Introduction
Demyelinating diseases, are neuropathological
entities most frequently observed in the central nervous system
with a preferential involvement of the major white matter tracts in
a periventricular distribution. These diseases are often difficult
to diagnose, since they do not exhibit a typical appearance on
magnetic resonance imaging (MRI) scans (1–3).
Tumefactive demyelinating lesions (TDLs), also referred to as
‘demyelinating pseudo tumors’, are a rare type of demyelinating
disease. They manifest as solitary lesions or a few separate
lesions that result in a mass effect, edema on the surrounding
tissue and enhancement (4,5). The correct diagnosis is often not
established by radiological study, but only after surgical biopsy
or resection (6–8). TDLs were first reported by van der
Velden et al (9) in 1979.
Pathologically, TDLs are considered to be intermediate lesions
between multiple sclerosis and acute disseminated encephalomyelitis
(8). The clinical presentation and
radiographic appearance of these lesions often result in the
diagnosis of intracranial tumors, including gliomas (8,10–12,13). Since
the majority of patients with TDLs respond favorably to
corticosteroid treatment, surgical resection is not required;
therefore, it is important for neurosurgeons to improve their
understanding of this uncommon pathological process in order to
avoid unnecessary resection or adjunctive therapy. The present
study analyzed the data of 14 patients with TDLs who underwent
surgical treatment in the Department of Neurosurgery, Beijing
Tiantan Hospital (Beijing, China) between January 2004 and January
2009 to help to establish the correct diagnosis prior to surgery,
in order to avoid unnecessary resection or adjunctive therapy in
future cases of TDLs.
Materials and methods
Patients
Between January 2004 and January 2009, 14 patients
(male, 9; female, 5) with cerebral TDLs were admitted and received
surgical treatment at the Department of Neurosurgery of the Beijing
Tiantan Hospital. The median age was 24 years (range, 4–51 years).
The diagnosis of TDLs was established following pathological
analysis. All the patients were examined neurologically and
radiologically, including the use of computed tomography (CT) and
MRI, prior to and following surgery. The clinical presentations,
imaging features and postoperative neurological deficits were
recorded. The study was approved by the Ethics Committee of Beijing
Tiantan Hospital Affiliated to Capital Medical University (Beijing,
China). Written informed consent was obtained from the
patients/patients' families.
Treatment
The surgical approach was decided according to the
preoperative diagnosis, which was performed based on the result of
medical history, presentations and the radiology examination (CT
and MRI scans). When the preoperative diagnosis was considered to
be a tumor, craniotomy and microsurgical resection were performed
with the aim of complete or partial resection depending on what was
feasible. However, in cases where it was not possible to affirm a
certain preoperative diagnosis, biopsy was indicated. Biopsy of
suspicious lesions was approached stereo tactically in order to
minimize tissue injury. In order to improve the positive rate and
accuracy of the biopsy, 9–12 targets were selected during
stereotactic biopsy. Immunohistopathological analysis was performed
for all cases, while the frozen-section procedure was performed
only in cases of surgical resection. For the cases that received
biopsy, corticosteroid therapy using high-dose methylprednisolone
was administered once the diagnosis of TDL was confirmed by
histopathological examination. The treatment regimen was as
follows: 500–1,000 mg of methylprednisolone within 500 ml of 0.9%
NaCl or 5% glucose was administrated intravenously by dropping in 3
h per day for 5 days, which was followed by oral administration of
60–80 mg per day for 1 week. Subsequently, 10 mg methylprednisolone
was administered every 5 days to allow withdrawal. Postoperatively,
patients with a history of seizure were treated continuously with
anticonvulsant therapy until 1–2 years free of seizures was
achieved. By contrast, for patients with no history of seizures, 3
months of prophylactic anticonvulsant therapy was routinely
administered.
Follow-up
Follow-up study was conducted for all the patients
by routine outpatient appointments with neurological and
neuroradiological examinations at 3–6 months following discharge,
and later by interview, telephone or post. The Karnofsky
performance scale (KPS) was used to evaluate the patients' status
(15).
