Introduction
EW02 is a polysaccharide-enriched crude extract from
black soybean (BS). BS has been used extensively by the Chinese as
food, traditional Chinese medicine or animal feed, for hundreds of
years (1). References to BS can be
found in traditional Chinese medicine pharmacopeias from 200 A.D.
onwards (2,3).
Review of the available traditional Chinese medical
literature at the China Academy of Chinese Medicine between 1988
and 2001 resulted in 100 references being found on BS in Chinese.
These contained safety and efficacy information on over 5,000
Chinese patients, with over 4,000 of these patients receiving BS
orally, either as monotherapy or in combination with other
foods/herbs. Recent studies, including those by Liao et al
(1) and Wu et al (4), have also described the ability of BS
polysaccharides to increase white cell counts, stimulate cytokine
production, enhance immunity and inhibit tumor growth.
In vitro assays using the enzyme-linked
immunosorbent assay method have demonstrated the ability of
polysaccharides from BS (PSBS) to stimulate the production of
cytokines in U937 cells, and colony-forming unit assays have
demonstrated the ability of EW02 to increase the number of
hematopoietic progenitor cells (5).
In vivo studies in mice showed that when administered after
5-FU, PSBS not only reduced the level of neutropenia compared with
the control, but also reduced the time for the neutrophil levels to
normalize. Bone marrow cells taken from the sacrificed PSBS-treated
mice showed increased myeloid colony formation (1).
The aforementioned findings have been replicated in
tumor-bearing (CT26 murine adenocarcinoma) and non-tumor-bearing
6–10-week-old BALB/c mice (weight, 20±5 g) when PSBS was
administered for 5 days following an intraperitoneal injection of
5-FU (6). A clear dose response was
observed between the 100-mg/kg and 400-mg/kg oral doses. No deaths
and no changes in body weights were observed during the study.
Another two studies also used genistein, an isoflavone found in BS,
to rescue irradiated mice, which subsequently showed hematopoietic
recovery (7,8). The production of granulocyte
colony-stimulating factor (G-CSF) and interleukin (IL)-6 was also
enhanced.
In patients on myelosuppressive chemotherapy, the
white blood cell (WBC) count nadir occurs between 11 and 19 days
post-chemotherapy. Blood samples taken around this time-point, with
or without EW02, may serve to provide preliminary information on
the effect of EW02 on absolute neutrophil count (ANC) and
circulatory cytokines (9). Under
clinician supervision, observational results on 8 breast cancer
(BC) patients for 12 days and 5 cervical cancer patients for 36
days have shown oral EW02 to be safe and well tolerated at the dose
of 100 mg three times per day. Furthermore, preliminary results on
4 BC patients observed for 12 days and 3 patients for 36 days also
showed this preparation to be safe and well-tolerated at the dose
of 300 mg three times per day. None of the three patients with 36
days EW02 therapy experienced leucopenia (9). Doses of BS in previous studies ranged
from <10 to 500 g, administered once daily, in 2 to 3 divided
doses, or as part of regular meals (10). The dosage of 700 mg EW02 three times a
day, which is equal to a daily dose of 300 g BS, is well tolerated
in the clinical setting. Furthermore, neutropenia is associated
with reduced myelopoietic proliferation (11). Therefore, we designed a randomized,
double-blinded trial to assess the efficacy for myelopoiesis, the
safety and the quality of life (QOL) conferred by EW02 administered
orally in BC patients receiving chemotherapy.
