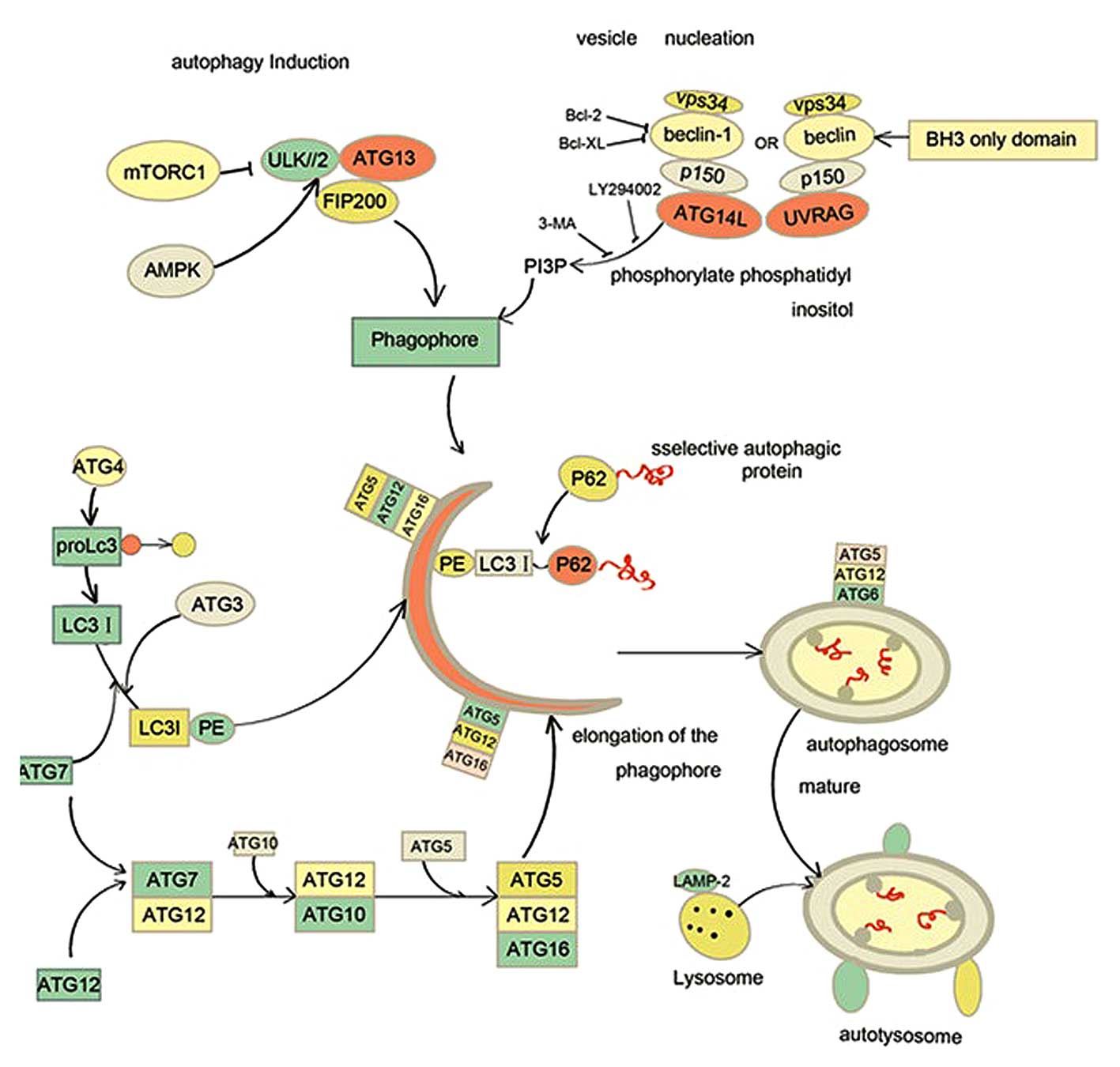
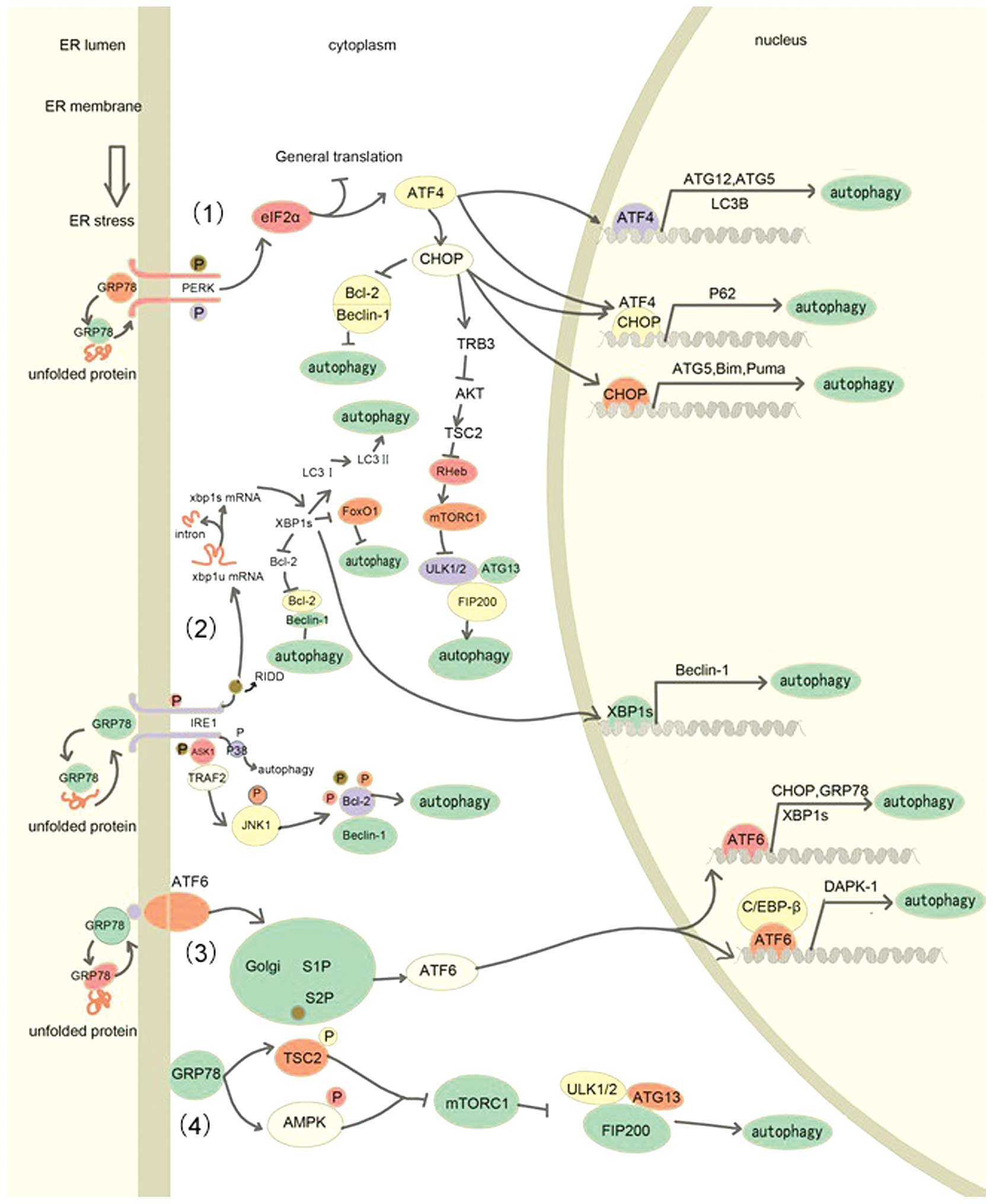
|
1
|
Mei Y, Thompson MD, Cohen RA and Tong X:
Endoplasmic reticulum stress and related pathological processes. J
Pharmacol Biomed Anal. 1:10001072013.PubMed/NCBI
|
|
2
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan F, Li J, Chen J, Hu Q, Gu C, Lin W and
Chen G: Endoplasmic reticulum stress is associated with
neuroprotection against apoptosis via autophagy activation in a rat
model of subarachnoid hemorrhage. Neurosci Lett. 563:160–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimodaira Y, Takahashi S, Kinouchi Y,
Endo K, Shiga H, Kakuta Y, Kuroha M and Shimosegawa T: Modulation
of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP
homologous protein (CHOP) and inositol-requiring enzyme 1α (IRE1α)
in human colon cancer cells. Biochem Biophys Res Commun.