Data presentation
Continuous variables were expressed as the mean ±
standard deviation or the median (range), and categorical variables
were expressed as proportions.
Results
Clinical presentations
From the available data of 14 patients, the
male-to-female ratio was ~1.8, with a male preponderance (Table I).
 | Table I.Clinical presentations of 14 patients
with tumefactive demyelinating lesions. |
Table I.
Clinical presentations of 14 patients
with tumefactive demyelinating lesions.
| Clinical
presentation | Cases, n | Proportion, % |
|---|
| Severity |
|
|
|
Acute | 4 | 28.6 |
|
Subacure | 6 | 42.8 |
|
Chronic | 4 | 28.6 |
| Symptoms and
sings |
|
|
| ICP | 4 | 28.6 |
| Local neurological
deficits |
|
|
|
Hemiplegia | 8 | 57.2 |
| Partial
aphasia | 1 | 7.1 |
| Seizures |
|
|
|
General | 1 | 7.1 |
|
Local | 3 | 21.4 |
| Vaccination
history | 1 | 7.1 |
| Infection
history | 0 | 0.0 |
TDLs were considered to have an acute (4 cases),
sub-acute (6 cases) or chronic (4 cases) onset with a duration
(defined as the time between onset and admission) of 10 days to 7
years. The main symptoms and signs of the TDLs included the
following: Increased intracranial pressure (ICP, 4 cases); local
neurological deficits, including hemiplegia (8 cases) and partial
aphasia (1 case); seizures, including general (1 case) and local (3
cases). Only 1 case had a history of vaccination and no cases were
observed to have an associated infection history.
Radiographic features
The radiographic features observed in the 14 TDL
cases are listed in Table II. The
locations of solitary lesions (12 cases) included the following:
Frontal lobe (3 cases), temporal lobe (4 cases), parietal lobe (3
cases), insular lobe (1 case) and basilar ganglia area (1 case).
Regarding the 2 cases with multiple lesions, in 1 case the lesions
were located at the frontal lobe and parietal lobe, while in the
other case the lesions were located in the frontal lobe and the
pons. The size of the lesions ranged between 2 and 5 cm. On the CT
scans, the TDLs were hypodense (9 cases) or heterogeneously dense