Materials and methods
Study design
The present study is a single-center,
double-blinded, placebo-controlled crossover pilot study of oral
EW02 in combination with chemotherapy versus placebo in combination
with chemotherapy in BC patients (ClinicalTrials.gov identifier,
NCT00555516). In total, 700 mg oral EW02 or placebo were
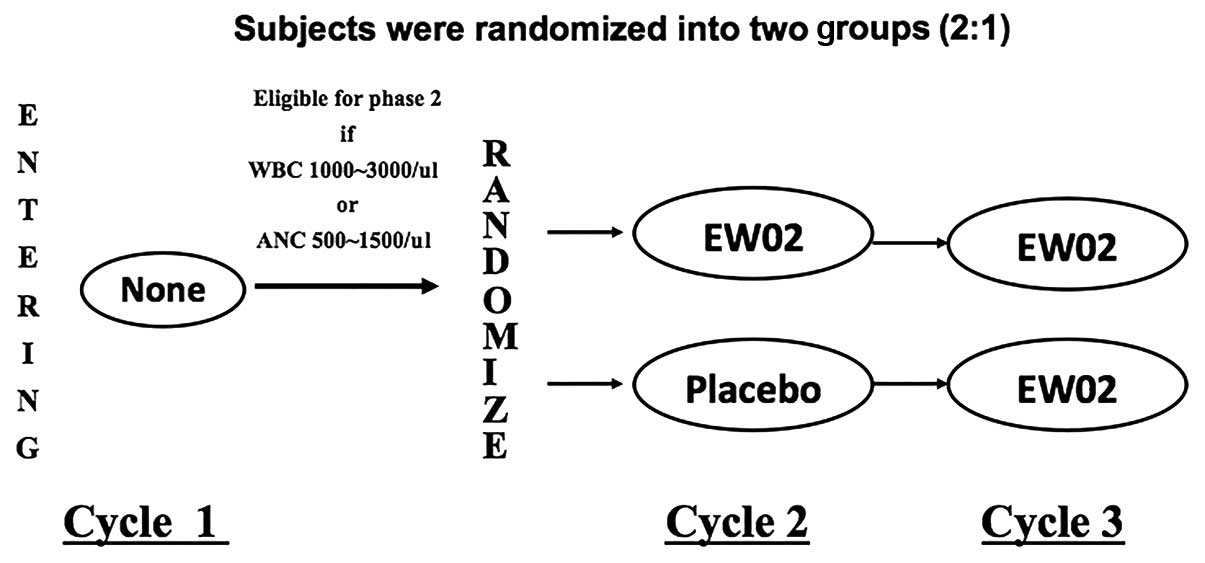
administered three times per day at the beginning of cycle (C)2 for
15 consecutive days (Fig. 1). Sample
size computation was based on the responder rate, defined as the
percentage of subjects who did not develop neutropenia during one
specific cycle (C1) of chemotherapy. With the assumption of a 51
and 17% responder rate in the EW02 and placebo groups,
respectively, in order to reject the null hypothesis at a two-sided
level of significance of 0.05, with a 2:1 randomization
(EW02:placebo), a total sample size of 60 would allow the detection
of a minimal difference with a power of 70%.
A total of 60 subjects were randomized 2:1 into two
groups in permuted blocks of six, with four subjects assigned to
group 1 and two subjects assigned to group 2. Group 1 received EW02
for 15 consecutive days during C2 and group 2 received 15
consecutive days of placebo during C2. C3 was designed as the
extension phase to collect additional safety data. All patients
received EW02 in C3 for 15 consecutive days.
The primary efficacy endpoint was the comparison of
surrogate markers, ANC/WBC (expressed as the mean percentage change
from baseline), between the two treatment groups during the C2
nadir. Patients underwent either one of the standard adjuvant
regimens listed in the inclusion criteria. The nadir of ANC/WBC was
defined on day (D)15 of each cycle and was compared with D1 to
calculate the net percentage of change. The secondary efficacy
endpoint was the intergroup comparison of the QOL survey collected
in C2.
The study was performed according to the guidelines
of the Helsinki Declaration and was approved by the Institutional
Review Board of the Tri-Service General Hospital (TSGHIRB; Taipei,
Taiwan, R.O.C; TSGHIRB Approval Number, 094-01-0001-II). All
patients provided written informed consent.
Eligibility criteria
Eligible patients were aged between 20 and 70 years,
and had histological confirmed and completely excised invasive BC
of stage I–IIIA, with neutropenia (WBC count of
<3,000/mm3 or ANC of <500/mm3) on D8 or
D15 of C1 of chemotherapy. The performance status, which was
defined by the Eastern Cooperative Oncology Group (ECOG) (12), was ≤2. Patients were treated with
chemotherapy as clinically indicated, but were restricted to one of
the following regimens: 60 mg/m2 Adriamycin + 600
mg/m2 cyclophosphamide (AC) every 3 weeks for 4 courses,
or 50 mg/m2 Adriamycin + 500 mg/m2
cyclophosphamide + 500 mg/m2 fluorouracil every 3 weeks
for six courses (CAF).