445:524–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubiolo JA, López-Alonso H, Martínez P,
Millán A, Cagide E, Vieytes MR, Vega FV and Botana LM: Yessotoxin
induces ER-stress followed by autophagic cell death in glioma cells
mediated by mTOR and BNIP3. Cell Signal. 26:419–432. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vandewynckel YP, Laukens D, Geerts A,
Bogaerts E, Paridaens A, Verhelst X, Janssens S, Heindryckx F and
Van Vlierberghe H: The paradox of the unfolded protein response in
cancer. Anticancer Res. 33:4683–4694. 2013.PubMed/NCBI
|
|
8
|
Lorin S, Hamaï A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen HG, Yang JC, Kung H, Shi X, Tilki
D, Lara PN Jr, De Vere White RW, Gao AC and Evans CP: Targeting
autophagy overcomes Enzalutamide resistance in castration-resistant
prostate cancer cells and improves therapeutic response in a
xenograft model. Oncogene. 33:4521–4530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Klionsky DJ: Autophagy revisited: A
conversation with Christian de Duve. Autophagy. 4:740–743. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MartinezBorra J and López-Larrea C:
Autophagy and self-defense. Adv Exp Med Biol. 738:169–184. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung CH, Jun CB, Ro SH, Kim YM, Otto NM,
Cao J, Kundu M and Kim DH: ULK-Atg13-FIP200 complexes mediate mTOR
signaling to the autophagy machinery. Mol Biol Cell. 20:1992–2003.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mizushima N: The role of the Atg1/ULK1
complex in autophagy regulation. Curr Opin Cell Biol. 22:132–139.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fader CM, Aguilera MO and Colombo MI:
Autophagy response: Manipulating the mTOR-controlled machinery by
amino acids and pathogens. Amino Acids. Sep 19–2014.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Simonsen A and Tooze SA: Coordination of
membrane events during autophagy by multiple class III PI3-kinase
complexes. J Cell Biol. 186:773–782. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song Z, An L, Ye Y, Wu J, Zou Y, He L and
Zhu H: Essential role for UVRAG in autophagy and maintenance of
cardiac function. Cardiovasc Res. 101:48–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Q, Zhang J, Fan W, Wong KN, Ding X,
Chen S and Zhong Q: The RUN domain of rubicon is important for
hVps34 binding, lipid kinase inhibition and autophagy suppression.
J Biol Chem. 286:185–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pattingre S, Tassa A, Qu X, Garuti R,
Liang XH, Mizushima N, Packer M, Schneider MD and Levine B: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiuri MC, Le Toumelin G, Criollo A, Rain
JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K,
Tavernarakis N, et al: Functional and physical interaction between
Bcl-X (L) and a BH3-like domain in Beclin-1. EMBO J. 26:2527–2539.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakatogawa H, Ichimura Y and Ohsumi Y:
Atg8, a ubiquitin-like protein required for autophagosome
formation, mediates membrane tethering and hemifusion. Cell.
130:165–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishida Y, Arakawa S, Fujitani K,
Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y
and Shimizu S: Discovery of Atg5/Atg7-independent alternative
macroautophagy. Nature. 461:654–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsumoto G, Wada K, Okuno M, Kurosawa M
and Nukina N: Serine 403 phosphorylation of p62/SQSTM1 regulates
selective autophagic clearance of ubiquitinated proteins. Mol Cell.
44:279–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rogov V, Dötsch V, Johansen T and Kirkin
V: Interactions between autophagy receptors and ubiquitin-like
proteins form the molecular basis for selective autophagy. Mol
Cell. 53:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eskelinen EL: Maturation of autophagic
vacuoles in Mammalian cells. Autophagy. 1:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Braakman I and Bulleid NJ: Protein folding
and modification in the mammalian endoplasmic reticulum. Annu Rev
Biochem. 80:71–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chistiakov DA, Sobenin IA, Orekhov AN and
Bobryshev YV: Role of endoplasmic reticulum stress in
atherosclerosis and diabetic macrovascular complications. Biomed
Res Int. 2014:6101402014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beard NA, Laver DR and Dulhunty AF:
Calsequestrin and the calcium release channel of skeletal and
cardiac muscle. Prog Biophys Mol Biol. 85:33–69. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aebi M, Bernasconi R, Clerc S and Molinari
M: N-glycan structures: Recognition and processing in the ER.
Trends Biochem Sci. 35:74–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hiramatsu N, Joseph VT and Lin JH:
Monitoring and manipulating mammalian unfolded protein response.