(5 cases) masses with a well-defined margin and little surrounding
edema. On the MRI scans, 12 cases demonstrated local subcortical
masses which were hypo-intense on T1-weighted images and
hyper-intense on T2-weighted images and fluid-attenuated inversion
recovery images, with the exception of 2 cases with heterogeneous
signal intensity. A total of 6 cases had a relatively sharp margin
and 8 cases had a poorly defined margin, with light to moderate
surrounding edema. Following Gd-DTPA administration, all the cases
presented variable enhancement, including 5 cases (35.7%) of
patchy, 6 cases of open ring-like (42.9%) and 3 cases of ring-like
(21.4%) enhancement. In addition, 6 cases demonstrated enhancing
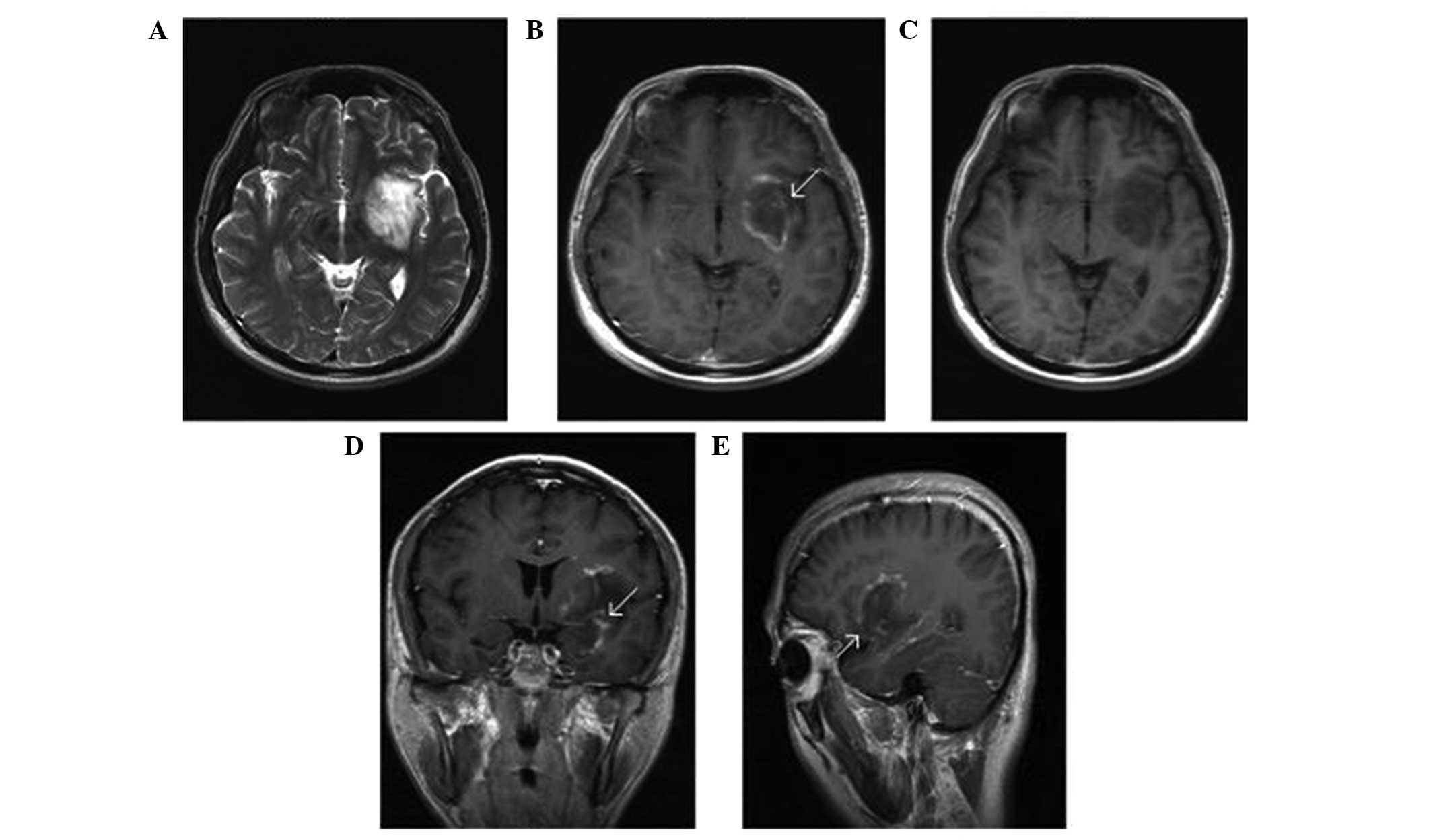
vein coursing undistorted through the lesion (Fig. 1).
 | Table II.Radiographic features of tumefactive
demyelinating lesions patients. |
Table II.
Radiographic features of tumefactive
demyelinating lesions patients.
| Feature | Cases, n | Proportion, % |
|---|
| Solitary lesions |
|
|
| Frontal
lobe | 3 | 21.4 |
| Temporal
lobe | 4 | 28.6 |
| Parietal
lobe | 3 | 21.4 |
| Insular
lobe | 1 | 7.2 |
| Basilar
ganglia area | 1 | 7.2 |
| Multiple
lesionsa |
|
|
| Frontal
lobe + parietal lobe | 1 | 7.2 |
| Frontal
lobe + pons | 1 | 7.2 |
| Computed
tomography scan |
|
|
|
Hypodenseb | 9 | 64.3 |
|
Heterogeneous density | 5 | 35.7 |
| Magnetic resonance
imagingc |
|
|
| Local
subcortical mass | 12 | 85.7 |
|
Heterogeneous intensity | 2 | 14.3 |
| Relative
sharp margind | 6 | 42.9 |
| Poor
defined margin | 8 | 57.1 |
| Variable
enhancement |
|
|
|
Patchy | 5 | 35.7 |
| Open
ring-like | 6 | 42.9 |
|
Ring-like | 3 | 21.4 |
| Vein
coursing | 6 | 42.9 |
Diagnosis, treatments and
outcomes
Among the 14 cases examined, 8 cases were
misdiagnosed preoperatively as gliomas and 1 case as metastasis
(Table III). In 5 cases, no certain
diagnosis was established. Microsurgical resection was performed in
9 cases, while 5 cases were approached by stereotactic biopsy.