Exclusion criteria included pregnancy or lactation,
hypersensitivity to bean products, and any prior systemic therapy
or radiotherapy for BC. Patients with an uncontrollable medical or
psychiatric disease, a history of myocardial infarction or a
secondary malignancy, those who were hepatitis B/C carriers, those
with neutropenia (WBC count of <4,000/mm3 or ANC of
<2,000/mm3 on C1D1) at the time of enrollment, or
those who had been part of other investigational drug studies
within the past 30 days were also excluded.
Rescue with G-CSF and withdrawal from
study
A rescue treatment was provided if a patient
suffered from febrile neutropenia and if the investigator believed
that G-CSF plus empiric antibiotics or equivalent treatment would
be necessary to relieve the situation. The criteria for G-CSF
rescue were febrile neutropenia and an ANC of
<500/mm3 or a WBC count of <1,000/mm3,
as defined by the National Health Insurance Bureau, Taiwan.
Follow-up procedure
Patients were evaluated at each visit, and blood was
drawn for determination of WBC counts (ANC), and serum electrolyte,
aspartate transaminase, alanine transaminase, blood urea nitrogen
and serum creatinine levels on D1, D8 and D15 of C2-C4. QOL was
evaluated at each cycle, using two questionnaires provided by the
European Organization for the Research and Treatment of Cancer
(EORTC) and the National Health Research Institutes (NHRI), Taiwan,
based on Chinese medicine theory, which included three sections:
EORTC QLQ-C30, EORTC QLQ-BR23 and QOL-NCH04 (13).
Biomarker tests
The serum of each patient was collected at C1D1, and
D15 of C2 and C3 for biomarker assay. Venous blood was drawn,
allowed to clot at room temperature for 30–60 min, centrifuged at
1,500 × g for 10 min to collect the serum and then stored at 4°C
for no more than 4 h. All sera were then stored at −80°C and only
thawed to room temperature immediately prior to the biomarker
assays. Serum IL-6 and G-CSF levels were determined using
commercial immunoassay kits according to the manufacturer's
instructions (RayBiotech Inc., Atlanta, GA, USA) and expressed in
pg/ml [intra-assay coefficient of variability (CV), <10%;
inter-assay CV, <12%].
Statistical analyses
Student's t-test and χ2 tests were used
to check the baseline characteristics of each group. An
intent-to-treat analysis was used. A two-sided Wilcoxon rank-sum
test was used for the comparison of percentage change of WBC/ANC
from pre-dose (D1) to D15 (nadir) in the two groups. The primary
efficacy endpoint was the comparison of surrogate markers, WBC/ANC,
using analysis of covariance (expressed as mean percentage change
from baseline), during the C2 nadir in the EW02 and placebo groups.
The secondary efficacy endpoints include inter-group and individual
comparisons of the QOL survey, and serum IL-6 and G-CSF levels.
Results
Patient characteristics
The demographics of the placebo and experimental
groups are shown in Table I. A total
of 60 patients were enrolled, with 40 patients in the EW02 group
and 20 patients in the placebo group. There was no significant
difference in any of the stratified variances.
 | Table I.Patient demographics and biomarker
evaluation. |
Table I.
Patient demographics and biomarker
evaluation.
| Factor | EW02 (n=40) | Placebo (n=20) | P-value |
|---|
| Age | 48.2±9.9 | 52.7±6.5 | 0.068a |
| ER |
|
|
|
|
Positive | 22 | 15 | 0.133b |
|
Negative | 18 | 5 |
|
| HER |
|
|
|
|
Positive | 12 | 4 | 0.409b |
|
Negative | 28 | 16 |
|
| Stage |
|
|
|
| I | 18 | 8 | 0.302b |
| II | 18 | 7 |
|
| III | 4 | 5 |
|
| Chemotherapy |
|
|
|
| AC | 30 | 12 | 0.232b |
| CAF | 10 | 8 |
|
Efficacy evaluation
The WBC count and ANC decreased on D15 after C1 in
the two groups (Table II). The WBC
count and ANC percentage changes between C2D1 and C2D15 decreased
with no significant difference between them (data not shown). There
was no significant difference in the increase in WBC count/ANC
between the EW02 and control groups in C2 compared with C1
(Table II). Patients receiving AC
treatment with EW02 presented with a slightly lower net decrease in
ANC than the placebo group (Table
III), however this was not statistically significant (P=0.052).
