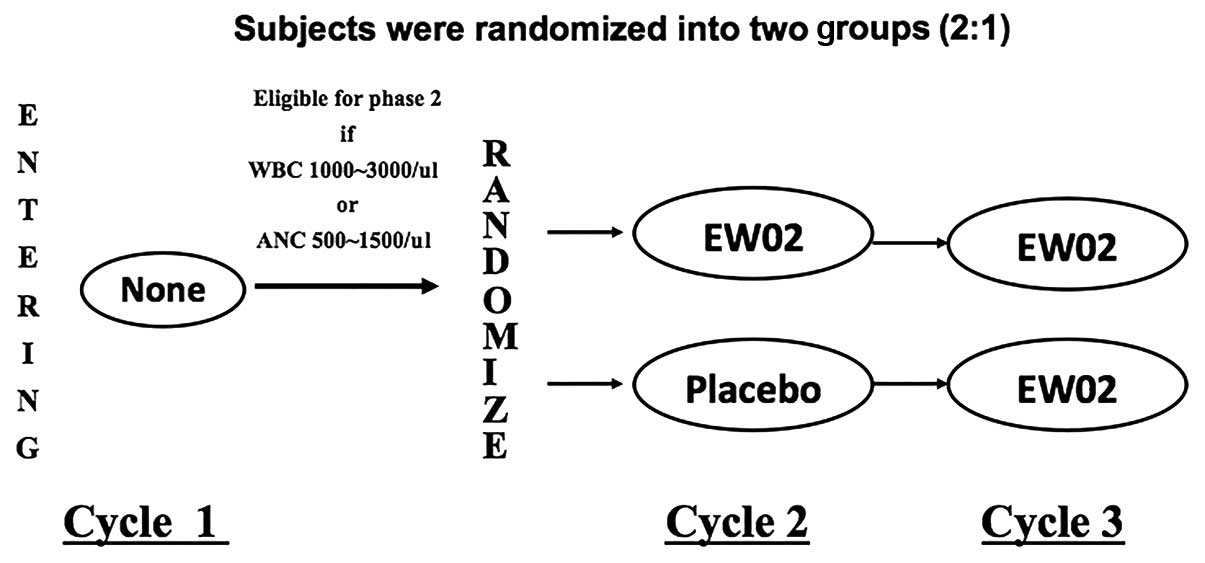
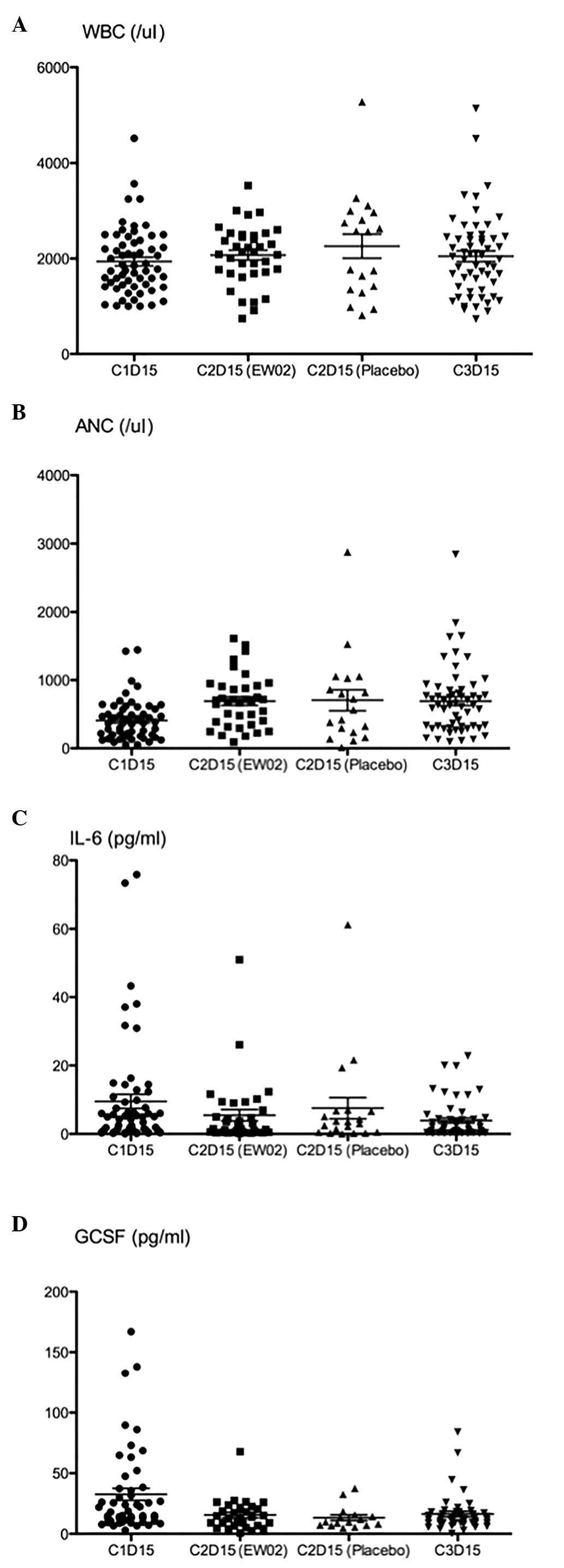
|
1
|
Liao HF, Chen YJ and Yang YC: A novel
polysaccharide of black soybean promotes myelopoiesis and
reconstitutes bone marrow after 5-flurouracil- and
irradiation-induced myelosuppression. Life Sci. 77:400–413. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang SZ: The Divine Farmer's Materia
Medica, 200 A.D. Blue Poppy Press; Boulder, CO: 1998
|
|
3
|
Li S: Compendium of Materia Medica (Bencao
Gangmu). Foreign Languages Press; Beijing: 2004
|
|
4
|
Wu MH, Lee YC, Tsai WJ, Yang WB, Chen YC,
Chuang KA, Liao JF, Wang CC and Kuo YC: Characterized
polysaccharides from black soybean induce granulocyte
colony-stimulated factor gene expression in a phosphoinositide
3-kinase-dependent manner. Immunol Invest. 40:39–61. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liao HF, Chou CJ, Wu SH, Khoo KH, Chen CF
and Wang SY: Isolation and characterization of an active compound
from black soybean [Glycine max (L.) Merr.] and its effect on
proliferation and differentiation of human leukemic U937 cells.