Methods Enzymol. 491:183–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI
|
|
34
|
Schroder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iwawaki T, Hosoda A, Okuda T, Kamigori Y,
NomuraFuruwatari C, Kimata Y, Tsuru A and Kohno K: Translational
control by the ER transmembrane kinase/ribonuclease IRE1 under ER
stress. Nat Cell Biol. 3:158–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y and Brandizzi F: IRE1: ER stress
sensor and cell fate executor. Trends Cell Biol. 23:547–555. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li M, Baumeister P, Roy B, Phan T, Foti D,
Luo S and Lee AS: ATF6 as a transcription activator of the
endoplasmic reticulum stress element: Thapsigargin stress-induced
changes and synergistic interactions with NF-Y and YY1. Mol Cell
Biol. 20:5096–5106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maurel M, Chevet E, Tavernier J and Gerlo
S: Getting RIDD of RNA: IRE1 in cell fate regulation. Trends
Biochem Sci. 39:245–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chow SE, Kao CH, Liu YT, Cheng ML, Yang
YW, Huang YK, Hsu CC and Wang JS: Resveratrol induced ER expansion
and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma
cells. Apoptosis. 19:527–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hetz C: The unfolded protein response:
Controlling cell fate decisions under ER stress and beyond. Nat Rev
Mol Cell Biol. 13:89–102. 2012.PubMed/NCBI
|
|
41
|
Kim R, Emi M, Tanabe K and Murakami S:
Role of the unfolded protein response in cell death. Apoptosis.
11:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jäger R, Bertrand MJ, Gorman AM,
Vandenabeele P and Samali A: The unfolded protein response at the
crossroads of cellular life and death during endoplasmic reticulum
stress. Biology of the Cell. 104:259–270. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hetz C, Martinon F, Rodriguez D and
Glimcher LH: The unfolded protein response: Integrating stress
signals through the stress sensor IRE1α. Physiol Rev. 91:1219–1243.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shajahan AN, Riggins RB and Clarke R: The
role of X-box binding protein-1 in tumorigenicity. Drug News
Perspect. 22:241–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Calfon M, Zeng H, Urano F, Till JH,
Hubbard SR, Harding HP, Clark SG and Ron D: IRE1 couples
endoplasmic reticulum load to secretory capacity by processing the
XBP-1 mRNA. Nature. 415:92–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gomez BP, Riggins RB, Shajahan AN, Klimach
U, Wang A, Crawford AC, Zhu Y, Zwart A, Wang M and Clarke R: Human
X-box binding protein-1 confers both estrogen independence and
antiestrogen resistance in breast cancer cell lines. FASEB J.
21:4013–4027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Margariti A, Li H, Chen T, Martin D,
VizcayBarrena G, Alam S, Karamariti E, Xiao Q, Zampetaki A, Zhang
Z, et al: XBP1 mRNA splicing triggers an autophagic response in
endothelial cells through BECLIN-1 transcriptional activation. J
Biol Chem. 288:859–872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Suzuki H, Kanekura K, Levine TP, Kohno K,
Olkkonen VM, Aiso S and Matsuoka M: ALS-linked P56S-VAPB, an
aggregated loss-of-function mutant of VAPB, predisposes motor
neurons to ER stress-related death by inducing aggregation of
co-expressed wild-type VAPB. J Neurochem. 108:973–985. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Vidal RL, Figueroa A, Court FA, Thielen P,
Molina C, Wirth C, Caballero B, Kiffin R, SeguraAguilar J, Cuervo
AM, et al: Targeting the UPR transcription factor XBP1 protects
against Huntington's disease through the regulation of FoxO1 and
autophagy. Hum Mol Genet. 21:2245–2262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li D, Wang L, Deng R, Tang J, Shen Y, Guo
J, Wang Y, Xia LP, Feng GK, Liu QQ, et al: The pivotal role of
c-Jun NH2-terminal kinase-mediated Beclin 1 expression during
anticancer agents-induced autophagy in cancer cells. Oncogene.
28:886–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nishitoh H, Matsuzawa A, Tobiume K,
Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A and Ichijo H: ASK1
is essential for endoplasmic reticulum stress-induced neuronal cell
death triggered by expanded polyglutamine repeats. Genes Dev.
16:1345–1355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ogata M, Hino SI, Saito A, Morikawa K,
Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K,
et al: Autophagy is activated for cell survival after endoplasmic
reticulum stress. Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wei Y, Pattingre S, Sinha S, Bassik M and
Levine B: JNK1-mediated phosphorylation of Bcl-2 regulates
starvation-induced autophagy. Mol Cell. 30:678–688. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jiang LC, Xin ZY, Deborah B, Zhang JS,
Yuan DY, Xu K, Liu XB, Jiang HQ, Fan QC, Zhang B and Li KY:
Inhibition of autophagy augments apoptosis in human oral squamous
cell carcinoma under nutrient depletion. J Oral Pathol Med.
44:361–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang MZ, Wang Y, Paueksakon P and Harris
RC: Epidermal growth factor receptor inhibition slows progression
of diabetic nephropathy in association with a decrease in
endoplasmic reticulum stress and an increase in autophagy.