Patients in the CAF treatment arm also presented with a lower, but
not significant reduction in ANC (P=0.245). Biomarker study
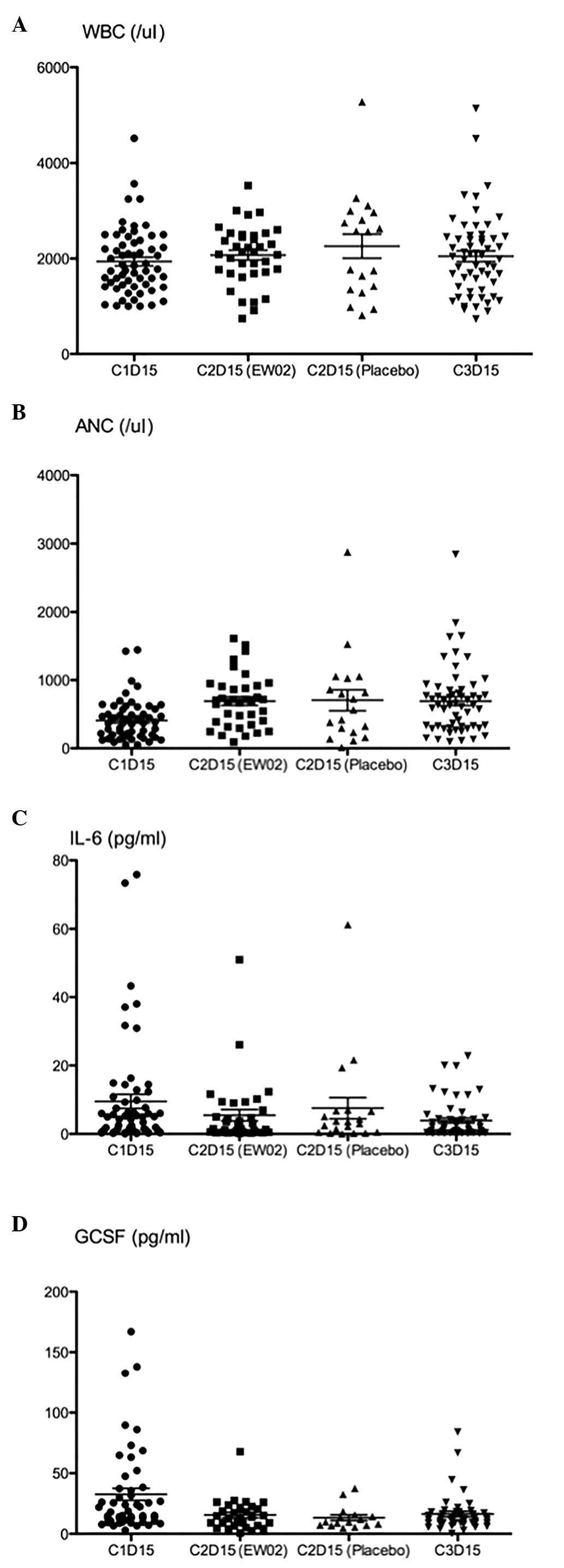
comparing IL-6 and G-CSF between each cycle showed no difference
between the change in the EW02 group and the placebo group
(Table II; Fig. 2).
 | Table II.Blood cell count and biomarkers during
treatment. |
Table II.
Blood cell count and biomarkers during
treatment.
| Factor | EW02 (n=40) | Placebo (n=20) | P-value |
|---|
| C1D1 |
|
|
|
| WBC |
6200±1490 |
6360±1760 | 0.783 |
| ANC |
3860±1320 |
3700±1430 | 0.836 |
| IL-6 |
0.53±8.32 |
0.43±10.28 | 0.872 |
|
G-CSF |
2.24±71.95 |
3.41±541.9 | 0.421 |
| C1D15 |
|
|
|
| WBC |
1860±520 |
1760±950 | 0.358 |
| ANC |
410±270 |
330±340 | 0.806 |
| IL-6 |
4.24±15.22 |
5.17±20.22 | 0.550 |
|
G-CSF |
3.17±57.88 |
3.35±448.69 | 0.524 |
| C2D15 |
|
|
|
| WBC |
2140±620 |
2560±1100 | 0.424 |
| ANC |
694.2±386.8 |
706.3±665.5 | 0.932 |
| IL-6 |
2.60±6.00 |
2.88±5.76 | 0.521 |
|
G-CSF |
2.39±58.23 |
3.12±370.2 | 0.541 |
 | Table III.Subgroup analysis of net absolute
neutrophil count change between C2D1 and C2D15 in different
chemotherapy regimens. |
Table III.