Gross complete resection was achieved in 8 patients and partial
resection in 1 patient, which was suspicious of TDL by
frozen-section (Table IV). For the 9
patients that underwent resection, the ICP was relieved completely
in 4 patients following surgery. The postoperative complications
included hemiplegia in 2 cases and mortality in 1 case (due to
postoperative intracranial infection). For all the biopsy cases,
there were no surgical complications in the present study. All the
biopsy cases received high-dose methylprednisolone therapy after
the diagnosis of TDLs was confirmed by histopathological
examination. Following steroid therapy, the preoperative symptoms
and signs improved (4 cases) or totally relieved (2 cases) for all
6 patients (improved and relieved) in 1–4 weeks (Table IV).
 | Table III.Diagnosis of patients with
tumefactive demyelinating lesions. |
Table III.
Diagnosis of patients with
tumefactive demyelinating lesions.
| Diagnosis | Cases, n | Proportion, % |
|---|
| Certain
diagnosis |
|
|
|
Gliomas | 8 | 57.1 |
|
Metastasis | 1 |
7.1 |
| No certain
diagnosis | 5 | 35.8 |
 | Table IV.Therapy methods and outcomes of
patients with tumefactive demyelinating lesions. |
Table IV.
Therapy methods and outcomes of
patients with tumefactive demyelinating lesions.
| Parameter | Cases, n | Proportion, % |
|---|
| Resection |
|
|
|
Total | 8 | 57.1 |
|
Subtotal (frozen-section) | 1 |
7.1 |
| Intracranial
hypotension | 4 | 28.6 |
| Complication |
|
|
|
Hemiplegia | 2 | 14.3 |
|
Mortality | 1 |
7.1 |
| Biopsy | 5 | 35.7 |
| Symptoms and
signs |
|
|
|
Improved | 4 | 28.6 |
|
Relieved | 2 | 14.3 |
Follow-up study
The follow-up study (range, 17–72 months; mean, 41
months) was successfully completed in all 14 cases, with the
exception of 1 mortality case. Among them, 2 resection cases and 2
biopsy cases still had hemiplegia, which was improved compared with
that at discharge (Table V). A total
of 2 cases with seizures were improved and the other 2 cases
achieved total seizure-control. The partial aphasia was completely
relieved in 3 months following biopsy. There were 2 recurrence
cases (14.3%) identified by MRI, at 26 and 51 months
postoperatively, which presented as multiple sclerosis and were
improved by steroid therapy. The KPS assessment demonstrated a
score of 100 for 7 cases (50%), 90 for 3 cases (21.4%), 80 for 3
cases (21.4%), and 0 for 1 case (7.1%) (16).
 | Table V.Follow-up study. |
Table V.
Follow-up study.
| Outcome | Cases, n (%) | Notes |
|---|
| Complications |
|
|
|
Hemiplegia | 4 (28.6) | 2 resection cases;
2 biopsy cases |
|
Seizures | 4 (28.6) | 2 cases improved; 2
cases achieved total seizure-control |
|
Aphasia | 1 | Totally relieved 3
months after biopsy |
| Recurrent
cases | 2 (14.3) | Identified by MRI,
26 and 51 months postoperatively |
| KPS |
|
|
|
100 | 7 (50.0) | – |
| 90 | 3 (21.4) | – |
| 80 | 3 (21.4) | – |
| 0 | 1 (7.1) | Mortality |
Discussion
The exact pathogenesis of TDLs is not clearly
understood. These lesions are considered to be an isolated middle
type between multiple sclerosis and acute-disseminated
encephalomyelitis plaques, which are the two common types of
demyelinating diseases in the central nervous system (1,8).