Diabetes. 63:2063–2072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Harding HP, Zhang Y, Bertolotti A, Zeng H
and Ron D: Perk is essential for translational regulation and cell
survival during the unfolded protein response. Mol Cell. 5:897–904.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shi Y, Vattem KM, Sood R, An J, Liang J,
Stramm L and Wek RC: Identification and characterization of
pancreatic eukaryotic initiation factor 2 alpha-subunit kinase,
PEK, involved in translational control. Mol Cell Biol.
18:7499–7509. 1998.PubMed/NCBI
|
|
61
|
Lu PD, Jousse C, Marciniak SJ, Zhang Y,
Novoa I, Scheuner D, Kaufman RJ, Ron D and Harding HP:
Cytoprotection by pre-emptive conditional phosphorylation of
translation initiation factor 2. EMBO J. 23:169–179. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Teske BF, Baird TD and Wek RC: Methods for
analyzing eIF2 kinases and translational control in the unfolded
protein response. Methods Enzymol. 490:333–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wek RC and Cavener DR: Translational
control and the unfolded protein response. Antioxid Redox Signal.
9:2357–2371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Palam LR, Baird TD and Wek RC:
Phosphorylation of eIF2 facilitates ribosomal bypass of an
inhibitory upstream ORF to enhance CHOP translation. J Biol Chem.
286:10939–10949. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kouroku Y, Fujita E, Tanida I, Ueno T,
Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E and Momoi T: ER
stress (PERK/eIF2alplha phosphorylation) mediates the
polyglutamine-induced LC3 conversion, an essential step for
autophagy formation. Cell Death Differ. 14:230–239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang J, Kang R, Huang H, Xi X, Wang B,
Wang J and Zhao Z: Hepatitis C virus core protein activates
autophagy through EIF2AK3 and ATF6 UPR pathway-mediated MAP1LC3B
and ATG12 expression. Autophagy. 10:766–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ma X, Piao S, Dey S, Mcafee Q, Karakousis
G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, et al: Targeting
ER stress-induced autophagy overcomes BRAF inhibitor resistance in
melanoma. J Clin Invest. 124:1406–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rzymski T, Milani M, Pike L, Buffa F,
Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I and Harris
AL: Regulation of autophagy by ATF4 in response to severe hypoxia.
Oncogene. 29:4424–4435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
B'Chir W, Chaveroux C, Carraro V, Averous
J, Maurin AC, Jousse C, Muranishi Y, Parry L, Fafournoux P and
Bruhat A: Dual role for CHOP in the crosstalk between autophagy and
apoptosis to determine cell fate in response to amino acid
deprivation. Cell Signal. 26:1385–1391. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao
M, Liu D, Qiao L, Li N, Zheng J and Chen D: CHOP mediates
ASPP2-induced autophagic apoptosis in hepatoma cells by releasing
Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2.
Cell Death Dis. 5:e13232014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rouschop KM, van den Beucken T, Dubois L,
Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W,
Voncken JW, et al: The unfolded protein response protects human
tumor cells during hypoxia through regulation of the autophagy
genes MAP1LC3B and ATG5. J Clin Invest. 120:127–141. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Puthalakath H, O'Reilly LA, Gunn P, Lee L,
Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin
J, Motoyama N, et al: ER stress triggers apoptosis by activating
BH3-only protein Bim. Cell. 129:1337–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Gorman AM, Healy SJ, Jager R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ohoka N, Yoshii S, Hattori T, Onozaki K
and Hayashi H: TRB3, a novel ER stress-inducible gene, is induced
via ATF4-CHOP pathway and is involved in cell death. EMBO J.
24:1243–1255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Du K, Herzig S, Kulkarni RN and Montminy
M: TRB3: A tribbles homolog that inhibits Akt/PKB activation by
insulin in liver. Science. 300:1574–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
B'Chir W, Maurin AC, Carraro V, Averous J,
Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P and Bruhat
A: The eIF2α/ATF4 pathway is essential for stress-induced autophagy
gene expression. Nucleic Acids Res. 41:7683–7699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shen J, Chen X, Hendershot L and Prywes R:
ER stress regulation of ATF6 localization by dissociation of
BiP/GRP78 binding and unmasking of Golgi localization signals. Dev
Cell. 3:99–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ye J, Rawson RB, Komuro R, Chen X, Davé
UP, Prywes R, Brown MS and Goldstein JL: ER stress induces cleavage
of membrane-bound ATF6 by the same proteases that process SREBPs.