Subgroup analysis of net absolute
neutrophil count change between C2D1 and C2D15 in different
chemotherapy regimens.
| Regimen | n | ∆ mean | 95% CI | P-value |
|---|
| AC |
|
|
|
|
|
EW02 | 27 | −2849 | −2980 to −2676 | 0.052 |
|
Placebo | 11 | −3057 | −3346 to −2870 |
|
| CAF |
|
|
| 0.245 |
|
EW02 | 10 | −2730 | −3418 to −2780 |
|
|
Placebo | 8 | −3284 | −3181 to −2465 |
|
QOL
The score for the QLQ-BR23 and NCH04 sections of the
questionnaire showed no significant difference between the two
groups in C2 (Table IV). The QLQ-C30
score increased at C2D8 compared with C1D8 (P=0.045), however, the
remaining comparisons between different cycles were not significant
(data not shown).
 | Table IV.Quality of life score difference
between EW02 and placebo group in C2. |
Table IV.
Quality of life score difference
between EW02 and placebo group in C2.
|
| C2D1 vs. C1D1 | C2D8 vs. C1D8 | C2D15 vs.
C1D15 |
|---|
|
|
|
|
|
|---|
| Questionnaire
section | Difference | P-value | Difference | P-value | Difference | P-value |
|---|
| EORTC QLQ-BR23 | 3.097 | 0.087 | 0.965 | 0.749 | 1.295 | 0.523 |
| EORTC QLQ-C30 | 1.264 | 0.644 | 7.515 | 0.045 | −1.172 | 0.745 |
| NCH04 | −0.807 | 0.674 | 0.502 | 0.867 | 2.685 | 0.344 |
Side-effects
All patients underwent chemotherapy with no
significant grade 3 or 4 side-effects in either group. Only mild
asthenia, neutropenia, insomnia and gastrointestinal discomfort
were recorded, as listed in Table V.
No severe side-effects, including hypotension, were reported to be
associated with EW02 in C2 and C3. No long-term side-effects were
recorded during follow-up.
 | Table V.Side-effects. |
Table V.
Side-effects.
| Symptom | EW02 (n=40) | Placebo (n=20) |
P-valuea |
|---|
| Asthenia | 16 | 9 | 0.711 |
| Digestive
system |
|
|
|
|
Anorexia | 16 | 7 | 0.707 |
|
Nausea | 19 | 13 | 0.200 |
|
Vomiting | 18 | 7 | 0.459 |
| Hematological
system |
|
|
|
|
Leucopenia | 7 | 5 | 0.494 |
|
Neutropenia | 8 | 6 | 0.388 |
|
Cardiovascular system | 1 | 0 | 0.476 |
|
Insomnia | 6 | 3 | 1.000 |
|
Skin | 8 | 2 | 0.327 |
Discussion
The present study showed that oral EW02 was a safe
supplement and that it did not aggravate the side-effects of
chemotherapy. However, EW02 did not provide a significant increase
in WBC count or ANC during C2 when the WBC nadir occurs compared
with the control group. Subgroup analysis showed a slightly lower
decrease in absolute neutrophil count after chemotherapy with AC
regimen in the EW02 group compared with the control group (Table III; P=0.052), although this result
was not statistically significant. This trend was not evident in
patients who received the CAF regimen, probably due to the small
sample size (n=18). All of these findings support the notion that
EW02 may partially assist myelopoiesis in EBC patients who undergo
adjuvant chemotherapy with the AC or CAF regimens. Further studies
with a larger sample size should be performed to validate it.
Biomarker studies showed that the IL-6 and G-CSF
levels were not changed between the two groups during C2. Chen
et al (14) showed that EW02
could increase the serum G-CSF level in cancer patients treated
with cisplatin-containing regimens. Possible reasons for this
discrepancy include the different target population, the different
dosage of EW02 and the different chemotherapeutic agents used. The
IL-6 level may have decreased in the patients who underwent
chemotherapy while inflammation was inhibited (15). As a result, EW02 may not have been
able to stimulate an increase in IL-6 in the present patients.