Pathological features demonstrated by demyelinated diseases include
hyper cellular lesions with confluent demyelination, abundant foamy
macrophages containing myelin debris, reactive astrogliosis,
‘relative’ axonal preservation and variable perivascular and
parenchymal lymphocytic inflammation. Infiltration by macrophages
and lymphocytes (in particular T cells) is a main pathological
feature of TDLs, which implies an association between TDLs and
virus infection. A number of previous reports support the
hypothesis that the pathogenesis of TDLs may be associated with
infections or vaccination, similar to other demyelinating disorders
(12–14). In the present study, only one case
presented a history of hepatitis B vaccination and no cases were
associated with infection, which may imply that TDLs is
pathogenically heterogeneous.
TDLs may occur at any age groups, but are most
commonly observed in adult patients in the second and third decades
of their life, as observed in previous studies (12–15). It
has also previously been reported that TDLs had a female prevalence
similar to that of multiple sclerosis, although no gender
predilection was reported (16,17).
However, a slight male prevalence was observed in the present
study. The common presentations of TDLs mimic tumors rather than
other demyelinating disorders, including increased ICP (headache
and vomiting), local neurological deficits (hemiplegia and aphasia)
and seizures (18). In the present
study, with the exception of the TDL patients with a history of
seizures, the onset of TDLs was found to be acute or sub-acute and
the neurological deficits progressed faster compared with the time
course for intracranial tumors, which may be as a result of the
different pathological processes. The duration between onset and
admission also depended on the clinical presentations. For patients
with increased ICP and local neurological deficits, the duration
usually ranged between days and weeks, or between months and years
for patients with seizures.
For radiological analysis, MRI is generally the most
sensitive imaging technique for depicting a demyelinating disease.
However, TDLs present a diagnostic challenge, since they usually
presents a subcortical mass in the hemisphere mimicking gliomas as
a solitary lesion or metastasis as a few separate lesions (7,8,12–14,17). A
number of features have previously been reported to aid diagnosis,
including ringlike or open-ring enhancement and central dilated
veins within the lesions (4,5,19). In the
present study, 9 cases (64.3%; Table
II) presented with ring like or open-ring enhancement. The
enhancement patterns of TDLs presented in the form of an open ring
with the incomplete portion of the ring on the gray matter side of
the lesion, while most typical active multiple sclerosis plaques
exhibited this open-ring or arc-like pattern of enhancement only in
9% of cases (20). The enhancing
portion of the ring was considered to represent the leading edge of
demyelination and thus favored the white matter side of the lesion.
The central non-enhancing core represented a more chronic phase of
the inflammatory process. In a proportion of the TDL cases in the
present study, a dilated vascular structure was observed running
centrally within the lesions on the T2-weighted and Gd-DTPA
enhanced images; in total, 6 such cases (42.9%) were identified in
the present study. These vascular structures have been hypothesized
to represent dilated veins draining towards the distended
subependymal veins (16,18,20). The
former sign is also observed in certain cases of glioma and
metastasis, while the latter sign appears to be more specific to
TDLs. The pathological diagnosis may also be challenging simply
based on the quality of the initial frozen-section specimen
(4). In the present study, only 1
case was diagnosed as a TDL based on the initial frozen-section
specimen result. The final diagnosis relies on the findings of
immunohistopathological analysis.
Clinically, TDLs have proven to be a diagnostic
dilemma for neurosurgeons. Among the spectrum of brain tumors, TDLs
are misdiagnosed as gliomas, which are common neoplastic lesions.