Mol Cell. 6:1355–1364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bommiasamy H, Back SH, Fagone P, Lee K,
Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ
and Brewer JW: ATF6alpha induces XBP1-independent expansion of the
endoplasmic reticulum. J Cell Sci. 122:1626–1636. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yoshida H, Matsui T, Yamamoto A, Okada T
and Mori K: XBP1 mRNA is induced by ATF6 and spliced by IRE1 in
response to ER stress to produce a highly active transcription
factor. Cell. 107:881–891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Adachi Y, Yamamoto K, Okada T, Yoshida H,
Harada A and Mori K: ATF6 is a transcription factor specializing in
the regulation of quality control proteins in the endoplasmic
reticulum. Cell Struct Funct. 33:75–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gade P, Ramachandran G, Maachani UB, Rizzo
MA, Okada T, Prywes R, Cross AS, Mori K and Kalvakolanu DV: An
IFN-γ-stimulated ATF6-C/EBP-β-signaling pathway critical for the
expression of Death Associated Protein Kinase 1 and induction of
autophagy. Proc Natl Acad Sci USA. 109:10316–10321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zalckvar E, Berissi H, Mizrachy L,
Idelchuk Y, Koren I, Eisenstein M, Sabanay H, PinkasKramarski R and
Kimchi A: DAP-kinase-mediated phosphorylation on the BH3 domain of
beclin 1 promotes dissociation of beclin 1 from Bcl-XL and
induction of autophagy. EMBO Rep. 10:285–292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li J, Ni M, Lee B, Barron E, Hinton DR and
Lee AS: The unfolded protein response regulator GRP78/BiP is
required for endoplasmic reticulum integrity and stress-induced
autophagy in mammalian cells. Cell Death Differ. 15:1460–1471.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cook KL, Shajahan AN, Wärri A, Jin L,
HilakiviClarke LA and Clarke R: Glucose-regulated protein 78
controls cross-talk between apoptosis and autophagy to determine
antiestrogen responsiveness. Cancer Res. 72:3337–3349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shimada Y, Kobayashi H, Kawagoe S, Aoki K,
Kaneshiro E, Shimizu H, Eto Y, Ida H and Ohashi T: Endoplasmic
reticulum stress induces autophagy through activation of p38 MAPK
in fibroblasts from Pompe disease patients carrying c.546G>T
mutation. Mol Genet Metab. 104:566–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kim DS, Kim JH, Lee GH, Kim HT, Lim JM,
Chae SW, Chae HJ and Kim HR: p38 Mitogen-activated protein kinase
is involved in endoplasmic reticulum stress-induced cell death and
autophagy in human gingival fibroblasts. Biol Pharm Bull.
33:545–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Carloni S, Albertini MC, Galluzzi L,
Buonocore G, Proietti F and Balduini W: Increased autophagy reduces
endoplasmic reticulum stress after neonatal hypoxia-ischemia: Role
of protein synthesis and autophagic pathways. Exp Neurol.
255:103–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Verchot J: The ER quality control and ER
associated degradation machineries are vital for viral
pathogenesis. Front Plant Sci. 5:662014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ding WX, Ni HM, Gao W, Hou YF, Melan MA,
Chen X, Stolz DB, Shao ZM and Yin XM: Differential Effects of
endoplasmic reticulum stress-induced autophagy on cell survival. J
Biol Chem. 282:4702–4710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
JoshiBarr S, Bett C, Chiang WC, Trejo M,
Goebel HH, Sikorska B, Liberski P, Raeber A, Lin JH, Masliah E and
Sigurdson CJ: De novo prion aggregates trigger autophagy in
skeletal muscle. J Virol. 88:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bernales S, McDonald KL and Walter P:
Autophagy counterbalances endoplasmic reticulum expansion during
the unfolded protein response. PLoS Biol. 4:e4232006. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vincenz L, Jäger R, O'Dwyer M and Samali
A: Endoplasmic reticulum stress and the unfolded protein response:
Targeting the Achilles heel of multiple myeloma. Mol Cancer Ther.
12:831–843. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Huang J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L, Tang D and Ni J: Targeting HMGB1-mediated
autophagy as a novel therapeutic strategy for osteosarcoma.