Although EW02 was found to increase IL-6 in a mouse model (1), the present study failed to show the same
results in humans.
Patients in the present study were prescribed 700 mg
EW02 three times a day, which is equals to a daily dose of 300 g
BS, which is equivalent to that used in the literature (10). Previous studies had reported that BS
increased the neutrophil count and induced differentiation in the
U937 leukemic cell line (5). Two
study groups also showed that BS could assist in the
myeloproliferation of irradiated rats (8,16).
Administering BS in rats with or without condition medium can
assist in reticulocyte count proliferation (1,17). BS was
also found to promote myelopoiesis in mouse splenocytes and bone
marrow cells in a previous study. Granulocyte/monocyte colony
formation increased in culture with BS plus splenocyte-conditioned
medium, but not when BS was used alone (1). It was concluded that BS indirectly
assisted hematopoiesis through increasing growth factors via T
lymphocytes, macrophages, fibroblasts and endothelial cells. The
dosage of EW02 in the present study was adequate, but did not
assist myelopoiesis in C2. Although previous studies showed
successfully increasing myelopoiesis in cell lines and rat models,
the present study did not find any increase in neutrophil count. We
postulated that the primary endpoint of this study should be able
to be met with a longer duration of usage and a larger study cohort
in the future. Another possible reason to explain why no effects on
myelopoiesis were observed in C2 is perhaps due to the fact that no
co-stimulating agents were used. Prolonged EW02 usage may be
required to achieve a stimulating effect to generate more
hematopoietic growth factors. We hypothesize that adding EW02 along
with another growth factor may further enhance the
myeloproliferative effect of these factors.
There were no observed adverse side-effects
attributable to EW02 in the present patient cohort. Although
soybean has been reported to cause hypotension in healthy
menopausal women, this was not found in the present study (18). Other side-effects, including anorexia,
nausea and insomnia, were experienced equally by the two groups,
and were attributed to the chemotherapy. EW02 only transiently
ameliorated the symptoms and discomforts of the patients in C2D8;
however, the QOL was equivalent for the majority of the time in the
two groups. Systematic meta-analysis had shown that soy products
ameliorate menopausal symptoms in post-menopausal women (19). However, BS does not reduce the hot
flash symptoms in BC patients, which may relate to the short
duration of usage or the placebo effect (20,21).
In conclusion, in the present study, EW02 was found
to be a relatively safe alternative supplement for BC patients who
underwent adjuvant chemotherapy. However, it did not meet the
primary endpoint successfully, with only a slightly lower net
decrease in ANC percentage in the subgroup analysis. The present
study population was small and the duration of EW02 usage was
short. Prolonged EW02 usage and a larger study cohort may represent
a potential strategy for future clinical trial design.
Acknowledgements
The authors would like to thank Ms. San-Yu Lin and
Ms. Su-Hui Lee of the Division of Hematology/Oncology, Department
of Medicine, Tri-Service General Hospital, for their assistance in
data collection and summarization. The authors would also like to
thank Dr Anthony Janckila for providing advice on the writing of
the original manuscript. This study was supported by a grant issued
by the Department of Health (no. DOH96-TD-I-111-003).
References
|
1
|
Liao HF, Chen YJ and Yang YC: A novel
polysaccharide of black soybean promotes myelopoiesis and
reconstitutes bone marrow after 5-flurouracil- and
irradiation-induced myelosuppression. Life Sci. 77:400–413. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang SZ: The Divine Farmer's Materia
Medica, 200 A.D. Blue Poppy Press; Boulder, CO: 1998
|
|
3
|
Li S: Compendium of Materia Medica (Bencao
Gangmu). Foreign Languages Press; Beijing: 2004
|
|
4
|
Wu MH, Lee YC, Tsai WJ, Yang WB, Chen YC,
Chuang KA, Liao JF, Wang CC and Kuo YC: Characterized
polysaccharides from black soybean induce granulocyte
colony-stimulated factor gene expression in a phosphoinositide
3-kinase-dependent manner. Immunol Invest. 40:39–61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao HF, Chou CJ, Wu SH, Khoo KH, Chen CF
and Wang SY: Isolation and characterization of an active compound
from black soybean [Glycine max (L.) Merr.] and its effect on
proliferation and differentiation of human leukemic U937 cells.