In general, gliomas with increased ICP or local neurological
deficits have a longer duration between onset and admission,
usually weeks to months. TDLs usually present with a more acute
onset and a shorter duration compared with gliomas (21). On an MRI scan, low-grade glioma
usually appears as a subcortical mass with little mass effect and
surrounding edema, but with no enhancement following Gd-DTPA
administration (22). However, the
majority of TDLs demonstrate enhancement. High-grade gliomas
present with enhancement, and with evident mass effect and
surrounding edema. Therefore, cerebral isolated masses with
clinical features including acute or subacute onset, neurological
deficits, enhancement (particularly ring like or open-ring
enhancement) with little mass effect and surrounding edema on an
MRI scans should alert neurosurgeons of the possible diagnosis of
TDLs.
The majority of patients with TDLs have been
reported to respond favorably to corticosteroid therapy and not
progress to multiple sclerosis (8).
However, a number of patients presented reduced response to steroid
therapy and in those cases plasma exchange was indicated (23–25). In
the present study, 5 patients with biopsy received high-dose
methylprednisolone therapy, demonstrating symptomatic improvement
or even total relief within 1–4 weeks. On the MRI scans, all the
patients demonstrated reduction or disappearance of radiographic
abnormalities following steroid therapy. In patients that underwent
resection, the lesions had disappeared in the neuroradiological
follow-up. In the follow-up results of the current study, ≥90% of
patients achieved a satisfactory prognosis (KPS≥80). However,
surgical complications occurred in 3 cases: Hemiplegia in 2
patients, while 1 patient succumbed to the disease. In comparison
with the confirmed positive effects and safety profile of steroid
therapy, resection resulted in further unnecessary trauma in
patients. Therefore, once TDLs is considered to be a possible
diagnosis, biopsy or trial steroid therapy may be indicated to
avoid unnecessary resection or adjunctive therapy. In the present
study, no trial steroid therapy was administrated prior to surgery
due to the lack of knowledge of TDLs.
A previous study has reported that TDLs are usually
isolated and rarely progress to typical multiple sclerosis
(9). However, a number of studies
have reported that the majority of TDL patients ultimately develop
clinically definite multiple sclerosis and that a minority subset
developed only TDLs at last follow-up. The median time to a second
clinical episode was ~2–5 years in 45–70% of patients (9,26,27). In the present study, 2 patients
(15.4%) experienced recurrence and all the cases presented with the
radiological features of multiple sclerosis following 2–4 years.
However, the natural progression of TDLs remains uncertain. Further
studies with larger sample sizes and longer follow-up are
required.
In conclusion, TDLs are a rare demyelinating
disorder in the central nervous system, resembling brain tumors in
their clinical and radiological features and usually responding
well to steroid therapy. By improving the understanding on the
clinical and radiographical features of these lesions, more
patients with TDLs may be correctly diagnosed prior to resection
and receive reasonable treatment, which may result in an improved
and satisfactory prognosis.
References
|
1
|
Love S: Demyelinating diseases. J Clin
Pathol. 59:1151–1159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barkhof F, Rocca M, Francis G, et al:
Validation of diagnostic magnetic resonance imaging criteria for
multiple sclerosis and response to interferon beta-1a. Ann Neurol.
53:718–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Triulzi F and Scotti G: Differential
diagnosis of multiple sclerosis: contribution of magnetic resonance
techniques. J Neurol Neurosurg Psychiatry. 64 (Suppl 1):S6–S14.