Autophagy. 8:275–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang L, Yang S, Liu J, Wang X, Ji J, Cao
Y, Lu K, Wang J and Gao Y: Expression of GRP78 predicts
taxane-based therapeutic resistance and recurrence of human gastric
cancer. Exp Mol Pathol. 96:235–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bifulco G, Miele C, Di Jeso B, Beguinot F,
Nappi C, Di Carlo C, Capuozzo S, Terrazzano G, Insabato L and
Ulianich L: Endoplasmic reticulum stress is activated in
endometrial adenocarcinoma. Gynecol Oncol. 125:220–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Visioli F, Wang Y, Alam GN, Ning Y, Rados
PV, Nör JE and Polverini PJ: Glucose-regulated protein 78 (Grp78)
confers chemoresistance to tumor endothelial cells under acidic
stress. PLoS ONE. 9:e1010532014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lin JA, Fang SU, Su CL, Hsiao CJ, Chang
CC, Lin YF and Cheng CW: Silencing glucose-regulated protein 78
induced renal cell carcinoma cell line G1 cell-cycle arrest and
resistance to conventional chemotherapy. Urol Oncol.
32:29.e1–29.e11. 2014. View Article : Google Scholar
|
|
102
|
KosakowskaCholody T, Lin J, Srideshikan
SM, Scheffer L, Tarasova NI and Acharya JK: HKH40A downregulates
GRP78/BiP expression in cancer cells. Cell Death Dis. 5:e12402014.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Reddy RK, Mao C, Baumeister P, Austin RC,
Kaufman RJ and Lee AS: Endoplasmic reticulum chaperone protein
GRP78 protects cells from apoptosis induced by topoisomerase
inhibitors: Role of ATP binding site in suppression of caspase-7
activation. J Biol Chem. 278:20915–20924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fu Y, Li J and Lee AS: GRP78/BiP inhibits
endoplasmic reticulum BIK and protects human breast cancer cells
against estrogen starvation-induced apoptosis. Cancer Res.
67:3734–3740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Zhou H, Zhang Y, Fu Y, Chan L and Lee AS:
Novel mechanism of anti-apoptotic function of 78-kDa
glucose-regulated protein (GRP78): Endocrine resistance factor in
breast cancer, through release of B-cell lymphoma 2 (BCL-2) from
BCL-2-interacting killer (BIK). J Biol Chem. 286:25687–25696. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Shani G, Fischer WH, Justice NJ, Kelber
JA, Vale W and Gray PC: GRP78 and Cripto form a complex at the cell
surface and collaborate to inhibit transforming growth factor beta
signaling and enhance cell growth. Mol Cell Biol. 28:666–677. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lee E, Nichols P, Spicer D, Groshen S, Yu
MC and Lee AS: GRP78 as a novel predictor of responsiveness to
chemotherapy in breast cancer. Cancer Res. 66:7849–7853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Suyama K, Watanabe M, Sakabe K, Okada Y,
Matsuyama D, Kuroiwa M and Mochida J: Overexpression of GRP78
protects glial cells from endoplasmic reticulum stress. Neurosci
Lett. 504:271–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT and
Dang CV: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li Z, Wang Y, Wu H, Zhang L, Yang P and Li
Z: GRP78 enhances the glutamine metabolism to support cell survival
from glucose deficiency by modulating the β-catenin signaling.
Oncotarget. 5:5369–5380. 2014.PubMed/NCBI
|
|
112
|
Li W, Wang W, Dong H, Li Y, Li L, Han L,
Han Z, Wang S, Ma D and Wang H: Cisplatin-induced senescence in
ovarian cancer cells is mediated by GRP78. Oncol Rep. 31:2525–2534.
2014.PubMed/NCBI
|
|
113
|
Auf G, Jabouille A, Delugin M, Guerit S,
Pineau R, North S, Platonova N, Maitre M, Favereaux A, Vajkoczy P,
et al: High epiregulin expression in human U87 glioma cells relies
on IRE1α and promotes autocrine growth through EGF receptor. BMC
Cancer. 13:5972013. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Auf G, Jabouille A, Guérit S, Pineau R,
Delugin M, Bouchecareilh M, Magnin N, Favereaux A, Maitre M, Gaiser
T, et al: Inositol-requiring enzyme 1alpha is a key regulator of
angiogenesis and invasion in malignant glioma. Proc Natl Acad Sci U
S A. 107:15553–15558. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tay KH, Luan Q, Croft A, Jiang CC, Jin L,
Zhang XD and Tseng HY: Sustained IRE1 and ATF6 signaling is
important for survival of melanoma cells undergoing ER stress. Cell
Signal. 26:287–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Thorpe JA and Schwarze SR: IRE1alpha
controls cyclin A1 expression and promotes cell proliferation
through XBP-1. Cell Stress Chaperones. 15:497–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Gambella M, Rocci A, Passera R, Gay F,
Omedè P, Crippa C, Corradini P, Romano A, Rossi D, Ladetto M, et
al: High XBP1 expression is a marker of better outcome in multiple
myeloma patients treated with bortezomib. Haematologica.