Anticancer Drugs. 12:841–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inagaki S, Morimura S, Gondo K, Tang Y,
Akutagawa H and Kida K: Isolation of tryptophol as an
apoptosis-inducing component of vinegar produced from boiled
extract of black soybean in human monoblastic leukemia U937 cells.
Biosci Biotechnol Biochem. 71:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh VK, Grace MB, Parekh VI, Whitnall MH
and Landauer MR: Effects of genistein administration on cytokine
induction in whole-body gamma irradiated mice. Int Immunopharmacol.
9:1401–1410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y and Mi MT: Genistein stimulates
hematopoiesis and increases survival in irradiated mice. J Radiat
Res. 46:425–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martin M, Villar A, Sole-Calvo A, Gonzalez
R, Massuti B, Lizon J, Camps C, Carrato A, Casado A, Candel MT, et
al: GEICAM Group (Spanish Breast Cancer Research Group), Spain:
Doxorubicin in combination with fluorouracil and cyclophosphamide
(i.v. FAC regimen, day 1, 21) versus methotrexate in combination
with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1,
21) as adjuvant chemotherapy for operable breast cancer: A study by
the GEICAM group. Ann Oncol. 14:833–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu YC: Clinical experiences on
therapeutic usge of black soybean. J He Bai TCM. 1:401984.
|
|
11
|
Wriedt K, Kauder E and Mauer AM: Defective
myelopoiesis in congenital neutropenia. N Engl J Med.
283:1072–1077. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sørensen JB, Klee M, Palshof T and Hansen
HH: Performance status assessment in cancer patients. An
inter-observer variability study. Br J Cancer. 67:773–775. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chie WC, Chang KJ, Huang CS and Kuo WH:
Quality of life of breast cancer patients in Taiwan: Validation of
the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23.
Psychooncology. 12:729–735. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YM, Whang-Peng J, Liu JM, Kuo BI,
Wang SY, Tsai CM and Perng RP: Serum cytokine level fluctuations in
chemotherapy-induced myelosuppression. Jpn J Clin Oncol. 26:18–23.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang XP, Yang DC, Elliott RL and Head JF:
Reduction in serum IL-6 after vaccination of breast cancer patients
with tumour-associated antigens is related to estrogen receptor
status. Cytokine. 12:458–465. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinomiya K, Tokunaga S, Shigemoto Y and
Kamei C: Effect of seed coat extract from black soybeans on radial
maze performance in rats. Clin Exp Pharmacol Physiol. 32:757–760.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hagiwara A, Imai N, Numano T, Nakashima H,
Tamano S, Sakaue K, Tanaka K, Yasuhara K and Hayashi SM: A twenty
eight-day repeated dose toxicity study of black soybean extract in
Sprague-Dawley rats. J Toxicol Sci. 35:87–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bloedon LT, Jeffcoat AR, Lopaczynski W,
Schell MJ, Black TM, Dix KJ, Thomas BF, Albright C, Busby MG,
Crowell JA, et al: Safety and pharmacokinetics of purified soy
isoflavones: Single-dose administration to postmenopausal women. Am
J Clin Nutr. 76:1126–1137. 2002.PubMed/NCBI
|
|
19
|
Shams T, Setia MS, Hemmings R, McCusker J,
Sewitch M and Ciampi A: Efficacy of black cohosh-containing
preparations on menopausal symptoms: A meta-analysis. Altern Ther
Health Med. 16:36–44. 2010.PubMed/NCBI
|
|
20
|
Quella SK, Loprinzi CL, Barton DL, Knost
JA, Sloan JA, LaVasseur BI, Swan D, Krupp KR, Miller KD and Novotny
PJ: Evaluation of soy phytoestrogens for the treatment of hot
flashes in breast cancer survivors: A North Central Cancer
Treatment Group Trial. J Clin Oncol. 18:1068–1074. 2000.PubMed/NCBI
|
|
21
|
Van Patten CL, Olivotto IA, Chambers GK,
Gelmon KA, Hislop TG, Templeton E, Wattie A and Prior JC: Effect of
soy phytoestrogens on hot flashes in postmenopausal women with
breast cancer: A randomized, controlled clinical trial. J Clin
Oncol. 20:1449–1455. 2002. View Article : Google Scholar : PubMed/NCBI
|