Anticancer Drugs. 12:841–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inagaki S, Morimura S, Gondo K, Tang Y,
Akutagawa H and Kida K: Isolation of tryptophol as an
apoptosis-inducing component of vinegar produced from boiled
extract of black soybean in human monoblastic leukemia U937 cells.
Biosci Biotechnol Biochem. 71:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh VK, Grace MB, Parekh VI, Whitnall MH
and Landauer MR: Effects of genistein administration on cytokine
induction in whole-body gamma irradiated mice. Int Immunopharmacol.
9:1401–1410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y and Mi MT: Genistein stimulates
hematopoiesis and increases survival in irradiated mice. J Radiat
Res. 46:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin M, Villar A, Sole-Calvo A, Gonzalez
R, Massuti B, Lizon J, Camps C, Carrato A, Casado A, Candel MT, et
al: GEICAM Group (Spanish Breast Cancer Research Group), Spain:
Doxorubicin in combination with fluorouracil and cyclophosphamide
(i.v. FAC regimen, day 1, 21) versus methotrexate in combination
with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1,
21) as adjuvant chemotherapy for operable breast cancer: A study by
the GEICAM group. Ann Oncol. 14:833–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YC: Clinical experiences on
therapeutic usge of black soybean. J He Bai TCM. 1:401984.
|
|
11
|
Wriedt K, Kauder E and Mauer AM: Defective
myelopoiesis in congenital neutropenia. N Engl J Med.
283:1072–1077. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sørensen JB, Klee M, Palshof T and Hansen
HH: Performance status assessment in cancer patients. An
inter-observer variability study. Br J Cancer. 67:773–775. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chie WC, Chang KJ, Huang CS and Kuo WH:
Quality of life of breast cancer patients in Taiwan: Validation of
the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23.
Psychooncology. 12:729–735. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YM, Whang-Peng J, Liu JM, Kuo BI,
Wang SY, Tsai CM and Perng RP: Serum cytokine level fluctuations in
chemotherapy-induced myelosuppression. Jpn J Clin Oncol. 26:18–23.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang XP, Yang DC, Elliott RL and Head JF:
Reduction in serum IL-6 after vaccination of breast cancer patients
with tumour-associated antigens is related to estrogen receptor
status. Cytokine. 12:458–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinomiya K, Tokunaga S, Shigemoto Y and
Kamei C: Effect of seed coat extract from black soybeans on radial
maze performance in rats. Clin Exp Pharmacol Physiol. 32:757–760.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hagiwara A, Imai N, Numano T, Nakashima H,
Tamano S, Sakaue K, Tanaka K, Yasuhara K and Hayashi SM: A twenty
eight-day repeated dose toxicity study of black soybean extract in
Sprague-Dawley rats. J Toxicol Sci. 35:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloedon LT, Jeffcoat AR, Lopaczynski W,
Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG,
Crowell JA, et al: Safety and pharmacokinetics of purified soy
isoflavones: Single-dose administration to postmenopausal women. Am
J Clin Nutr. 76:1126–1137. 2002.PubMed/NCBI
|
|
19
|
Shams T, Setia MS, Hemmings R, McCusker J,
Sewitch M and Ciampi A: Efficacy of black cohosh-containing
preparations on menopausal symptoms: A meta-analysis. Altern Ther
Health Med. 16:36–44. 2010.PubMed/NCBI
|
|
20
|
Quella SK, Loprinzi CL, Barton DL, Knost
JA, Sloan JA, LaVasseur BI, Swan D, Krupp KR, Miller KD and Novotny
PJ: Evaluation of soy phytoestrogens for the treatment of hot
flashes in breast cancer survivors: A North Central Cancer
Treatment Group Trial. J Clin Oncol. 18:1068–1074. 2000.PubMed/NCBI
|
|
21
|
Van Patten CL, Olivotto IA, Chambers GK,
Gelmon KA, Hislop TG, Templeton E, Wattie A and Prior JC: Effect of
soy phytoestrogens on hot flashes in postmenopausal women with
breast cancer: A randomized, controlled clinical trial. J Clin
Oncol. 20:1449–1455. 2002. View Article : Google Scholar : PubMed/NCBI
|