99:e14–e16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Bagratuni T, Wu P, Gonzalez de Castro D,
Davenport EL, Dickens NJ, Walker BA, Boyd K, Johnson DC, Gregory W,
Morgan GJ and Davies FE: XBP1s levels are implicated in the biology
and outcome of myeloma mediating different clinical outcomes to
thalidomide-based treatments. Blood. 116:250–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Chen X, Iliopoulos D, Zhang Q, Tang Q,
Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, et
al: XBP1 promotes triple-negative breast cancer by controlling the
HIF1α pathway. Nature. 508:103–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mimura N, Fulciniti M, Gorgun G, Tai YT,
Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H, et al:
Blockade of XBP1 splicing by inhibition of IRE1α is a promising
therapeutic option in multiple myeloma. Blood. 119:5772–5781. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Schroder M and Kaufman RJ: Divergent roles
of IRE1alpha and PERK in the unfolded protein response. Curr Mol
Med. 6:5–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cullinan SB and Diehl JA: PERK-dependent
activation of Nrf2 contributes to redox homeostasis and cell
survival following endoplasmic reticulum stress. J Biol Chem.
279:20108–20117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
BobrovnikovaMarjon E, Grigoriadou C, Pytel
D, Zhang F, Ye J, Koumenis C, Cavener D and Diehl JA: PERK promotes
cancer cell proliferation and tumor growth by limiting oxidative
DNA damage. Oncogene. 29:3881–3895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Krishnamoorthy J, Rajesh K, Mirzajani F,
Kesoglidou P, Papadakis AI and Koromilas AE: Evidence for eIF2α
phosphorylation-independent effects of GSK2656157, a novel
catalytic inhibitor of PERK with clinical implications. Cell Cycle.
13:801–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Milani M, Rzymski T, Mellor HR, Pike L,
Bottini A, Generali D and Harris AL: The role of ATF4 stabilization
and autophagy in resistance of breast cancer cells treated with
bortezomib. Cancer Research. 69:4415–4423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hamanaka RB, BobrovnikovaMarjon E, Ji X,
Liebhaber SA and Diehl JA: PERK-dependent regulation of IAP
translation during ER stress. Oncogene. 28:910–920. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
KusioKobialka M, PodszywalowBartnicka P,
Peidis P, GlodkowskaMrowka E, Wolanin K, Leszak G, Seferynska I,
Stoklosa T, Koromilas AE and Piwocka K: The PERK-eIF2α
phosphorylation arm is a pro-survival pathway of BCR-ABL signaling
and confers resistance to imatinib treatment in chronic myeloid
leukemia cells. Cell Cycle. 11:4069–4078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Higa A, Taouji S, Lhomond S, Jensen D,
FernandezZapico ME, Simpson JC, Pasquet JM, Schekman R and Chevet
E: Endoplasmic reticulum stress-activated transcription factor
ATF6α requires the disulfide isomerase PDIA5 to modulate
chemoresistance. Mol Cell Biol. 34:1839–1849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Schewe DM and Aguirre-Ghiso JA:
ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor
cells in vivo. Proc Natl Acad Sci U S A. 105:10519–10524. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Shimodaira Y, Takahashi S, Kinouchi Y,
Endo K, Shiga H, Kakuta Y, Kuroha M and Shimosegawa T: Modulation
of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP
homologous protein (CHOP) and inositol-requiring enzyme 1α (IRE1α)
in human colon cancer cells. Biochem Biophys Res Commun.
445:524–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Mahoney E, Lucas DM, Gupta SV, Wagner AJ,
Herman SE, Smith LL, Yeh YY, Andritsos L, Jones JA, Flynn JM, et
al: ER stress and autophagy: New discoveries in the mechanism of
action and drug resistance of the cyclin-dependent kinase inhibitor
flavopiridol. Blood. 120:1262–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Dey S, Tameire F and Koumenis C: PERK-ing
up autophagy during MYC-induced tumorigenesis. Autophagy.
9:612–614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ma X, Piao S, Dey S, Mcafee Q, Karakousis
G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G, et al: Targeting
ER stress-induced autophagy overcomes BRAF inhibitor resistance in
melanoma. J Clin Invest. 124:1406–1417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hart LS, Cunningham JT, Datta T, Dey S,
Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, et al: ER
stress-mediated autophagy promotes Myc-dependent transformation and
tumor growth. J Clin Invest. 122:4621–4634. 2012. View Article : Google Scholar : PubMed/NCBI
|