001 and P=0.003, respectively).
In conclusion, the results of the present study
demonstrated that NLRC5 was widely expressed in eight common human
tumor tissues in the nucleus as well as the cytoplasm. Furthermore,
the nuclear expression was found to be correlated with MHC class I.
MHC class I heavy chain loss has been validated in a previous study
to be a predictor of patient survival (22); the present study demonstrated that the
expression of NLRC5 in the nucleus acted as a negative prognostic
indicator in NSCLC patients. Therefore NLRC5 and MHC class I may be
used in conjunction with other independent prognostic factors in
order to further stratify patients for adjuvant therapy. However,
in order to validate the use of these factors as cancer biomarkers
to predict the patients' prognosis, randomized screening trials are
required. In vitro and in vivo experiments may also
be required in order to fully elucidate the effect of MHC class I
and NLRC5 on the tumor growth and differentiation.
Acknowledgements
The authors would like to thank Miss. Li Li for
advice and instruction in IHC and Professor Chen Xian-Cheng for
assistance with microscopy and image processing.
References
|
1
|
Igney FH and Krammer PH: Immune escape of
tumors: Apoptosis resistance and tumor counterattack. J Leukoc
Biol. 71:907–920. 2002.PubMed/NCBI
|
|
2
|
Garcia-Lora A, Algarra I and Garrido F:
MHC class I antigens, immune surveillance and tumor immune escape.
J Cell Physiol. 195:346–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garrido F, Cabrera T, Concha A, et al:
Natural history of HLA expression during tumour development.
Immunol Today. 14:491–499. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kärre K: NK cells, MHC class I molecules
and the missing self. Scand J Immunol. 55:221–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson JA, Al-Attar A, Watson NF,
Scholefield JH, Ilyas M and Durrant LG: Intratumoral T cell
infiltration, MHC class I and STAT1 as biomarkers of good prognosis
in colorectal cancer. Gut. 59:926–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Lora A, Martinez M, Algarra I,
Gaforio JJ and Garrido F: MHC class I-deficient metastatic tumor
variants immunoselected by T lymphocytes originate from the
coordinated downregulation of APM components. Int J Cancer.
106:521–527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Madjd Z, Spendlove I, Pinder SE, Ellis IO
and Durrant LG: Total loss of MHC class I is an independent
indicator of good prognosis in breast cancer. Int J Cancer.
117:248–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramnath N, Tan D, Li Q, et al: Is
downregulation of MHC class I antigen expression in human non-small
cell lung cancer associated with prolonged survival? Cancer Immunol
Immunother. 55:891–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meissner TB, Li A, Liu YJ, Gagnon E and
Kobayashi KS: The nucleotide-binding domain of NLRC5 is critical
for nuclear import and transactivation activity. Biochem Biophys
Res Commun. 418:786–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staehli F, Ludigs K, Heinz LX, et al:
NLRC5 deficiency selectively impairs MHC Class I- dependent
lymphocyte killing by cytotoxic T cells. J Immunol. 188:3820–3828.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magalhaes JG, Sorbara MT, Girardin SE and
Philpott DJ: What is new with Nods? Curr Opin Immunol. 23:29–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barker BR, Taxman DJ and Ting JP:
Cross-regulation between the IL-1β/IL-18 processing inflammasome
and other inflammatory cytokines. Curr Opin Immunol. 23:591–597.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benko S, Magalhaes JG, Philpott DJ and
Girardin SE: NLRC5 limits the activation of inflammatory pathways.
J Immunol. 185:1681–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davis BK, Roberts RA, Huang MT, et al:
Cutting Edge: NLRC5-dependent activation of the inflammasome. J
Immunol. 186:1333–1337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui J, Zhu L, Xia X, et al: NLRC5
negatively regulates the NF-κB and type I interferon signaling
pathways. Cell. 141:483–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neerincx A, Lautz K, Menning M, et al: A
role for the human nucleotide-binding domain, leucine-rich
repeat-containing family member NLRC5 in antiviral responses. J
Biol Chem. 285:26223–26232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao Y and Qian Y: Expression regulation
and function of NLRC5. Protein Cell. 4:168–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biswas A, Meissner TB, Kawai T and
Kobayashi KS: Cutting edge: Impaired MHC class I expression in mice
deficient for Nlrc5/Class I transactivator. J Immunol. 189:516–520.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brambilla E, Travis WD, Colby TV, et al:
The new World Health Organization classification of lung tumours.
Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan L, Li P, Yin Z, et al: Ribosomal s6
protein kinase 4: A prognostic factor for renal cell carcinoma. Br
J Cancer. 109:1137–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rimsza LM, Roberts RA, Miller TP, et al:
Loss of MHC class II gene and protein expression in diffuse large
B-cell lymphoma is related to decreased tumor immunosurveillance
and poor patient survival regardless of other prognostic factors: A
follow-up study from the Leukemia and Lymphoma Molecular Profiling
Project. Blood. 103:4251–4258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bijen CB, Bantema-Joppe EJ, de Jong RA, et
al: The prognostic role of classical and nonclassical MHC class I
expression in endometrial cancer. Int J Cancer. 126:1417–1427.
2010.PubMed/NCBI
|
|
23
|
Hicklin DJ, Marincola FM and Ferrone S:
HLA class I antigen downregulation in human cancers: T-cell
immunotherapy revives an old story. Mol Med Today. 5:178–186. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagata Y, Hanagiri T, Mizukami M, et al:
Clinical significance of HLA class I alleles on postoperative
prognosis of lung cancer patients in Japan. Lung Cancer. 65:91–97.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nijman HW, van Diest PJ, Poort-Keesom RJ,
et al: T cell infiltration and MHC I and II expression in the
presence of tumor antigens: An immunohistochemical study in
patients with serous epithelial ovarian cancer. Eur J Obstet
Gynecol Reprod Biol. 94:114–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cromme FV, van Bommel PF, Walboomers JM,
Gallee MP, Stern PL, Kenemans P, Helmerhorst TJ, Stukart MJ and
Meijer CJ: Differences in MHC and TAP-1 expression in cervical
cancer lymph node metastases as compared with the primary tumours.
Br J Cancer. 69:1176–1181. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cromme FV, Meijer CJ, Snijders PJ,
Uyterlinde A, Kenemans P, Helmerhorst T, Stern PL, van den Brule AJ
and Walboomers JM: Analysis of MHC class I and II expression in
relation to presence of HPV genotypes in premalignant and malignant
cervical lesions. Br J Cancer. 67:1372–1380. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Atkins D, Ferrone S, Schmahl GE, Störkel S
and Seliger B: Down-regulation of HLA class I antigen processing
molecules: An immune escape mechanism of renal cell carcinoma? J
Urol. 171:885–889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu JD, Higgins LM, Steinle A, Cosman D,
Haugk K and Plymate SR: Prevalent expression of the
immunostimulatory MHC class I chain-related molecule is
counteracted by shedding in prostate cancer. J Clin Invest.
114:560–568. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meissner TB, Li A, Biswas A, Lee KH, Liu
YJ, Bayir E, Iliopoulos D, van den Elsen PJ and Kobayashi KS: NLR
family member NLRC5 is a transcriptional regulator of MHC class I
genes. Proc Natl Acad Sci USA. 107:13794–13799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meissner TB, Liu YJ, Lee KH, Li A, Biswas
A, van Eggermond MC, van den Elsen PJ and Kobayashi KS: NLRC5
cooperates with the RFX transcription factor complex to induce MHC
class I gene expression. J Immunol. 188:4951–4958. 2012. View Article : Google Scholar : PubMed/NCBI
|