1998.PubMed/NCBI
|
|
4
|
Masdeu JC, Quinto C, Olivera C, Tenner M,
Leslie D and Visintainer P: Open-ring imaging sign: Highly specific
for atypical brain demyelination. Neurology. 54:1427–1433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klawiter EC, Benzinger T, Roy A, Naismith
RT, Parks BJ and Cross AH: Spinal cord ring enhancement in multiple
sclerosis. Arch Neurol. 67:1395–1398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Patre PL, Castillo V, Delavelle J,
Vuillemoz S, Picard F and Landis T: ‘Tumor-mimicking’ multiple
sclerosis. Clin Neuropathol. 22:235–239. 2003.PubMed/NCBI
|
|
7
|
Dagher AP and Smirniotopoulos J:
Tumefactive demyelinating lesions. Neuroradiology. 38:560–565.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucchinetti CF, Gavrilova RH, Metz I, et
al: Clinical and radiographic spectrum of pathologically confirmed
tumefactive multiple sclerosis. Brain. 131:1759–1775. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Velden M, Bots GT and Endtz LJ:
Cranial CT in multiple sclerosis showing a mass effect. Surg
Neurol. 12:307–310. 1979.PubMed/NCBI
|
|
10
|
Wurm G, Parsaei B, Silye R and Fellner FA:
Distinct supratentorial lesions mimicking cerebral gliomas. Acta
Neurochir (Wien). 146:19–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwamoto K, Oka H, Utsuki S, Ozawa T and
Fujii K: Late-onset multiple sclerosis mimicking brain tumor: A
case report. Brain Tumor Pathol. 21:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagzag D, Miller DC, Kleinman GM, Abati A,
Donnenfeld H and Budzilovich GN: Demyelinating disease versus tumor
in surgical neuropathology. Clues to a correct pathological
diagnosis. Am J Surg Pathol. 17:537–545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peterson K, Rosenblum MK, Powers JM,
Alvord E, Walker RW and Posner JB: Effect of brain irradiation on
demyelinating lesions. Neurology. 43:2105–2112. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hunter SB, Ballinger WE Jr and Rubin JJ:
Multiple sclerosis mimicking primary brain tumor. Arch Pathol Lab
Med. 111:464–468. 1987.PubMed/NCBI
|
|
15
|
Lucchinetti CF, Gavrilova RH, Metz I,
Parisi JE, Scheithauer BW, Weigand S, et al: Clinical and
radiographic spectrum of pathologically confirmed tumefactive
multiple sclerosis. Brain. 131:1759–1775. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crooks V, Waller S, Smith T and Hahn TJ:
The use of the Karnofsky Performance Scale in determining outcomes
and risk in geriatric outpatients. J Gerontol. 46:M139–M144. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Comi G: Mutiple sclerosis: Pseudotumoral
forms. Neurol Sci. 25 (Suppl 4):S374–S379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitha AP, Scott JN, George D, Hanson A,
MacRae ME and Bell RB: Tumefactive demyelinating lesions. Can J
Neurol Sci. 34:362–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Given CA II, Stevens BS and Lee C: The MRI
appearance of tumefactive demyelinating lesions. AJR Am J
Roentgenol. 182:195–199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He J, Grossman RI, Ge Y and Mannon LJ:
Enhancing patterns in multiple sclerosis: evolution and
persistence. AJNR Am J Neuroradiol. 22:664–669. 2001.PubMed/NCBI
|
|
21
|
Kim DS, Na DG, Kim KH, et al:
Distinguishing tumefactive demyelinating lesions from glioma or
central nervous system lymphoma: Added value of unenhanced CT
compared with conventional contrast-enhanced MR Imaging. Radiology.
251:467–475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enzinger C, Strasser-Fuchs S, Ropele S, et
al: Tumefactive demyelinating lesions: Conventional and advanced
magnetic resonance imaging. Mult Scler. 11:135–139. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cha S, Pierce S, Knopp EA, et al: Dynamic
contrast-enhanced T2-weighted MR imaging of tumefactive
demyelinating lesions. AJNR Am J Neuroradiol. 22:1109–1116.
2001.PubMed/NCBI
|
|
24
|
Weinshenker BG: Therapeutic plasma
exchange for acute inflammatory demyelinating syndromes of the
central nervous system. J Clin Apheresis. 14:144–148. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keegan M, Pineda AA, McClelland RL, Darby
CH, Rodriguez M and Weinshenker BG: Plasma exchange for severe
attacks of CNS demyelination: predictors of response. Neurology.
58:143–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Champs Study Group, . Interferon beta-1a
for optic neuritis patients at high risk for multiple sclerosis. Am
J Ophthalmol. 132:463–471. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Confavreux C and Vukusic S: Natural
history of multiple sclerosis: implications for counselling and
therapy. Curr Opin Neurol. 15:257–266. 2002. View Article : Google Scholar : PubMed/NCBI
|